Purification and Characterization of Alcayota Gum. Experimental Reviews
Received: 15-Mar-2018 / Accepted Date: 18-Mar-2018 / Published Date: 23-Mar-2018
Abstract
In this work by means of alkaline hydrolysis, the extraction of alcayota gum from Cucurbita ficifolia was carried out. The gum is purified by hydroalcoholic precipitations. This rubber is characterized by measurements of viscositydensity, Dynamic Light Scattering (DLS), and rheological. The intrinsic viscosity of the gum was 149.83 cm3/g and the molecular weight of 1867 kDa (whose Mark-Houwink parameters were k=0.00263 and a=0.7583), a shape factor of 3.12 and a hydration value of 47.63 g/g. From DLS the hydrodynamic radius was determined with a value of 53 nm, a molecular weight of 1722 kDa and with Mark-Houwink parameters of aD=0.5998 and kD=0.002543. The studies carried out for CyOH(d) showed a thixotropic behavior for the dispersed solutions that increased with the increase in hydrolysis and glycerin and decreased with the increase in temperature. The degree of thixotropy is higher for CyOH(d) compared to Cy(d). This polysaccharide can be applied in the film forming technology, as a thickening agent, etc.
Keywords: Cucurbita ficifolia; Gum; Polysaccharide; Dynamic light scattering; Intrinsic viscosity; Molecular weight
Introduction
New polysaccharides
Then we are going to analyze a reviews will be made on the latest advances about the processes of extraction and purification of novel polysaccharides.
Morales-Ortega et al. [1] carried out the extraction of water extractable arabinoxylans from a Mexican spring wheat flour. They found arabinoxylans possess an arabinose/xylose ratio of 0.66. The intrinsic viscosity and molecular weight values for arabinoxylans were 3.5 dL/g and 504 kDa, respectively.
Vinod, Sashidhar & Cerník [2] studied adsorption and metal interaction behavior with various toxic heavy metals (Pb, Cd, Ni, Cr and Fe) in Kondagogu ( Cochlospermum gossypium) gum (KG). They found that Kondagogu-Cd complex suggests that Cd is strongly bound to Kondagogu compared to the other metals.
Yao et al. [3] performed aqueous and alkaline extraction of quinoa polysaccharides. The aqueous extraction has a chemical structure composed of rhamnose, arabinose, galactose and galactic acid; while the alkaline extraction has rhamnose, arabinose, mannose, galactose and galactic acid. The molecular weights determined were from 22 to 37 kDa, repectively.
Fimbres-Olivarría et al. [4] extracted sulfated polysaccharide from Navicula sp. This sulfated polysaccharide possesses glucose (29%), galactose (21%), rhamnose (10%), xylose (5%) and mannose (4%), presenting a molecular weight of 107 kDa. The polysaccharide at 1% (w/v) solution in water formed in the presence of 0.4% (w/v) FeCl3, showing elastic and viscous moduli of 1 and 0.7 Pa, respectively.
Chen et al. [5] worked with ultrasonic extraction of Tuber huidongense polysaccharides (Chinese truffle). They used ultrasonic power 99.65 W, extraction time 40.39 min, ratio of water to raw material 24.65 mL/g, and extraction temperature 70.1°C with a yield of 7.17%. From this technique, they obtained two polysaccharide fractions of with molecular weights of 128 kDa and 729 kDa, where the former has glucose, mannose and galactose and the latter contains glucose.
Zhao et al. [6] characterized a polysaccharide fraction isolated from the gonads of Haliotis discus hannai Ino (Abalone), it contains mannose, glucose and galactose, was prepared by partial acid hydrolysis. The results showed that polysaccharide with higher molecular weight and sulfate content demonstrated greater anticoagulant activity.
Huang et al. [7] performed the extraction and characterization of two polysaccharides obtained from Dendrobium officinale with molecular weights of 394 kDa and 362 kDa, respectively. They contain mannose and glucose in their chemical structure; possessed antioxidant activity and mild immunostimulatory activity.
Liu et al. [8] extracted a polysaccharide from Arachis hypogaea using hot water, whose optimal extraction conditions were 85°C, 3 h and 20:1 (mL/g) respectively. The average molecular weight of the polysaccharide was 238.3 kDa and it is composed of glucose, galactose, arabinose and xylose.
Li et al. [9] studied the extraction of some polysaccharides from Opuntia dillenii Haw cactus. These polysaccharides have a yield of approximately 30% with a molecular weight range of 339 to 943 kDa, respectively.
Liu et al. [10] carried out the fractional characterization of polysaccharides obtained from Gleditsia sinensis and Gleditsia microphylla with a galactomannan recovery of 33.3% in ethanol at ≈82% in isopropanol. They conclude that a higher mannose/galactose ratio and a higher molecular weight were obtained in a lower concentration of alcohols.
Hammi et al. [11] optimized the extraction of polysaccharide yield with high uronic acid content and antioxidant property from edible Zizyphus lotus fruit. They found that the optimal conditions were: extraction time of 3 h 15 min, extraction temperature of 91.2°C and water to solid ratio of 39 mL/g. The Zizyphus gum with an average molecular weight of 2720 kDa, are composed of arabinose, rhamnose, glucose, fructose, galactose and xylose.
Qu et al. [12] performed the optimization of the extraction by infrared-assisted of Bletilla striata polysaccharides. Where the optimum extraction parameters as follows: 75°C extraction temperature, 2.5 h extraction time; and water to solid ratio (53 ml / g); obtaining a yield of 43.95%.
Vasile et al. [13] carried out physicochemical and emulsifying properties of a novel exudate gum from Prosopis alba were assessed in comparison with arabic gum. They concluded that exudate gum from Prosopis alba is a gum that acts in a similar way to arabic gum in emulsions.
