Physiological and Ultrastructural Alterations in the Crayfish Procambarus clarkii Treated with Spinosad (Bacterial Derived Insecticide)
Received: 07-Nov-2017 / Accepted Date: 12-Jan-2018 / Published Date: 20-Jan-2018 DOI: 10.4172/2168-9652.1000226
Abstract
Physiological and ultrastructural investigations have been carried out on Procambarus clarkii exposed to sublethal concentrations of Spinosad insecticide. The study showed that the highest mortality percentage was 88% for males and 70% for female occurred at 64 × 10³ ppm Spinosad. The lethal toxicity (LC50) was 24.1 g/l and 29.8 g/l for males and females respectively. Exposure to 1/2 LC50 (12.07 g/l). Spinosad for 7 days caused many physiological disorders including decrease the haemolymph glucose levels and elevated uric acid levels.12.07 g/l Spinosad resulted in a significant decrease in the total proteins of haemolymph, hepatopancreas and muscles in both males and females. The total cholesterol and triglycerides levels were significantly increased in both haemolymph and hepatopancreas and decreased in muscles of males. On the other hand, cholesterol and triglycerides levels were significantly decreased in haemolymph, hepatopancreas and muscles of females. TEM examinations of male hepatopancreas exposed to 12.07 g/l Spinosad for 7 days revealed ruptured microvilli of absorptive cells, deformed mitochondria, destructed rough endoplasmic reticulum, vacuolated cytoplasm, pyknotic nuclei and appearance of vesicles containing small dark granules.
Keywords: Physiological; Ultrastructure alterations; P. clarkii spinosad
Introduction
Procambarus clarkii was introduced in the early 1980’s into Egypt for aquaculture [1]. They were left without control and had invaded most of the governorates of Upper and Lower Egypt and its distribution has extended from northern Delta to Assiute governorate [2]. Fresh water crayfish can largely prey on snails and mosquitoes thus can be used as a biological control agent [3,4]. Crayfish utilized by man in many countries including Egypt because they are cheaper than the marine prawns [5]. In California, Hawaii, Spain, Japan and Egypt their burrowing activity has caused considerable agricultural damage to irrigation structures such as dams besides eating the roots and shoots of various aquatic crops [6,7]. P. clarkii was known to cause a lot of damage to the fisheries of the Nile possibly by eating the fry and the young fish and damaging the nets of fishermen [8]. The international introduction of P. clarkii into different countries has almost negative consequences, so considerable effort has been paid to control its dispersal with pesticides [9,10]. Pesticides attract public concern due to their potential transport from one environment to another and their effect on non-target biota [11]. The intensive application of chemical pesticides near an aquatic environment may cause hazards to the organisms inhabiting those areas including non- target animals such as fish and shrimp [12]. Spinosad is a bacterial-derived insecticide that contains two active ingredients, spinosyn A and D which are derivatives of the actinomycete, Saccharopolyspora spinosa that occurs naturally in the soil [13,14]. It Kills through contact and ingestion action. Spinosad causes neuronal excitation in insects and after periods of hyper excitation, lice become paralyzed and die [15]. Due to its safety to the environment, mammals, fishes and beneficial insects, Spinosad was registered under the US Environmental Protection Agency reduced risk program [16]. Little works have been carried out on the use of Spinosad in controlling P. clarkii [17]. Therefore, the present study aimed to spot the light on some physiological and ultrastructural alterations in the freshwater crayfish P. clarkii after being exposed to Spinosad.
Materials and Methods
Collection and acclimation of specimens
Adult P. clarkii were collected by using 0.7 cm diagonal net size from Bany Helal irrigation canal at Sharkia Governorate during April 2017. The collected specimens were transferred alive to the laboratory, where they maintained in glass aquaria (17.5 h × 38.5 l × 23 w cm). The conditions were maintained at 25°C and a 12:12 h light-dark regime. Water was changed every four days. Animals were fed with carrot.
Determination of Spinosad LC50: Stock solutions of Spinosad were prepared by using distilled water as a solvent to give 4 × 103, 8 × 103, 16 × 103, 32 × 103 ppm. Three replicates per each concentration were used to determine the LC50. Ten full mature animals either males and females (9- 12 cm) were placed in each aquarium. An equal number from each sex were left without treatment as a control. Experiments were checked at 24 h intervals up to 96 h. The dead crayfish were counted and reported. LC50 was determined according to Finney [18] by the graphic method of the curve dose-effect, using the profit analysis.
Chronic exposure
In the long-term exposure, 1/4 and 1/2 of 96 h LC50of the tested pesticides was used and redosed every 4 days in a static renewal manner. Living animals, surviving the effect of the tested pesticide were sacrificed after 7 days of exposure.
