Production of Invertase by Aspergillus niger Under Solid State Fermentation Using Orange Fruit Peel as Substrate
Received: 30-Nov-2016 / Accepted Date: 07-Dec-2016 / Published Date: 12-Dec-2016 DOI: 10.4172/2329-8863.1000247
Abstract
The Aspergillus niger was tested for its ability to produce invertase on natural agricultural residues. Orange peel was selected as substrate due to its high peak enzyme activity, the maximum invertase activity was obtained in preliminary studies by incubating flasks containing orange peel as substrate supplemented with sucrose (1.0% w/v), yeast extract (1% w/v), and 4 ml of A. niger conidial suspension for 96 hours at 30°C.
Keywords: Aspergillus niger; Invertase; Agricultural residues; Solid state fermentation; Secondary metabolites
405821Introduction
Invertase
Invertase [β-fructofuranosidases (EC.3.2.1.26)] is an enzyme that is widely distributed among the biosphere. It is broadly used in the food and beverage industry to produce candies, chocolates, lactic acid and glycerol etc. [1]. Invertase is produced by different strains of microorganisms, Saccharomyces cerevisiae commonly called Baker’s yeast is the primary strain used for the production of Invertase commercially. They are found in wild growing on the skin of grapes, oranges and other fruits. Though plants like pineapple (Ananas comosus), oat (Avena sativa), pea (Pisum sativum), can also be used, but in common microorganisms like A. niger, S. cerevisiae, Candida utilis, are considered ideal for their study [2,3].
The present study carried out with the objective to utilize the fruit peel waste to convert commercially valuable products. Beside their pollution and hazardous aspects, in many cases, food processing wastes might have potential for recycling raw materials or for conversion into useful product of higher. When compared to submerged fermentation, the production of Enzymes by solid state fermentation have potential advantages i.e., simplicity in operation, high productivity fermentation, less favorable for growth contaminants and concentrated product formation [4,5].
Materials and Methods
Microorganism and culture medium
The microorganism Aspergillus niger MTCC 282 was produced from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. The production of Invertase enzyme requires the preparation of inoculum scraped and washed from the slant culture with 10 ml sterile water; and 2 ml of the inoculum was added to each 250 ml flasks containing production medium. The medium used for the production of enzyme under solid-state fermentation composed of (gm/L): Sucrose 25, yeast extract 10, KH2PO4-4.38, (NH4)2SO4-8.76, CaCl2·2H2O 0.088, MgSO4·7H2O - 0.88, pH - 5.0.
Substrates
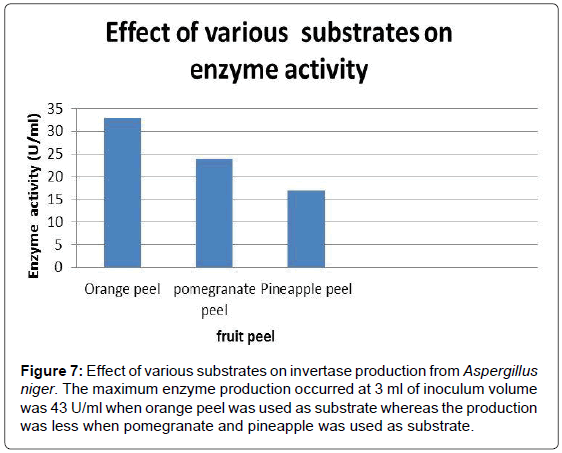
Locally available fruit substrates were collected such as orange peel, pomegranate peel, pineapple peel washed, shade dried, grinded and stored in the polyethylene bags at room temperature. They were autoclaved at 15 lbs for 20 minutes before use [4].