Chen et al. [14] conducted a review of polysaccharide extraction and purification techniques of Traditional Chinese medicines. They showed the example of the following polysaccharides: Bush sophora root polysaccharide, whose average molecular weight is 22.4 kDa; Euphorbia fischeriana polysaccharide, whose average molecular weight is 11.2 kDa; Lactarius deliciosus Gray polysaccharide, whose average molecular weight is 11 kDa; Tricholoma matsutake polysaccharide, whose average molecular weight is 88.9 kDa; and Hericium erinaceus polysaccharide, whose average molecular weight is 15 kDa.
Romdhane et al. [15], carried out the extraction in hot water of polysaccharides from watermelon rinds. Ellos mostraron the results proved that galactose was the dominant sugar in the extracted polysaccharides, followed by arabinose, glucose, galacturonic acid, rhamnose, mannose, xylose and traces of glucuronic acid. The results suggested that watermelon rinds gum presents a promising natural source of antioxidants and antihypertensive agents.
Varma and Kumar [16], released study of water soluble polysaccharide from seeds of Albizia lebbeck L. They showed a apparent molecular weight of polysaccharide of 198 kDa; with a monosaccharide composition of mannose (4.06%), rhamnose (22.79%), glucose (38.9%), galactose (17.84%) and xylose (16.42%). They authors suggested its use as additive in food preparations for hight water sorption.
Zou et al. [17] obteined two polysaccharides from Gentiana crassicaulis at 100°C. They showed that two fractions are typical pectic polysaccharides, and used as a potential natural immunomodulator.
Li et al. [18], evaluated polysaccharides form Schisandra chinensis and and Schisandra sphenanthera. They showed it’s the application as bioactive macromolecules in future daily life and medicine.
Yu et al. [18] presented a revision of polysaccharides as an important class of bioactive natural products, including its diversity pharmacological applications, such as immunoregulatory, anti-tumor, anti-virus, antioxidation, and hypoglycemic activity.
Gao et al. [19] investigated the characteristics, antioxidative, anti-inflammative and renoprotective effects of mycelia selenized polysaccharides from Oudemansiella radicata on lipopolysaccharide (LPS)-induced kidney damaged mice. They showed that homogeneous heteropolysaccharide with an average molecular weight of 31.2 kDa.
Chi et al. [20] relesased the structural characteristics of polysaccharides of Enteromorpha prolifera, contains special sulfated rhamnose-rich polysaccharides, with a molecular weight of 41.1 kDa and sulfate content of 16.2%, and good iron(III) chelating capacity of 20.85%.
Nie et al. [21], studied polysaccharides of Stem lettuce of extraction optimization, characterization, and bioactivities. They found two polysaccharide, SLP-1 and SLP-2, with a molecular weight of 90 kDa and 44 kDa, respeactively.
Fu et al. [22], investigated structural characteristic and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii by human fecal inoculums, with a molecular weight of 48,788 Da, and consisted of arabinose, galactose, glucose, xylose, and mannose.
Cai et al. [23] extracted two polysaccharides, STRP1 and STRP2, purified from Sophorae tonkinensis Radix; consisted of mannose, rhamnose, glucuronic acid, glucose, galactose and arabinose in a similar molar ratio with main backbones of (1→3)-linked-α-D-Gal and (1→4)-linked-α-D-Glc, while average molecular weights were 13 and 198 kDa, respectively. They observed a strong chelating ability on ferrous ions; substantial radical scavenging activities on DPPH, hydroxyl and superoxide anion radicals in vitro; and significant attenuation on acetaminophen-induced hepatic oxidative damage.
Yi et al. [24], studied in vitro activities of 39 polysaccharides from different parts of 13 lotus root varieties. Chen et al. [25], investigated the chemical characterization and antitumor effects of a polysaccharide from Ramulus mori. This water-soluble polysaccharide present estimated molecular weight of 137 kDa, was isolated and purified by gel permeation chromatography.
Once we have observed the state of the art in the purification and extraction of new polysaccharides, we will focus on describing the similar biopolymers under study in Cucurbits.
Polysaccharides in Cucurbits genus
Thomas & Webb [26], evaluated the possibility of galactose as highest proportion of monosaccharide present in several species of the Cucurbitaceae family, such as Cucurbita pepo [26].
Sakurai et al. [27], studied polysaccharides from Cucurbita maxima Duch. of the hypocotyl cell walls when it reduced the growth, and this affected syntheses of some galactosic polysaccharides in pectin and hemicellulose.
Wakabayashi et al. [28], evaluated effects of indole-3-acetic acid (IAA) on the mechanical properties of cell walls and structures of cell wall polysaccharides in outer and inner tissues of segments of dark grown squash (Cucurbita maxima Duch.) hypocotyls. They showed extract of hemicellulosic xyloglucans derived from outer tissues had a molecular weight about two times as large as in inner tissues, and the molecular weight of xyloglucans in both outer and inner tissues decreased during incubation, and accelerated depolymerization of hemicellulosic xyloglucans in outer tissues is involved in the cell wall loosening processes.
Wakabayashi et al. [29], studied cell-wall metabolism (Cucurbita maxima Duch.) during aging of intact growing stem tissues differs markedly between outer and inner tissues, and the absence of a simple relationship between the molecular weights of xyloglucans and the mechanical properties.
Ptitchkina et al. [30], researched gel properties of high-methoxy pectin from pumpkins by small-deformation oscillatory measurements of storage modulus (G’). They observed were tentatively ascribed to stable association of unesterified galacturonate chain segments at low pH, where electrostatic repulsion is suppressed.
Shkodina et al. [31] extracted Pectins from pumpkin raw material with 0.1 M HCl and with three enzymic preparations. They compared to the commercial citrus pectin with pectins from pumpkin raw material and found lower uronide content and a higher content of neutral sugars (glucose and galactose), and pumpkin pectin molecules may contain a large number of branched regions formed from neutral sugars.