Haemolymph and tissue sampling
Haemolymph sampling: Haemolymph was obtained from conscious P. clarkii by direct puncture of the heart using a syringe containing EDTA as anticoagulant for subsequent analysis.
Tissue sampling: After decapitation of P. clarkii, pieces of muscle and hepatopancreas were taken for physiological and ultrastructural studies.
Physiological studies
Haemolymph glucose and uric acid levels were measured according to GOD-PAP method [19]. Haemolymph, hepatopancreatic and muscle total proteins were determined according to the method of the Biuret method [20].
Triglycerides and total cholesterol in haemolymph, hepatopancreas and muscle were measured according to the method of Fernandez et al. [21] (Tables 1-4).
| Exposure period | Pesticide | |||
|---|---|---|---|---|
| ½ LC50for 15 days | 96 h LC50 | |||
| Female | Male | Female | Male | |
| 14.9 | 12.07 | 29.8 | 24.1 | Spinosad (g/l) |
Table 1: (LC50) values and sub lethal concentrations of both male and female P. clarkii exposed to different Spinosad concentrations under laboratory conditions.
| Glucose | Total proteins | Total cholesterol | Triglycerides | Uric acid | |
|---|---|---|---|---|---|
| Control male | 104.8 ± 2.54 | 3.9 ± 0.98 | 11 ± 1.73 | 26.07 ± 2.52 | 1.31 ± 0.12 |
| Treated male | 87 ± 1.53** | 4.3 ± 0.17 | 13 ± 1.15* | 27.53 ± 4.17 | 1.66 ± 0.33 |
| Control female | 90.2 ± 1.43 | 3.5 ± 0.29 | 13.9 ± 1.59 42.17 ± 2.17 |
42.17 ± 2.17 | 0.43 ± 0.20 |
| Treated female | 74.7 ± 3.46* | 3.97 ± 0.03 | 8.2 ± 2.72* | 54.8 ± 1.98* | 0.98 ± 0.56 |
Data are represented as means of three samples ± SE, * Significant difference (p = 0.05)
**Highly significant difference (P = 0.01)
Exposure of mature male and female P. clarkii to 1/2 LC50 (12.07 g/l) Spinosad for 7 days caused many physiological disorders such as decreasing the haemolymph
glucose levels and elevating uric acid levels. 12.07 g/l Spinosad resulted in also a significant decrease in the total proteins of haemolymph, hepatopancreas and muscles
in both males and females
Table 2: Changes in haemolymph biochemical parameters in both male and female P. clarkii exposed to 12.07 g/l Spinosad for 15 days.
| Total proteins | Total cholesterol | Triglycerides | |
|---|---|---|---|
| Control male | 25 ± 1.73 | 9 ± 1.15 | 238 ± 3.7 |
| Treated male | 19.1 ± 0.49* | 13.1 ± 0.85* | 432.3 ± 33.9** |
| Control female | 28 ± 0.58 | 20 ± 2.65 | 265 ± 8.7 |
| Treated female | 22.2 ± 1.59* | 18 ± 2.15* | 249.9 ± 11.5* |
Data are represented as means of three samples ± SE
* Significant difference (p = 0.05)
**Highly significant difference (P = 0.01)
The total cholesterol and triglycerides levels were significantly increased in both
haemolymph and hepatopancreas while decreased in muscles of males
Table 3: Changes in hepatopancreas biochemical parameters in both male and female P. clarkii exposed to 12.07 g/l Spinosad for 15 days.
| Total proteins | Total cholesterol | Triglycerides | |
|---|---|---|---|
| Control male | 60.14 ± 1.8 | 96.1 ± 0.4 | 94 ± 3.46 |
| Treated male | 53.17 ± 1.05* | 4.3 ± 0.3* | 70.17 ± 1.14** |
| Control female | 48.7 ± 0.86 | 8.15 ± 0.94 | 97.6 ± 4.33 |
| Treated female | 44.42 ± 0.94* | 5.7 ± 0.29* | 79.3 ± 0.64* |
Data are represented as means of three samples ± SE
* Significant difference (p = 0.05)
**Highly significant difference (P = 0.01)
On the other hand, cholesterol and triglyceride levels were significantly decreased
in haemolymph, hepatopancreas and muscles of females
Table 4: Changes in muscles biochemical parameters in both male and female P. clarkii exposed to 12.07 g/l Spinosad for 15 days.
Transmission electron microscopy preparations
The dissected hepatopancreas of crayfish exposed to either clean water or 1/2 LC50 of Spinosad for 7dayswas fixed in 2.5% glutraldehyde in 0.05 M cocodylate buffer containing 0.15 sucrose at pH 7.2 for 2 h. Ultra-thin sections stained with aqueous urinyl acetate and lead citrate and then examined using a JOEL Transmission Electron Microscopic at the regional center for Mycology and Biotechnology in El-Azhar University, Nasr city, Cairo, Egypt.