Solid state fermentation
Substrate (peels of orange/pomegranate/pineapple) was collected and dried. 5 g of substrate powder was taken in 250 ml erlenmeyer flask and mixed with water to a solid liquid ratio of 5:4 (w/v). The thoroughly mixed substrate was autoclaved for 20 min at 121°C, 15 lb and cooled to room temperature. Under aseptic conditions, the sterilized solid state medium was inoculated with 2 ml (23 × 106 cfu/ml) spore inoculum. The contents were mixed thoroughly and the flaks were placed in an incubator at 32°C for 24 h intervals [4]. All the sets were prepared in duplicate.
Enzyme extraction
After fermentation, the 250 ml conical flask containing the fermented solid (5 g) was added with the extractant (0.1% Tween 80) in 1:5 w/v ratio, mixed thoroughly on a rotary shaker (150 rev/ min) for 30 min and filtered. After extracting twice the extracts were pooled, centrifuged at 4°C for 20 min at 10,000 rev/min and the clear supernatant was used as crude enzyme for assay and purification studies.
Enzyme assay
DNS method for assay procedure (Miller): DNS method tests for the presence of free carbonyl group (C=O), the so-called reducing sugars. This involves the oxidation of the aldehyde functional group present in glucose [6]. Simultaneously, 3,5-dinitrosalicylic acid (DNS) gets reduced to 3-amino,5-nitrosalicylic acid under alkaline conditions. Reduced sugar (glucose) concentrations were determined at a wavelength of 540 nm by using a UV spectrophotometer and a preconstructed calibration curve.
Reagents: 1. DNS reagent consists of: NaOH -10 g/L, Sodium potassium tartarate - 182 g/L, 3, 5-Dinitrosalysilic acid - 10 g/L, Phenol - 2.0 g/L, Sodium sulphite - 0.5 g/L
2. NaOH - 0.1 M
3. Substrate solution - 10% (w/v) colloidal chitin in phosphate buffer 0.2 M at pH 8.0
Procedure: All reagents making up the DNS reagent were mixed and dissolved in distilled water. 1.0 ml of sample (samples/standard NAG solutions/blank) was added to 1.0 ml of substrate and incubated at 35°C for 1 hour. 1.0 ml of NaOH was added and boiled for 5 minutes to stop enzyme reaction. Mixture was then centrifuged at 4000 rpm for 5 minutes. 1.0 ml of supernatant was added to 1.0 ml of DNS reagent and boiled for 5 minutes until red brown color appear. The solution was cooled to room temperature under running tap water. Absorbance of the solution was determined at 540 nm using spectrophotometer. The enzyme activity was quantified according to DNS standard curve [2,6].
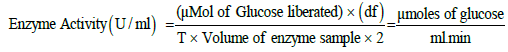
Where, df=dilution factor; Volume of enzyme sample=0.1 ml; T=Incubation time
Results and Discussion
Optimization studies
Both the physico-chemical (i.e., incubation time, incubation temperature, inoculum age, initial pH) and nutritional (i.e., addition of carbon sources, nitrogen sources) parameters are known to influence the production of microbial metabolites during a fermentation process. They were optimized by the conventional method of optimizing one independent parameter at a time, while fixing all others at a certain value [7]. The parameter optimized in one experiment was maintained in the subsequent experiments. The same production medium used in secondary screening was used for optimization studies. In all the experiments the flasks were inoculated with 2 ml of inoculum prepared from the slant cultures [8].
The parameters namely Effect of Incubation time, Effect of Incubation temperature, Inoculum volume, Effect of pH, Effect of Carbon source and Nitrogen sources were chosen for the optimization of Invertase production [2,9].