Jun et al. [32], obteined polysaccharide from pumpkin (Cucurbita moschata Duch) peels, and they were fractionated subsequently into water soluble pectic substance (WSP) with 2 molecular weigth 13 and 205 kDa, EDTA soluble pectic substance (ESP) and alkali soluble pectic substance (ASP) fractions.
Yang et al. [33], conducted quantitative analysis of the component monosaccharides of an acidic polysaccharide extracted from pumpkin, and was hydrolyzed into component monosaccharides with 2.0 M trifluoroacetic acid at 100°C for 6 h and then labeled with 1-phenyl-3-methyl-5-pyrazolone. They founded glucose (21.7%) and glucuronic acid (18.9%) were identified to be the main component monosaccharides, followed by galactose (11.5%), arabinose (9.8%), xylose (4.4%), and rhamnose (2.8%). Finally, found that pumpkin polysaccharide possessed significant cytoprotective effect and antioxidative activity.
Kurz et al. [34], studied cell wall polysaccharides (Cucurbits genus) for their suitability as markers for quality and authenticity control of fruit products. They proposed, the alcohol-insoluble residue (AIR) from several cultivars of apricots and peaches of different harvest seasons, provenances, and stages of ripeness was extracted and subsequently fractionated into acid- and EDTA/alkali-soluble pectins, hemicellulose, and cellulose. Each fraction were analysed for its neutral sugar composition, and found sugar composition of glucose, galactose, arabinose and rhamnose.
Kosťalova et al. [35], performed to evaluate the chemical composition of the seeded fruit oil pumpkin biomass (Oil pumpkin (Cucurbita pepo L. var. Styriaca)) dried by solvent-exchange using etanol, with 20.9% pectin (as uronic acids), 38.1% neutral carbohydrates, and other biopolymers.
Yadav et al. [36], released various important medicinal properties including anti-diabetic, antioxidant, anti-carcinogenic, antiinflammatory and others properties of pumkins for human health benefits.
Kostalova et al. [37], obteined pectic polysaccharides from oil pumpkin (Cucurbita pepo L. var. Styriaca) by a three-step extraction procedure at various reaction conditions, where 0.01 M ethylendiaminotetraacetic acid was used as extractant in the first step and 1% and 5% NaOH in the second and third steps. The pumpkin polysaccharide fractions represent dietary fibers with a high content of pectin and antioxidant properties.
Nosaľova et al. [38], isolated from the pectic polysaccharides from pumpkin fruit biomass and characterized by composition, structural features and molecular properties.
Moreover, the application of these polysaccharides provoked any side effects what is their advantage towards the conventional opioidderived antitussive agents.
Huang et al. [39], extracted and deproteinized polysaccharides from pumpkin by hot water method. The percentages of deproteinization and polysaccharide loss were compared as indexes using the trichloroacetic acid (TCA) method, the NaCl method, the and CaCl2 method, respectively. They results showed that the TCA method exhibited the lowest percentage of deproteinization than CaCl2 excelled over the NaCl method.
Song et al. [40], studied crude water-soluble heteropolysaccharide from Lady Godiva pumpkins (Cucurbita pepo Lady Godiva), comprised L-fucose, D-galactose, D-glucose, and D-mannose, the glycosidic linkages consisted of 1,2,6-trisubstitued-galactopyranosyl, 1,6-disubstitued-galactopyranosyl, 1,4,6-trisubstitued-glucopyranosyl, 1,3-disubstituedglucopyranosyl, terminal-glucopyranosyl, and terminal-fucopyranosyl, with a molar proportion of 1:4:2:2:2:1. They found an molecular weight of 10.1 kDa.
Sedigheh et al. [41], studied the hypoglycaemic and hypolipidemic effects of different doses of pumpkin (Cucurbita pepo L.) powder in male diabetic rats and concluded that pumpkin might be beneficial in diabetic patients.
Kostálová et al. [42], studied pectic polysaccharides from the Styrian oil-pumpkin biomass usied two series of acidic polysaccharide fractions were isolated using in succession hot water, EDTA and dilute HCl. They found that chemical and spectroscopic (FTIR, NMR) analyses of the fractions revealed the predominance of partially methylesterified and acetylated pectins containing homogalacturonan and ramified rhamnogalacturonan elements, and a minority of phenolic compounds, protein and hemicelluloses.
Kostalova et al. [43], evaluated the seeded fruit biomass of the Styrian oil-pumpkin in view of its pectin component, a series of acidic polysaccharides were isolated by a six-step sequential extraction using hot water, EDTA, dilute HCl and dilute and stronger NaOH solutions. They founded the first four fractions comprised partially methyl-esterified and acetylated pectins with varying proportions of rhamnogalacturonan regions ramified with galactose- and arabinosecontaining side chains and showed considerable polymolecularity. The alkali-extracted polysaccharides contained lower amounts of pectins with homogalacturonan and arabinose-rich rhamnogalacturonan regions next to hemicelluloses prevailing in the last polysaccharide.
Song et al. [44,45], released acetylation of pumpkin (Cucurbita pepo, lady godiva variety) polysaccharide using acetic anhydride with pyridines as catalyst. They found, antioxidant activities and cytoprotective effects of pumpkin polysaccharide and its acetylated derivatives were very promising produced phosphorylated derivatives of pumpkin polysaccharide using POCl3 and pyridine. They evaluated antioxidant activities and cytoprotective effects of unmodified polysaccharide and phosphorylated derivatives and found good results.
Koštálová et al. [46], released microwave-assisted extraction polysaccharides from pumpkin biomass and compared to the usual extraction by conventional heating. Heating times ranged from 2 to 10 min, L/S ratio from 30/1 to 50/1 and temperature from 80 to 120˚C. And found the liquid/solid ratio had the greatest influence on yield and molecular weight, respectively.
Wang et al. [47], conducted studies anti-diabetic activity of the polysaccharides obtained from the dried pumpkin pulp. They foud pumkim polysaccharide were composed of rhamnose, arabinose, glucose and galactose. These results suggested the potential hypoglycemic effect of the pumpkin polysaccharides.