Statistical analysis
The statistical analysis was performed using SPSS 14.00. The mean values obtained in the different groups were compared by unpaired student’s t-test.
Results
Mortality tests of Spinosad on P. clarkii
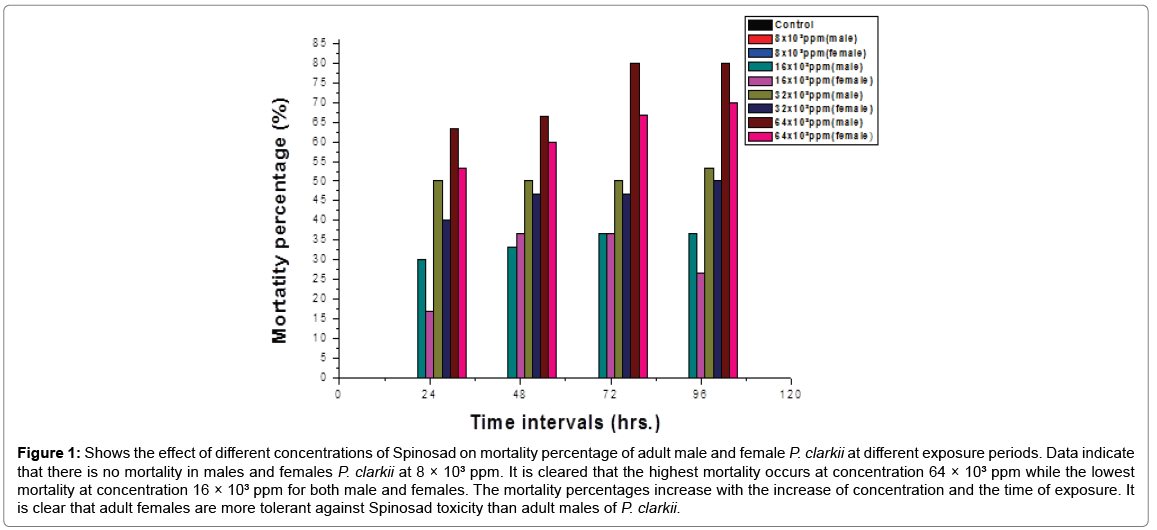
Effect of different concentrations of Spinosad on mortality percentages of adult males and females P. clarkii at different exposure periods (Figure 1).
Figure 1: Shows the effect of different concentrations of Spinosad on mortality percentage of adult male and female P. clarkii at different exposure periods. Data indicate that there is no mortality in males and females P. clarkii at 8 × 103 ppm. It is cleared that the highest mortality occurs at concentration 64 × 103 ppm while the lowest mortality at concentration 16 × 103 ppm for both male and females. The mortality percentages increase with the increase of concentration and the time of exposure. It is clear that adult females are more tolerant against Spinosad toxicity than adult males of P. clarkii.
Lethal toxicities (LC50) of Spinosad pesticide
The different LC50values of male and female P. clarkii exposed to Spinosad were illustrated in Table 1.
Analytical studies
Transmission Electron Microscopy examinations of hepatopancreas of P. clarkii:
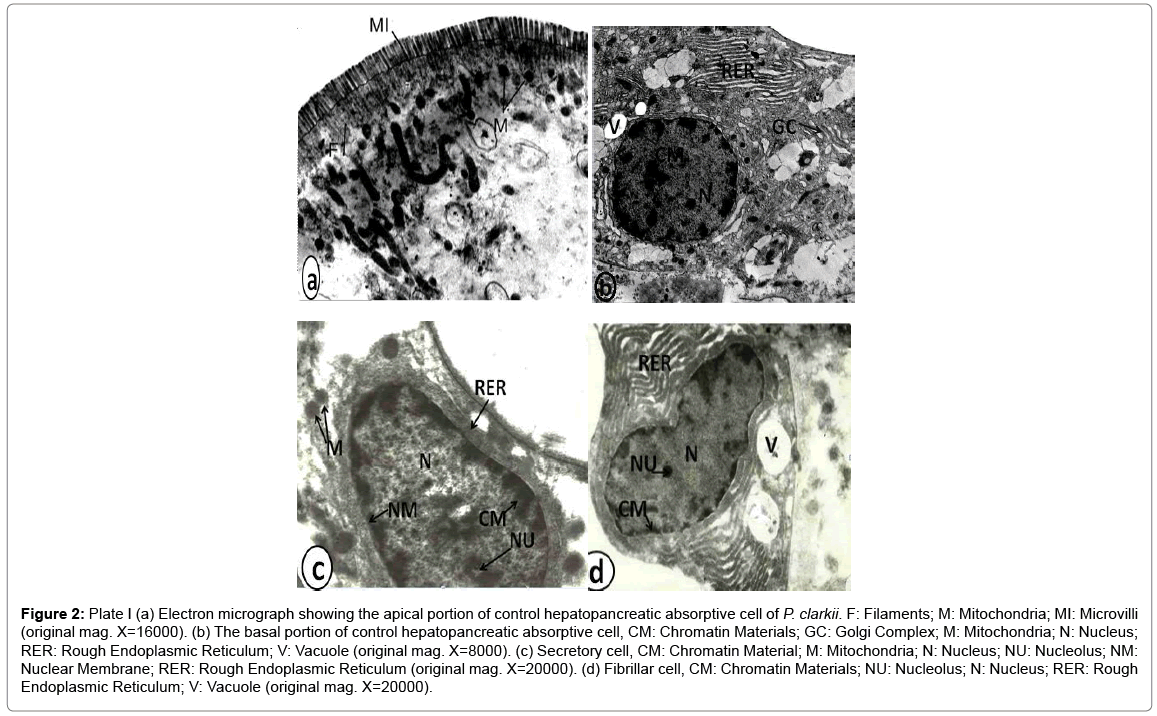
Normal hepatopancreas: Transmission electron microscopy examinations have been revealed the presence of three distinct cell types in the hepatopancreas of P. clarkii. These three types are the absorptive cells (Ac), secretory cells (Sc) and fibrillar cells (Fc). The absorptive cell is large columnar with a centrally located nucleus (N). The apical surface of absorptive cell has numerous microvilli (MV). The cytoplasm contains a number of irregularly shaped mitochondria (M) and parallel tubules of rough endoplasmic reticulum (RER) (plate I: a and b) (Figure 2). The secretory cell is the largest cell type and is characterized by the presence of