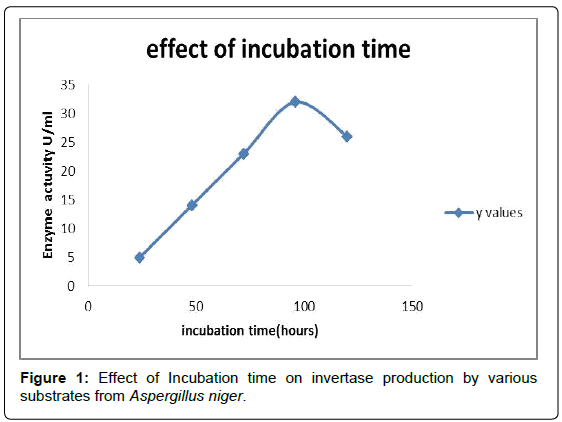
Effect of incubation time
To determine the effect of incubation time on Invertase production, 50 ml of the production medium was inoculated with 4% inoculum prepared from 5 day old culture and incubated at varying fermentation times ranging from 1 day to 4 days. After every 24 hours, the extracts were evaluated for Invertase activity [9]. Invertase production by Aspergillus niger has increased steadily from 24 hours to 96 hours and a further increase in incubation time resulted in a decrease in Invertase production (Figure 1; Tables 1 and 2). The decrease in Invertase production after 96 hours might be due to a reduction in the available nutrients and accumulation of toxic products of metabolism.
| Conditions | Enzyme activity (U/ml) |
|---|---|
| Carbon source | 45 |
| Fructose | 38 |
| Glucose | 31 |
| Lactose | 47 |
| Sucrose | |
| Nitrogen source | |
| Peptone | 46 |
| Urea | 34 |
| Yeast | 51 |
| Malt | 43 |
Table 1: Effect of Carbon and Nitrogen sources on Invertase production by Aspergillus niger.
| Optimized parameter | Optimized condition |
|---|---|
| Incubation time | 96 hours |
| Incubation temperature | 30°C |
| Inoculum volume | 3 ml |
| Initial pH | 5.0 |
| Carbon source | Sucrose |
| Nitrogen source | Yeast |
Table 2: Optimized conditions for Invertase production by Aspergillus niger.
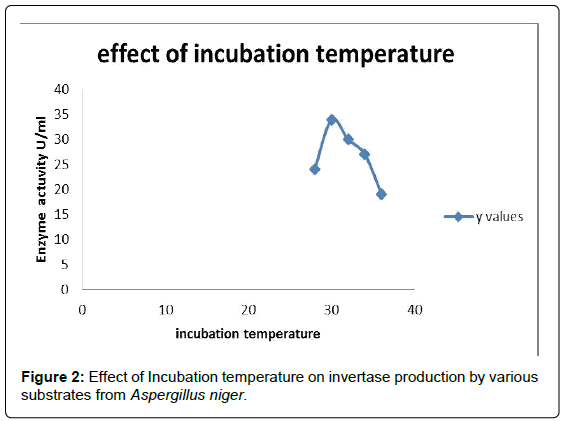
Effect of incubation temperature
To determine the effect of incubation temperature on Invertase production, 50 ml of the production medium was inoculated with 4% inoculum prepared from 5 day old culture and incubated at varying fermentation temperatures of 28°C, 30°C, 32°C, 34°C and 36°C. After 4 days, the extracts were evaluated for Invertase activity. The strain has shown maximum Enzyme production at a temperature of 30°C (Figure 2 and Table 2). From the result, we can observe that temperature had a profound impact on Invertase production [10].
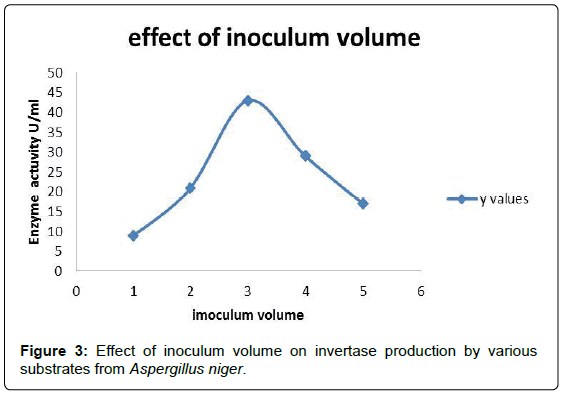
Effect of inoculum volume
To determine the effect of inoculum volume on invertase production, 50 ml of the production medium inoculated with 1 ml, 2 ml, 3 ml, 4 ml and 5 ml inoculum prepared from 5 day old culture and incubated at 30°C. The strain has shown maximum enzyme production 43 U/ml when the volume of the medium was 3 ml (Figure 3 and Table 2).