Wang et al. [48], produced crude polysaccharides from pumpkin seeds were obtained with hot water extraction and etanol precipitation. They founded that pumpkin seeds gum was composed of mannose, glucose and galactose in a molar ratio of 1.0:12.9:5.3 with molecular weight of 21,100 g/mol. And founded with 1 HNMR and 13 CNMR spectra of pumpkin seeds gum consisted of (1/4)-linked β-Dglucopyranosyl, (1/2)-linked α-D-galactose and (1/6)-linked α-Dmannopyranosyl, and protein with 17 general amino acids and was rich in glutamic acid, alanine and glycine. They showed antioxidative and strong antibacterial activity of this pumpkin seeds gum.
Li et al. [49], studied oxidative stress is known to impair architecture and function of cells (as cucurbits), which may lead to various chronic diseases, and therefore therapeutic and nutritional interventions to reduce oxidative damages represent a viable strategy in the amelioration of oxidative stress-related disorders, including neurodegenerative diseases. They worked with antioxidant polysaccharides and were also found to attenuate neuronal damages and alleviate cognitive and motor decline in a range of neurodegenerative models. They concluded that status of antioxidant function of food-derived polysaccharides and then attempt to appraise their anti-neurodegeneration activities.
Research of Cucurbita ficifolia
Alarcon-Aguilar et al. [50], researched hypoglycemic effects of freeze-dried juice of Cucurbita ficifolia Bouche´ (Cucurbitaceae) fruits were studied in healthy and alloxan-diabetic mice.
Xia & Wang [51], studied the antihyperglycemic effects of Cucurbita ficifolia fruit extract by experimental diabetes in rats.
Simpson & Morris [52], released investigation of antidiabetes compounds of cucurbits, betwee them Cucurbita ficifolia. They propoused anti-diabetic drugs as cucurbits polysaccharides with a global economic potential.
Garcia Gonzalez et al.[53], performed aqueous extract of Cucurbita ficifolia fruit. They demonstrated hypoglycemic effect, which may be attributed to some components in the extract.
Dye solutions of varying concentrations were prepared and their absorbances determined at the wavelength of 605 nm for which it was found to show maximum absorbance and the validity of Beer-Lambert law established by a linear correlation between the dye concentration and the optical density [10].
The Cucurbitaceae family
Cucurbitaceae are among the oldest crop plants in America. They offered primitive man abundant food, of easy and rapid propagation, which could grow optimally in open places rich in organic waste [54].
The Cucurbitaceae family includes 118 gender and 825 species [55]. All of them are very sensitive to cold. They originated in the tropical and subtropical areas of the world and most have developed long guides or branches with tendrils to adapt to the competition for light. Both native and cultivated species have annual or perennial plants, usually grown in temperate climates. They are prolific in production of seeds, since they live a season until they die from frost [56].
The great diversity of pumpkins and squashes, melon, watermelon, cucumber and alcayota are part of this family (Figure 1) [57].
Cucurbita gender
The genus Cucurbita is native to the American continent. It includes about 27 species that can be annual or perennial and are cultivated mainly for the consumption of their fruits to the mature or immature state. Other parts of the plant are also consumed, such as the leaves, flowers and seeds of the fruits.
Five species have been domesticated for the consumption of their fruits: Cucurbita maxima, C. moschata, C. pepo, C. argyrosperma with annual plants, and C. ficifolia, with perennial plants (Figure 1) [56]. The first four species are squash and the last is known as alcayota or cayote, this fruit is used for confectionery products, mainly for the production of sweets with special characteristics due to the pulp’s fibrousness that gives it an excellent consistency and a mild flavor [57].
Botanical description of Cucurbita genus
Root: The root system of the squash is characterized by having a thick pivoting root that can penetrate up to 1.80 m deep at maturity, although ramifications below the level of 0.60 m are not important. The ramifications are very expansive and can cover a diameter of 6 m with numerous secondary branches that measure from 0.50 m to 2.40 m and weave a network of rootlets around the plant.
Kindred: In general, they have prostrate and climbing stems, but some varieties are semierguided. The growth of the branches is very vigorous and with a growth rate so high that it can hardly be matched by other species of herbaceous and annual plants. The stems are grossly pentagonal, hollow at maturity and carry glandular hairs.
Leaves: The leaves are large, cordiform, petiolate and generally have 3-5 lobes, varying the size of them according to the species and variety.
Flowers: All Cucurbita species are monoecious, with yellow flowers, large and visible, and usually isolated in the axils of the leaves; they have a flared corolla with five lobes, which, together with the five basal lobes of the chalice, form the perianth. The flowers are composed of five sepals and a five-petalled corolla that measures 6 to 15 cm long by 8 to 16 cm wide and is usually covered with fine hairs.
Fruit: it is one of the largest in the vegetable kingdom. It is indehiscent, with the fleshy pericarp adhering to the pericarp, and it is classified as an iberian berry. The surface can be smooth, warty, or covered with spines or other types of formations. The young fruits of almost all varieties have hairs of one or several kinds that can persist or not until maturity. The bark can be white, green, yellow, red or irregularly stained or with spots arranged in bands.
Seeds: The seeds are large, flat, oval, and one of the ends ends in a point. The approximate weight is 50 mg for small fruit cultivars and 250 mg for larger fruit cultivars. In its composition, lipids and proteins predominate, contributing up to 80-85% of the embryo’s dry weight [56].
Floor: Sandy soils, airy, deep, are the most suitable for the cultivation of squash. Although good crops can also be achieved in heavy and sandy soils if they are well supplied with organic matter and fertilized properly. Generally, clayey, and little permeable, hinder the development of the root system and favor the development of mycotic and bacterial diseases; sandy ones have low water retention capacity and low fertility. It is a medium resistant species, but very sensitive to sodium soils. It is convenient to rotate every three years to reduce the incidence of diseases [56].