a basely located nucleus (N) with small nucleolus (NU). The cytoplasm of this cell contain very thin layer of rough endoplasmic reticulum (RER) found in a perinuclear position and small numbers of mitochondria (plate I: c). the fibrillar cell (FC) has a large nucleus (N) with prominent nucleolus (NU) and massive rough endoplasmic reticulum (RER) (plate I: d).
Figure 2: Plate I (a) Electron micrograph showing the apical portion of control hepatopancreatic absorptive cell of P. clarkii. F: Filaments; M: Mitochondria; MI: Microvilli (original mag. X=16000). (b) The basal portion of control hepatopancreatic absorptive cell, CM: Chromatin Materials; GC: Golgi Complex; M: Mitochondria; N: Nucleus; RER: Rough Endoplasmic Reticulum; V: Vacuole (original mag. X=8000). (c) Secretory cell, CM: Chromatin Material; M: Mitochondria; N: Nucleus; NU: Nucleolus; NM: Nuclear Membrane; RER: Rough Endoplasmic Reticulum (original mag. X=20000). (d) Fibrillar cell, CM: Chromatin Materials; NU: Nucleolus; N: Nucleus; RER: Rough Endoplasmic Reticulum; V: Vacuole (original mag. X=20000).
Effect of spinosad
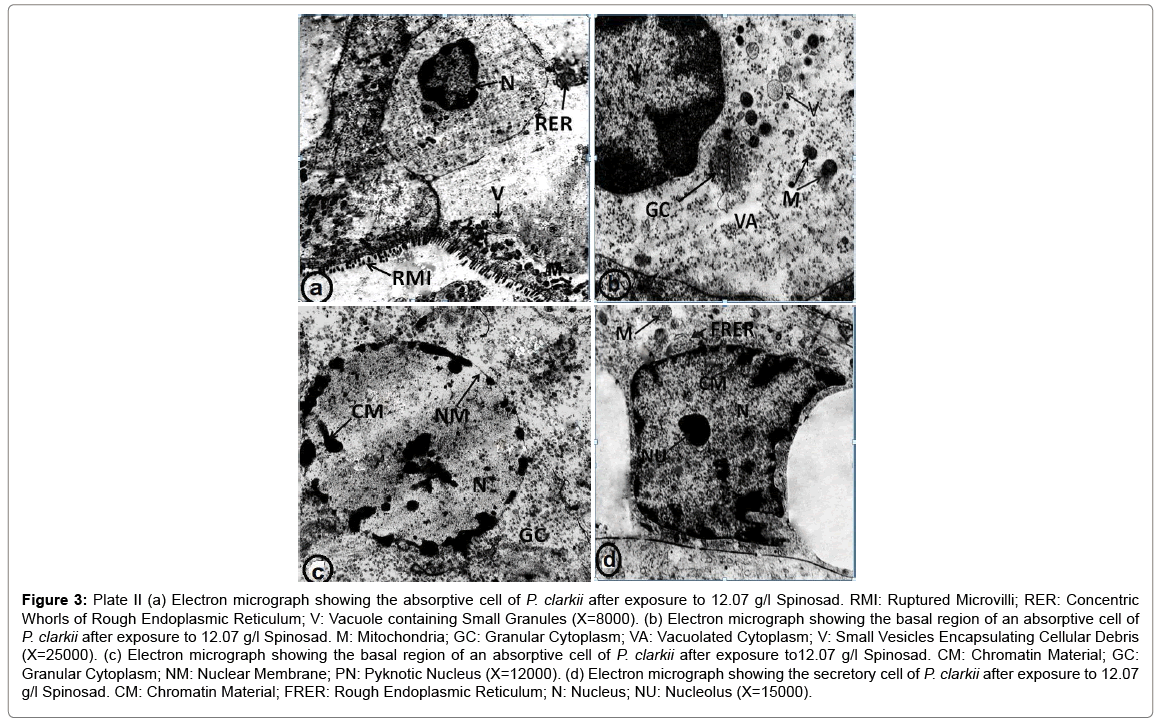
Plate II (Figures 3a-3d) shows electron micrographs of hepatopancreatic cells of P. clarkii after exposure to 1/2 LC50 (12.07 g/l) Spinosad for 7 days. Microvilli of the absorptive cells become ruptured and lost their filaments within the cytoplasm, the rough endoplasmic reticulum had the form of short and thin cisternae and also had the form of concentric whorls (Figure 3a). The cytoplasm of absorptive cell lost its density and became granular and vacuolated, vacuoles contain cellular debris are also noted (Figure 3b). The nucleus become pyknotic (PN), heterochromatin appeared as dense masses scattered in the nucleoplasm and as electron dense aggregates along the inner nuclear envelope (Figure 3c). The rough endoplasmic reticulum become fragmented (Figure 3d).