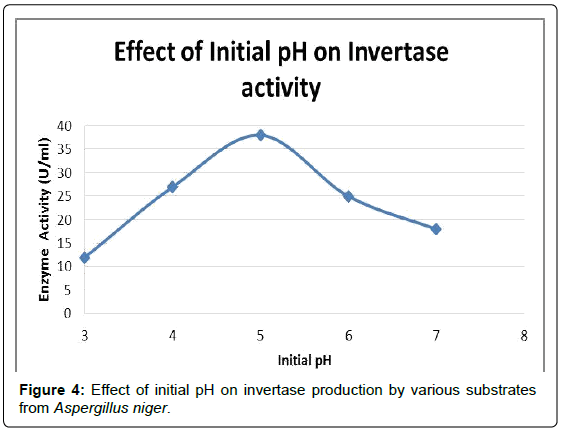
Effect of pH
To determine the optimum pH for Invertase production, pH of the production medium was adjusted [11] (with 0.1 N HCl and 0.1 N NaOH) to different pH values of 3, 4, 5, 6, 7 and then inoculated with 4% inoculum prepared from a 5 day old slant culture and incubated at 30°C for 4 days. The strain has shown maximum Invertase production when the initial pH of the medium was 5 (Figure 4 and Table 2).
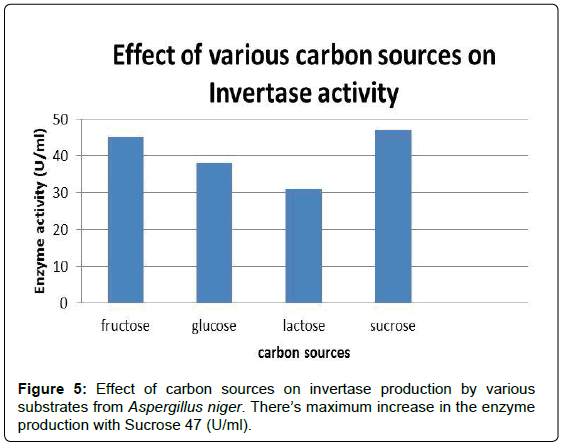
Effect of carbon source
To determine the effect of carbon source on Invertase production, different carbon sources such as fructose, glucose, lactose and sucrose were added at a concentration of 1% w/v to the production medium (pH 5) and inoculated with 4% inoculum from a 5 day old culture. The flasks were incubated at 30°C for 4 days and subsequently evaluated for Invertase activity. The strain has shown maximum enzyme production when sucrose was used as the carbon source (Figure 5 and Table 2). Sucrose has resulted in a significant increase in Invertase production compared to control where Glucose was used as the carbon source [12].
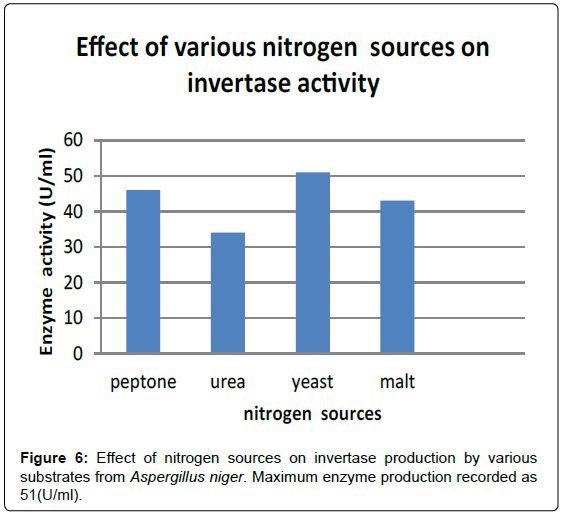
Effect of nitrogen source
To determine the effect of nitrogen source on Invertase production, different nitrogen sources such as Peptone, Urea, Yeast and Malt were added at a concentration of 1% w/v to the production medium (pH- 5) containing sucrose as the carbon source instead of glucose and inoculated with 4% inoculum from a 5 day old culture [13]. The flasks were incubated at 30°C for 96 hours and subsequently evaluated for Invertase activity. The strain has shown maximum enzyme production when Yeast was used as the nitrogen source (Figures 6 and 7; Table 2).