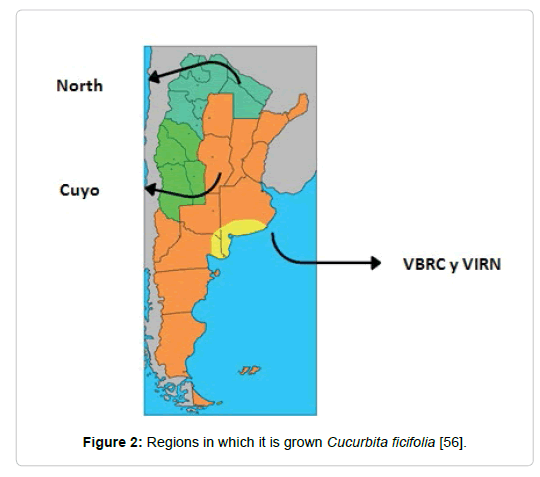
Seedtime: The appropriate sowing season is past the frost period, with a minimum germination soil temperature between 10 and 15ºC, and humidity must be available for the seeds to trigger germination processes. Figure 2 shows the cultivation areas in Argentina.
Figure 2: Regions in which it is grown Cucurbita ficifolia [56].
In the region of the Bonaerense Valley of the Colorado River (VBRC), even with some risk, it takes place from mid-October. The first week of November is the recommended planting season for the lower Rio Negro valley region (VIRN).
In the northern zone of the country there are different sowing dates that allow counter - season cultivation. The first dates are in May - June, but with much risk of losses due to frost. The second date is in July - August. Therefore, it is not feasible to carry out sowing in this wide region because intense rains that are harmful to this crop begin.
In the Cuyo region you can also distinguish two sowing dates, the first is in the months of September - October, which takes place in the north of Mendoza and in the province of San Juan. The second is in the months of October - November, and can be carried out both in the northern zone of the region and in the south of the province of Mendoza [56].
Irrigation: Although the seeds need very little water to germinate. In later stages the water deficit manifests itself notably: at the beginning there is loss of color of the leaves and later it harms the yield and quality of the fruits.
Most crops are irrigated by furrows. To avoid diseases caused by soil fungi, water should reach the plant by capillarity. The greatest need for irrigation of this crop is during flowering and thickening of the fruits. With drip irrigation a very efficient use of water is achieved and yields are increased
Harvest: They are usually harvested when the fruits have taken the typical color of the variety. It is likely that the seeds have fully matured at this stage.
A fruit in the process of maturation undergoes a series of marked changes in color, texture and flavor, which indicate that changes are being made in its composition. When these changes are completed, the fruit reaches the optimum quality of consumption. This is only achieved if the fruits are harvested in a state of appropriate maturity, since the immature fruits will not reach a satisfactory quality even when they have completed the suitable changes of maturation. During the conservation the hydrolysis of the starch in sugars takes place, which usually produces the softening of the fruits. The sugars, either free or combined with other constituents, are important for the fruits to achieve a pleasant taste, through a balance in the acid-sugar ratio, attractive color (derived from anthocyanins) and a pleasant pulp texture (if they are combined with structural polysaccharides). As the fruit ripens quantitative and qualitative metabolic transformations are carried out and, in addition to the starch, most other polysaccharides are completely metabolized [56].
Harvest indexes: There are several maturity indices that are based on different methodologies [56].
Visual aids: generally the small-scale producer should rely on visual inspection of the fruits. The size alone does not serve to determine the optimum ripeness of harvest. Some very large fruits may still be immature to be harvested and others very young may be overmature. The characteristic of greater use is the color of the fruits. In this type, this index can be associated with the change of coloration of the peduncle of the fruit. Generally it passes from light green or medium to ocher green or ocher accompanied by lignification and interruption of the passage of the sap.
Physical means: the fingernail penetration resistance test is common to determine commercial maturity, and although it is a highly subjective method, if it is associated with the typical coloration of the variety, it indicates that the fruit’s crust has achieved harden as much as possible.
Physiological: are methods that consist in expressing the age of the fruits based on the respiration rate. The breath is measured at different harvest dates and it is decided which is the best. What is normally determined is the rate of oxygen utilization or the production of carbon dioxide. The ratio of carbon dioxide to oxygen is called the respiration ratio (CR). This index is not applicable to the producer, using it only for research purposes
Cucurbita ficifolia
The origin of the alcayota ( Cucurbita ficifolia) is uncertain. We have two theories. Some authors affirm that, due to the linguistic evidence, its origin is Mexican, since the names used have Nahuatl origin (chilacayote, lacayote), the dialect of the region, however, the oldest archaeological remains preserved come from Peru. The wild variety from which it originated is unknown and the hypotheses point to a species, possibly native to the eastern region of the Andean mountain range [58].
Taxonomic information [59]
Kingdom: Plantae
Division: Magnoliophyta
Class: Magnoliopsida
Order: Violales
Family: Cucurbitaceae
Genre: Cucurbita
Species: Cucurbita ficifolia
Botanical description of Cucurbita ficifolia: Cucurbita ficifolia is a creeping or climbing plant, monoecious, belonging to the great family of dicotyledons. They have a fleshy fruit (Figure 3), round and elongated, with a thick, rough or smooth skin, resistant to low temperatures, but not to severe frosts [54].
The fruits can reach to measure between 15 to 50 cm long, ovoidelliptical, sometimes slightly compressed at the apex, which joins the fruit with the stem [59]. Its epicarp (shell) is rigid, persistent, three colorations can usually be seen:
• Light or dark green, with or without longitudinal stripes towards the apex.
• Green with small white spots.
• Whites or cream.
The mesocarp (pulp) is white with a granular and fibrous texture. In the center of the fruit there are follicles containing elongated seeds [56].
All the species of this genus, Cucurbita ficifolia is one of the most fruit produced, with more than 50 fruits per plant [57].