Figure 3: Plate II (a) Electron micrograph showing the absorptive cell of P. clarkii after exposure to 12.07 g/l Spinosad. RMI: Ruptured Microvilli; RER: Concentric Whorls of Rough Endoplasmic Reticulum; V: Vacuole containing Small Granules (X=8000). (b) Electron micrograph showing the basal region of an absorptive cell of P. clarkii after exposure to 12.07 g/l Spinosad. M: Mitochondria; GC: Granular Cytoplasm; VA: Vacuolated Cytoplasm; V: Small Vesicles Encapsulating Cellular Debris (X=25000). (c) Electron micrograph showing the basal region of an absorptive cell of P. clarkii after exposure to12.07 g/l Spinosad. CM: Chromatin Material; GC: Granular Cytoplasm; NM: Nuclear Membrane; PN: Pyknotic Nucleus (X=12000). (d) Electron micrograph showing the secretory cell of P. clarkii after exposure to 12.07 g/l Spinosad. CM: Chromatin Material; FRER: Rough Endoplasmic Reticulum; N: Nucleus; NU: Nucleolus (X=15000).
Discussion and Conclusion
The red swamp crayfish, Procambarus clarkii was introduced worldwide and has become the dominant freshwater crayfish in almost all areas it occupies [22]. P. clarkii greatly spread all over the River Nile [10]. Considerable efforts have been paid to control its dispersal with pesticides [23,24]. Spinosad may be used to control agricultural pests. In the present study, toxicological effects of Spinosad on P. clarkii were determined. This study indicated that there is no mortality in males and females P. clarkii at 8 × 103 ppm Spinosad. The highest mortality occurs at concentration 64 × 103 ppm while the lowest mortality at concentration 16 × 103 ppm for both male and females. It is also clear that adult females are more tolerant to toxicity than adult males of P. clarkii. LC50 of Spinosad for 96 h. for P. clarkii has been determined to be 24.1 and 29.8 g/l for adults male and female, respectively. This result disagree with Abdel-Kader [25] who calculated LC50 of Spinosad for 5 days to be 858 and 4338 ppm for adults male and female P. clarkii, respectively.