Conclusion
The current study points to the successful utilization of agroindustrial waste, Orange peel as substrate for invertase production by A. niger under solid state fermentation. Considering the production cost, reutilization of bio-resources, feasibility of the solid state fermentation and therapeutically favourable features of the enzymes of eukaryotic origin, utilizing of fungi for production of invertase was a promising approach. Therefore, solid state fermentation seems to be the prospective technique for large-scale production of microbial metabolites of biotechnological importance.
Acknowledgements
The authors would like to convey thanks to the Department of Chemical Engineering, Andhra University (A), Visakhapatnam, for providing the laboratory facilities. The authors wish to express gratitude to Coworkers and Friends for their support and encouragement through the duration of the research work.
References
- Aehle W (2004) Industrial enzymes: Enzymes in food applications. Enzymes in industry: Production and applications. Wiley.
- Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures.
- Schuster E, Dunn Coleman N, Frisvad J, Van Dijck P (2002) On the safety of Aspergillus niger - a review. Applied Microbiology and Biotechnology 59: 426-435.
- Uma C, Gomathi D, Multhulakshmi C, Gopalkrishana VK (2010) Production, Purification and characterization of invertase by Aspergillus flavus using fruit peel waste as substrate. Advances in Biological Research 4: 31-36.
- Singhania RR, Patel AK, Soccol CR, Pandey A (2009) Recent Advances in solid-state fermentation. Biochem Eng J 44: 13-18.
- Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426-429.
- Durand A (2003) Bioreactor designs for solid state fermentation. Biochem Eng J 13: 113-125.
- Neumann NP, Lampen JO (1967) Purification and properties of yeast invertase. Biochemistry 6: 468-475.
- Mehta K, Duhan JS (2014) Production of Invertase from Aspergillus niger Using Fruit Peel Waste As Substrate. Int J Pharm Bio Sci 5: 353-360.
- Benkeblia N, Varoquaux P (2003) Effect of irradiation, temperature and storage time on glucose, fructose and sucrose status of onion bulbs Allium cepa L. Int Agrophysics 17: 1-5.
- Vitolo M, Duranti MA, Pellegrim MB (1995) Effect of pH, aeration and sucrose feeding on the invertase activity of intact S. cerevisiae cells grown in sugarcane blackstrap molasses. Journal of Industrial Microbiology 15: 75-79.
- Vitolo M, Yassuda MT (1991) Effect of sucrose concentration on the invertase activity of intact yeast cells. Biotechnol Lett 13: 53-56.
- Kamble Suresh P, Borate Jyotsna C (2012) Effect of nitrogen sources on the production of invertase by yeast Saccharomyces cerevisiae 3090. International Journal of Applied Biology and Pharmaceutical Technology 3: 297-300.
Citation: Raju AI-CH, Pulipati K, Jetti A (2016) Production of Invertase by Aspergillus niger Under Solid State Fermentation Using Orange Fruit Peel as Substrate. Adv Crop Sci Tech 4:247. DOI: 10.4172/2329-8863.1000247
Copyright: © 2016 Raju AI-CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15049
- [From(publication date): 0-2016 - Aug 18, 2025]
- Breakdown by view type
- HTML page views: 13489
- PDF downloads: 1560