Chemical composition: The approximate chemical composition of the alcayota fruit is shown in (Table 1). The data shown is approximate since the chemical composition varies in a range that depends on the cultivation conditions, the climate, the use of fertilizers or fertilizers, the time of harvest, the frequency of irrigation, etc. [59].
| Component | Tender fruit | Mature fruit |
|---|---|---|
| Humidity (%) | 94.5 | 91.4 |
| Protein (%) | 0.3 | 0.2 |
| Grease (%) | 0.1 | 0.5 |
| Total carbohydrates (%) | 4.4 | 6.9 |
| Raw fiber (%) | 0.5 | 0.6 |
| Ash (%) | 0.2 | 0.4 |
Table 1: Chemical composition of alcayota on a wet basis [59].
The main characteristic of the alcayota is that its firm structural polysaccharide, polygalactoglucomannan, which is hyperhydrophilic but insoluble in water. This gives it the ability to increase its size and volume several times. In addition, it has very positive characteristics related to its molecular weight reduction and generation of molecular associations (hydrogen bridge bonds) through hydrolysis.
The alcayota is a fruit known for its sweets, which is grown in San Luis and other regions of the country as described above. It is proposed to add another application for these fruits, in addition to the current confectionery products and redirect their use towards the biopackaging of food, being this an original and novel alternative of great scientific-technological interest [60].
Hypothesis and objectives: This work will study the separation and purification of alcayota gum extracted from Cucurbita ficifolia. This polysaccharide was purified by hydroalcoholic solutions. The alcayota gum was characterized by measurements as viscosity-density, Dynamic Light Scattering, and rheology in order to determine the physico-chemical characteristics
Experimental Study
The polymers present remarkable features that distinguish them from any other class of compounds and that make them ideal for diverse applications. Its fibrillar nature and large size results, in short, in forces that extend at the macroscopic level [61].Some of these properties of polysaccharides are: the large average size of the macromolecules, the size distribution, their architecture, the specific nature of their chemical groups, the arrangement of these groups in the chains and the state of aggregation of the molecules [62]. For this reason, it is important to carry out a series of tests that allow knowing its structure and its behavior against external factors, in order to determine its best applications [62].
The tests carried out can be divided into two groups:
• Structural analysis: they characterize the internal structure of the material through techniques such as dynamic light scattering and viscosimetry.
• Tests or functional analysis: they are directed to the knowledge of the behavior of the material in front of agents such as water, gases, the application of a force, etc.
Below, the techniques and materials used in each of them are detailed. The tests carried out on alcayota gum solutions as dynamic light scattering, density, viscosimetry and analysis of the rheological characteristics will be described first.
Raw material
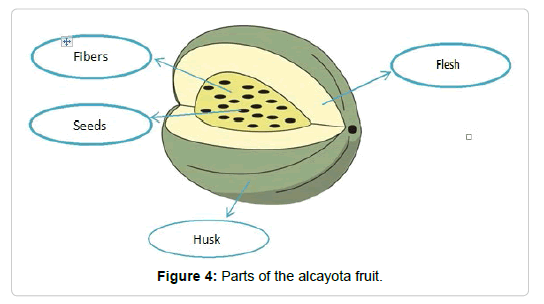
Alcayota fruits of between 3 and 4 kg of initial weight were used. The fruit was separated into three parts: husk, pulp, fiber and seeds (Figure 4). The pulp was dried and later ground to obtain the flour from which different determinations will be made.
Drying: Drying is defined as the operation in which the liquid contained in a solid is removed by evaporation.
The process involves the simultaneous transfer of mass and energy:
•Transfer of the internal moisture of the solid towards the surface of the solid and its subsequent evaporation. The movement of moisture within the solid is a function of the physical nature of the solid, its temperature and its moisture content.
•Transfer of energy in the form of heat to evaporate moisture from its surface. This process depends on the external conditions of temperature, humidity, air flow, pressure, exposure area and type of dryer used.
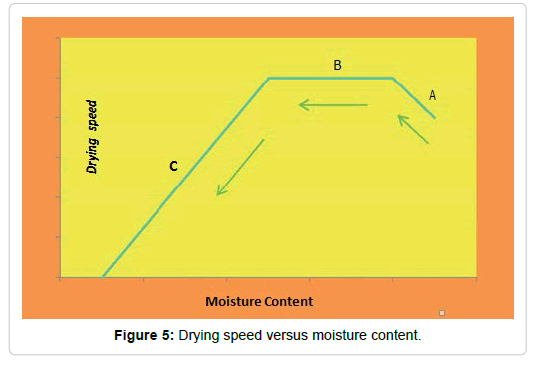
There are three stages in the drying process, as shown in Figure 5.
During the first stage of drying (step A), the solid is heated. It is a stage of short duration in which the evaporation is not significant neither by its intensity nor by its quantity. In this stage, the solid is heated from room temperature until the equilibrium between cooling by evaporation and the absorption of heat from the air is reached. The second stage (stage B) is the so-called first drying period or constant drying rate period, where free or unbound moisture evaporates. The third stage (stage C) is the second drying period or period of decreasing drying speed (which is not necessarily linear), where the bound moisture of the material evaporates. The process ends when the moisture content of the solid is in equilibrium with the humidity of the drying air [63].
The part of the fruit used to extract the polysaccharides was the pulp, so the peel was removed and the seeds were separated. The pulp was cut into pieces of approximately 5 cm on each side and placed in trays leaving enough space between them to allow the air passage and thus not harm the drying process. The equipment used was a stove at 50˚C, in which the trays were kept for a week, obtaining 151 g of dried pulp from a 3.5 kg fute. The final acceptable moisture content is 3% as maximum value because a higher content produces the nuisance of the grinding process.
Grinding process: Grinding is defined as the operation in which powdered products are obtained by the application of shear, impact or compression forces.