Physiological measurements have been used as indicators of the state of animal health condition and as a biochemical method for assessing the possible mode of action of stressors [26,27]. Analyses of haemolymph constituents have proved to be useful in the detection and diagnosis of metabolic disturbances and disease processes [28]. Blood glucose appeared to be a sensitive and reliable indicator of environmental stress [29]. The present study showed clearly that glucose level in haemolymph of male and female P. clarkii was significantly decreased after treatment with Spinosad. This increase may be due to an increase in haemolymph concentration of catecholamines and corticosetroids [30] as a stress response of animal subjected to environmental alterations. This finding is in agreement with Winkaler et al. [31] reported a high plasma glucose levels in the fish Prochilodus lineatus exposed to neem extract. The present study showed clearly that uric acid in haemolymph of male and female P. clarkii significantly increased after treatment with Spinosad. Low values of uric acid have insignificant meaning but increasing values indicate several disturbances in the kidney [32]. This increase in haemolymph uric acid concentrations may be attributed to the action of pesticides as well as oxygen insufficiency which causes pathological changes of the green glands [33]. Moreover, accumulation of pesticide in the green gland which may cause damage of cells followed by an increase in haemolymph uric acid. This finding is in agreement with Hamdi [34] who showed that there is increase in haemolymph uric acid of crayfish P. clarkii after treatment with Malathion. Proteins are important biochemical components and an assessment of the total protein content in haemolymph, hepatopancreas and muscle could be used as a diagnostic tool for determining the physiological status of an organism [35]. This study shows also that Spinosad cause an increase in haemolymph total protein, but they cause a decrease in hepatopancreatic and muscle total protein .This finding is not in agreement with El-Sheikh [36] who reported a decrease in haemolymph total protein of Spodoptera littoralis exposed to Spinosad and he explained that this decrease may be due to inhibition of DNA and RNA synthesis. This finding is also in agreement with Hamdi [34] who reported a decrease in total protein of both hepatopancreas and muscle of crayfish P. clarkii exposed to Malathion and she explained that this depletion may be attributed to the toxic effect of the pesticide, however. The depletion of the total protein levels in haemolymph and the tissues of hepatopancreas and muscle of test crayfish may be due to the enhanced proteolytic activity in these organs under pesticide stress. Also, this increase in haemolymph total protein may be due increased biosynthesis process occurred by the high enzyme stress to withstand pesticide entrances defensive case. This finding is in agreement with Senthil et al. [37] and Narra et al. [38] reported decreasing in total protein level in haemolymph, hepatopancreas and muscle of freshwater field crabs Spiralothelphusa hydrodroma and Barytelphusaguerini, respectively exposed to Chlorpyrifos.
Cholesterol is a major concern of diet-conscious persons [39]. The present study showed that Spinosad have induced decreasing in total cholesterol and triglyceride concentrations in haemolymph, hepatopancreas and muscle in both sexes. The decrement in the cholesterol and triglyceride may be due to the increased activity levels of lipase, the enzymes responsible for the breakdown of lipid into free fatty acid and cholesterol. This decline may be due to increase hormonal secretions that enhance metabolic rate which in turn reduce the metabolic reserve of the triglyceride [40]. Also, it may be due to the imposition of high energy demands to counter the toxic stress. A decrease in hepatopancreatic and muscle total cholesterol and triglyceride has been reported in P. clarkii exposed to Malathion [34] and this decrease might be attributed to the alteration of the hepatopancreas which were manifested by vacuolar degeneration of the hepatocytes. On the other hand, the increase in cholesterol and triglyceride in haemolymph and hepatopancreas of males may be due to inhibition of lipase activity and other enzymes of lipid metabolism.
In Crustacea, there is a correlation between the physiological condition of the organism and the structure and the appearance of hepatopancreas [41]. The hepatopancreas represents a corner stone in the body metabolism and considered to be the most sensitive organ to pollutants and toxicants [42] and the main site of accumulation and detoxification in crayfish bodies [43]. The present study showed clearly that there are three main cell types forming the digestive tubules of P. clarkii. These cells were the absorptive, secretory and fibrillar cells. These findings are in agreement with Abdelmonem et al. [5]. The obtained results showed that Spinosad caused ruptured microvilli of the absorptive cells, granular and vacuolated cytoplasm; rough endoplasmic reticulum had the form of concentric whorls and nuclear pyknosis. Heterochromatin condensation and marginalization may be due to progressive inactivation of nuclear component [44]. Electron micrographs showed also the presence of vacuoles containing cytoplasmic debris in the cytoplasm. These vacuoles probably arisen to digest the destructed cellular organelles as a result of treatment with the tested pesticides. Asztalos et al. [45] proposed that the focal development of empty vacuoles might be the starting point of cellular autolysis process. The presences of autophagic vacuole in this study improve the detoxification role of hepatopancreatic cells against pesticides. The fragmentation of RER might be a consequence of final hyperactivity prior to cell necrosis [46].
References
- Ibrahim AM, Khalil MT, Mubarak MF (1995) On the feeding behavior of the exotic crayfish, P. clarkii in Egypt and its prospects in the bicontrol of local vector snails. J Union Arab Biol Cairo 4: 321-340.
- Saad AA, Emam WM (1998) Field and semifield studies on the crayfish P. clarkii in Egypt. Egypt J Aquat Biol Fish 2: 331-344.