The reduction in size was made with two objectives.
• Favor the extraction of the soluble components present in the pulp. Due to the increase in the surface of the solid when grinding, a greater area of contact with the solvent is generated, improving the extraction process and decreasing the operation time.
• Obtain homogeneous appearance membranes.
The equipment that was used is a gravitational mill of concentric cylinders (Fritsch, Germany). This type of apparatus uses at the same time forces of shear and impact for the reduction of size.
It is formed by a rotating cylinder inside which are concentric steel cylinders. As the outer cylinder rotates the concentric cylinders collide with each other, hitting the shredded product that is filling the clearances between the cylinders. The cylinders slide, in turn, with each other, producing the shearing of the raw material. This combination of shear and impact forces produces a very effective size reduction. The size of the cylinders is generally 10-30 cm in diameter [64]. The lowest of the speeds provided by the equipment was selected (speed I). The grinding time was 10 minutes, obtaining particles between 1 and 12 μm in diameter (Figure 6). From here on, it will be called Cy to the flour obtained from the grinding of the dried pulp.
Process: Aqueous dispersions were prepared at 3 to 6% of alcayota meal (Cy). Each solution was made from 100 ml of dispersion.
Leaching: Leaching is defined as the separation of a desired solute or removal of an undesirable solute from the solid phase, when it is brought into contact with a liquid phase. Bothphases come into intimate contact and the solute diffuses from the solid to the liquid phase, which allows a separation of the original components of the solid.
The general leaching process consists of the following steps:
1. The solvent is transferred from the volume of solution to the surface of the solid.
2. The solvent diffuses into the solid.
3. The solute dissolves in the solvent.
4. The solute diffuses through the mixture of solid and solvent to the surface of the particle.
5. The solute is transferred to the general solution.
The rate of diffusion of the solute through the solid, and that of the solvent to the surface of the solid are usually the resistances that control the global leaching process and depend on several factors, mainly the temperature, since increasing it increases the diffusivity. Another factor is the particle size, as it was said before when decreasing the size increases the contact area and, finally, the agitation of the solution since it eliminates the film of fluid that covers the surface of the solid at rest increasing the transfer coefficient [65]. In this procedure, tannins, fats and oils from the alcayota fruit are eliminated. Remaining a solid rich in polysaccharide.
Process: The solutions were stirred magnetically for two hours maintaining a temperature of 65°C. Agitation and temperature favor the solid-liquid extraction process of the soluble components. After two hours of agitation, the heating is removed and the stirring continues until the temperature drops to 40°C. The temperature should not exceed 80°C since undesirable reactions of the polysaccharides present such as thermal hydrolysis or nonenzymatic browning reactions (caramelization and Maillard reaction) begin to occur at this temperature. Then, 100 ml of ethanol are added and the mixture is stirred cold for 30 minutes. Subsequently, it is filtered using a common plastic filter reserving the precipitate and eliminating the filtrate. The precipitate is redissolved in 100 ml of water. This procedure is carried out several times until the filtrate is translucent. The dispersions obtained were designated Cy(d). Ethanol precipitates the polysaccharides leaving in solution low molecular weight biomolecules, water soluble polyphenols, salts, fats and oils that are eliminated in the filtration. In this way the dispersion is purified [66].
Alkaline hydrolysis
The hydrolysis of the polysaccharides can be carried out by different techniques. Acids, bases, enzymes and even microorganisms can be used. The goal is to break down the polysaccharides into molecules of lower molecular weight. Many times, in addition to small molecules of polysaccharides salts and volatile acids are obtained [67]. During hydrolysis, a series of undesirable side reactions also occur that can only be reduced by controlling the conditions under which the reaction occurs [68].
The bases can act in reducing terminal units as well as oxydryl groups. In the first case, the degradation begins at the ends of the chains and advances step by step through the entire chain. In the second case, the oxydryl groups are substituted, to a degree that depends on the reactivity of the groups and on the nature of the reaction. Degradation can also occur at other sites such as glycosidic linkages, acid groups or ester linkages [68].
Process: A controlled basic hydrolysis is carried out with sodium hydroxide on the dispersion obtained in the previous stage. The amount of base, temperature and reaction time are used to regulate the amount of oxydryl groups in the resulting molecule [69].
The objectives sought are:
• Hydrolyze the polysaccharides and thus generate oxydryl groups and possibly carboxyl and carboxylates that facilitate the solubilization and obtention of the desired polysaccharide.
• Achieve ruptures of the ester and ether bonds in the polysaccharide by decreasing its molecular weight and thus obtaining a fine dispersion. The procedure consists in adding to the dispersion Cy(d), 10 ml of 0.1 M sodium hydroxide, stirring at 65ºC for two hours and then precipitating with ethanol, in the same way as in the previous stage. The precipitate is washed with ethanol twice, obtaining a polysaccharide of average molecular weight having free oxydryl groups. After the ethanolic washes, the precipitate is redispersed in 100 ml of distilled water. The dispersion obtained was called CyOH(d).
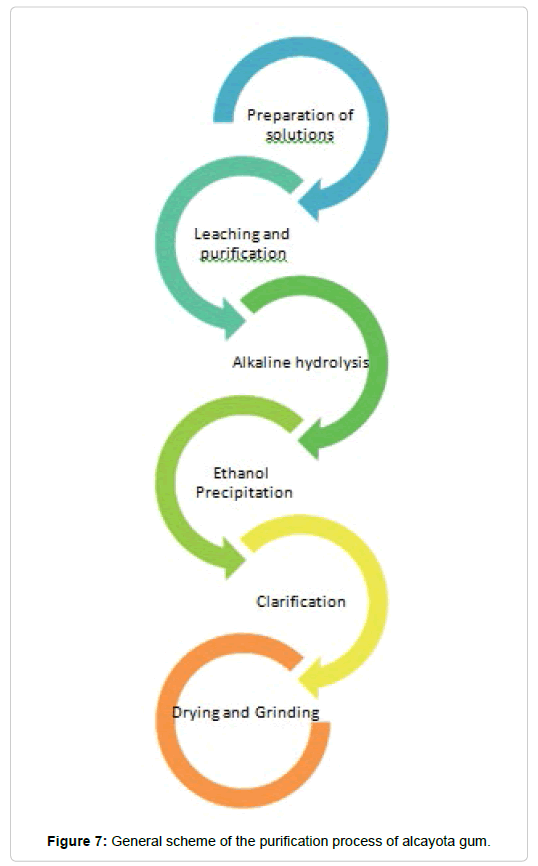
Finally, Figure 7 shows an outline of the process followed to make alcayota gum.