- Feminella JW, Resh VH (1986) Effects of crayfish grazing on mosquito habitat at Coyote Hills Marsh. Proceeding and papers of the annual conference of the California Mosquitoes and Vector Control Association 54: 101-104.
- Hofkin BV, Hofinger DM, Koech ES, Locker ES (1992) Predation of Biomphalariaand non-target molluscs by the crayfish Procambarus clarkii: Implications for the biological control of schistosomiasis. Ann Trop Med Parasitol 86: 663-670.
- Abdel MK, Mahmoud AS, Zeinab ZK, Radwa MS (2015) Effect of chlorpyrifos and neem seed extract (Azadirechtein) on hepatopancreatic cellular structures of the freshwater crayfish Procambarus clarkii. Egypt J Aquat Biol 19: 125-136.
- Huner JV, Barr JE (1991) Red swamp crawfish: Biology and exploitation. In: Elizabith, B.C. (3rd edn). The Louisiana sea grant program, Center for wetland resources, Baton Rouge, Louisiana, p: 128.
- Abd El-Atti MS (2002) Effect of water quality and some parasites on some biological aspects of freshwater crayfish Procambarus clarkii at Sharkia governorate. Ph.D. Thesis, Fac Sci, Zagazig Univ, Egypt, p: 152.
- Soliman GN, El-Assal F, El-Deen MS, Hamdi SAH (1998) The reproductive biology of the red swamp crawfish Procambarus clarkii (Girard, 1852) (Decapoda: Cambridae) in the River Nile, Egypt. Egypt J Zool 30: 311-325.
- Lang WH, Chang VCS (1967) Laboratory and field evaluation of selected pesticides for control of the red crayfish in California rice fields. Ecotoxicol Environ 60: 473-477.
- Ibrahim AM, Emam WM, Abdel-Rahman S (2005) Ridding of undesirable crayfish Procambarus clarkii from certain habitat in Egypt by means of organophosphorus insecticide. J Egypt Acad Soc Environ Develop 6: 267-276.
- Klassen CD (1986) Cassarett's and Doulls Toxicology: The Basic Science of Poisons (3rd edn) MacMillan publ. Co., NY, pp: 1628-1650.
- Barbee GC, Stout MJ (2009) Comparative acute toxicity of neonicotinoid and pyrethroid insecticides to non-target crayfish (Procambarus clarkii) associated with rice–crayfish crop rotations. Pest Manag Sci 65: 1250-1256.
- Mertz FP, Yao RC (1990) Saccharopolyspora spinosa sp. isolated from soil collected in sugar mill rum still. Inter System Bacteriol 40: 34-39.
- http://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/reference/ReferencesPapers.aspx?ReferenceID=1523616
- Kirst HA (2010) The spinosyn family of insecticides: Realizing the potential of natural products research. J Antibiot 63: 101-111.
- Flye MP (2003) Efficacy of Spinosad against cherry fruit flies in Washington state. At the 6th Slovenian Conference on Plant Protection, pp: 211-218.
- Radwa MS (2015) Toxicological effects of some natural and synthetic pesticides on the fresh water crayfish Procambarus clarkii. MSc Thesis, Fac Sci Zagazig Univ, Egypt, p: 195.
- Finney DJ (1971) Probit analysis, 3rd edn. Cambrige. Univ. Press, London
- Trinder P (1969) Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol 22: 246.
- Wooton LDP (1964) Micro-analysis in medical biochemistry. In: Churchill ltd, London, Basal, Karger pp.264.
- Fernandez, MAS, Mendoca MIR, Marques JC, Madeira CM (1994) Energy and nutrient storage capacity of Procambarus clarkii in the lower Mondego River (Portogal). Freshwater Crayfish 10: 98-104.
- Henttonen P, Huner JV (1999) The introduction of alien species in Europe: A historical introduction. In: Gherardi F and Holdich DM, Editors, 1999.Crayfish in Europe as Alien Species. Crustacean Issues 11, A.A. Balkema, Rotterdam, pp: 13-22.
- Hobbs HH, Jass JP, Huner JV (1989) A review of global crayfish introductions with particular emphasis on two north American species (Decapoda, Cambaridae). Crustaceana 56: 299-316.
- Aly RH (2000) Effect of natural and chemical insecticides on some organs of the female crayfish, Procambarus clarkii (Crustacea: Decapoda) from the River Nile, Egypt J Aquat Bio Fish 4: 83-103.
- Abdel-Kader SM (2011) Some biochemical and histopathological changes in Procambarus clarkii exposed to acute toxicity of neem extract, Neemix, Abbassa, Int J Aquat 4: 93-113.
- Heath WA (1975) Water pollution and fish physiology. In: Crc Press. Boca Raton, Florida, pp: 130-136.
- Schreck CB, Molyle PM (1990) Methods for fish biology. Am Fish Soc Bathesda Maryland, pp: 115-119.
- Aldrin JF, Messager GL, Baudin LF (1982) La biochemie Clinique en aquaculture Interst et perspective. Cnexo Acets Colloq 19: 291-326.
- Nemcsok M, Boross L (1982) Comparative studies on the sensitivity of different fish species to metal pollution. Acta Biol Acad Sci Hung 33: 23-27.
- Pickering AD (1981) Stress and compensation in teleostean fishes. Response to social and physical factors. In: Stress and Fish, Pickering, A.D. (ed.) Academic press, New York/London, pp: 295-322.Â
- Winkaler EU, Santos TRM, Machado-Neto JG, Martinez CBR (2007) Acute lethal and sublethal effects of neem leaf extracts on the neotropical freshwater fish Prochilodus lineatus. Comp Biochem Physiol C Toxicol Pharmacol 145: 236-244.
- Maxine M, Benjamin BS (1985) Outline of veterinary clinical pathology. In: (3rded), Colorado State University. Printed in India at Rekha printers Pvt. Ltd, New Delhi, 110020.
- Abd El-Atti MS (2005 Histological and ultrastructural alterations in the antennal gland of the crayfish Procambarus clarkii exposed to mercury. Egypt J Aquat Biol Fish 9: 489-503.
- Hamdi SAH (2001) Some physiological changes in the haemolymph, hepatopancreas and muscle of the red swamp crawfish Procambarus clarkii (Girard, 1852) exposed to Malathion. Egypt. Ph.D. Thesis, Fac Sci, Cairo Univ, p: 150.
- Winkaler EU, Santos TRM, Machado-Neto JG, Martinez CBR (2007) Acute lethal and sublethal effects of neem leaf extracts on the neotropical freshwater fish Prochilodus lineatus. Comp Biochem Physiol C Toxicol Pharmacol 145: 236-244.
- Prasath PMD Arivoli S (2008) Biochemical study of freshwater fish Catla catla with reference to mercury chloride. Iran J Environ Health Sci Eng 3: 109-116.
- El- Sheikh TAA (2012) Biological, biochemical and histological effects of spinosad, Bacillus thuringiensis var. kurstaki and cypermethrinon the cotton leaf worm, Spodoptera littoralis. Egypt Acad J Biol Sci 4: 113-124.
- Narra MR, Regatte RR, Kodimyala R (2012) Alterations of energy metabolites in fresh water field crab Barytelphus aguerini treated with chlorpyrifos. Inter Environ Sci Res 1: 67-71.
- Deris NL, Druilhet R (1988) Analysis of crawfish tail meat and fat. University of Southwestern Louisiana, Lafayette, LA 70504.
- Turner CD, Bargnara JT (1976) Endocrine mechanism in the invertebrates In: General Endocrinology Saunders WB Co, Philadelphia, London, Toronnto, pp: 545-558.
- Popescu MV, Manolache V, Nastasescu M, Marinescu C (1997)Structural modifications induced by copper in Astacus leptodactylus (Crustacea: Decapoda) hepatopancreas. Rom J Biol Sci 1: 99-105.
- Senthil KP, Samyappan K, Jaikumar S,Deecaraman M (2007) Effect of chlorpyrifos on the nutritive value in a freshwater field crab, Spiralothelphusa hydrodroma. Res J Agr Biol Sci 3: 760-766.
- Jaiswal K, Sarojini R (1990) Histopathological lesion in the hepatopancreas of freshwater prawn Macrobrachium kistnensis induced by naphthalene poisoning. Environ Pollut Health Hazards Environ Series III: 151-157.
- Lauren DJ, Rice S(1985): Significance of active in passive depuration in the clearance of naphthalene from the tissue of Hemigrapsus nudus (Crustacea: Decapod). Mar Biol 88: 135-142.
- Braunbeck T, Burkhardt HP, Storch V (1990) Liver pathology in eels (Anguilla anguilla) from Rhine river exposed to the chemical spill at Basle in November 1986. Limnol Aktuell 1: 371-391.
- Asztalos B, Nemcsok J, Benedeczky I, Gabriel R, Szabo A (1988) Comparison of effects of paraquat and methidation on enzyme activity and tissue necrosis of carp following exposure to the pesticides singly or in combination. Environ Pollut 55: 123-135.
Citation: El-Atti MSA, Saied RM (2018) Physiological and Ultrastructural Alterationsin the Crayfish Procambarus clarkii Treated with Spinosad (Bacterial DerivedInsecticide). Biochem Physiol 7:226. DOI: 10.4172/2168-9652.1000226
Copyright: © 2018 El-Atti MSA, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the originalauthor and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6240
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 5213
- PDF downloads: 1027