Addition of the plasticizing agent
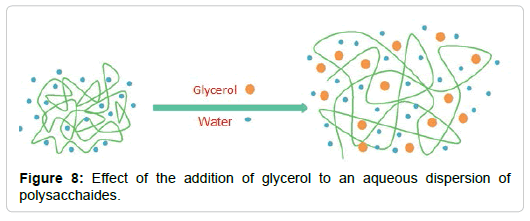
Plasticizers are commonly used to facilitate processing and/ or increase the flexibility of the film. Water, some oligosaccharides, polyols and lipids are different types of plasticizers widely used in films based on hydrocolloids. Their combination could lead to synergistic effects between the components improving the properties of the films. Various theories have been raised about how these compounds work. The theory of lubrication postulates that plasticizers intermingle and act as internal lubricants by reducing the friction forces between the polymer chains. The theory of the gel postulates that the rigidity of the polymeric network comes from its three-dimensional structure, then the plasticizers would act by breaking polymer-polymer interactions, that is interposed between them. The theory of free volume postulates that the addition of plasticizers is a way to increase free volume, reducing the interactions between chains [70,71]. All the proposed theories agree that the effects generated by the addition of glycerol, are due to these small molecules are located between the polymer chains, as shown in Figure 8.
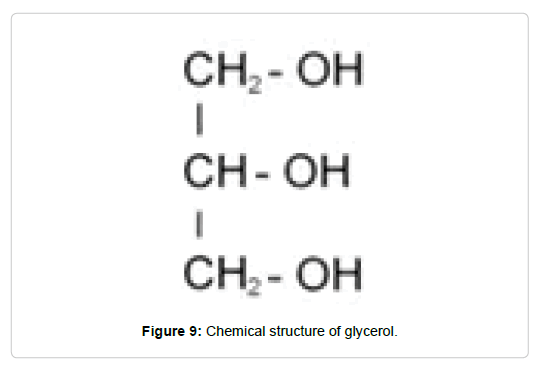
In general, plasticizing agents are low molecular weight molecules with hydrophilic components that interact with the polar groups of the polymers. Examples of these are glycerol, sorbitol, sucrose, starch and cellulose derivatives, among others. Glycerol is characterized by three oxydryl groups and an asymmetric structure (Figure 9), consequently the interactions with the chains of the polysaccharides become complex, giving rise to a poorly ordered network [72]. Glycerol has hydrophilic characteristics which contributes to increase the water vapor permeability and the susceptibility of the matrix to environmental humidity [73].
Generally, the transmission of water vapor through a hydrophilic film depends both on the diffusivity and the solubility of the water molecules in the film matrix. An increase in the space between the polymer chains due to the inclusion of glycerol molecules between the polymer molecules promotes the diffusivity of water vapor through the film, accelerating the transmission of the vapor [70].
Process: To the previous dispersion (CyOH(d)) was added 5 ml of glycerol and stirred cold for a few minutes to homogenize.
Characterization
Dynamic light scattering: The Dynamic Light Scattering (DLS), also called Photon Correlation Spectroscopy is a technique designed to measure the size of particles. DLS measures Brownian motion and relates this to the size of the particles. The Brownian movement is the random movement of particles due to the bombardment of the solvent molecules that surround them. The larger the particles, the slower the Brownian movement will be. Small particles are bombarded more frequently by solvent molecules so they move more quickly. It is necessary to perform the test at a stable temperature, otherwise the convection currents in the sample could cause non-random movements that would impair the correct interpretation of the particle size.
The radius that is measured in DLS is a value that represents how the particle diffuses into a fluid, so it is called hydrodynamic radius. This radius corresponds to that of a sphere that has the same translational diffusion coefficient as the particle under study [74].
The first order autocorrelation function as a single exponential decay, as:
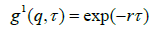 (1)
(1)
where Γ is the decay rate. The translational diffusion coefficient Dt may be derived at a single angle or at a range of angles depending on the wave vector q.
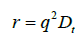 with (2)
with (2)
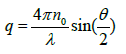 (3)
(3)
where λ is the incident laser wavelength, n0 is the refractive index of the sample and θ is angle at which the detector is located with respect to the sample cell.
Depending on the anisotropy and polydispersity of the system, a resulting plot of Γ/q2 vs. q2 may or may not show an angular dependence. Small spherical particles will show no angular dependence, hence no anisotropy. The intercept will be in any case the Dt.
Dt is often used to calculate the hydrodynamic radius, RH, of a sphere through the Stokes– Einstein equation.
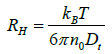 (4)
(4)
where kB is the Boltzmann constant (1.381 ×10-16 erg K-1); T is the absolute temperature (293 K) and η0 is viscosity of the solvent.
The autocorrelation function is a sum of the exponential decays corresponding to each of the species in the population.
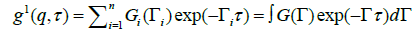 (5)
(5)
It is tempting to obtain data for and attempt to invert the above to extract G(Γ). Since
G(Γ) is proportional to the relative scattering from each species, it contains information on the distribution of sizes.
One of the most common methods is the cumulant method, from which in addition to the sum of the exponentials above, more information can be derived about the variance of the system as follows:
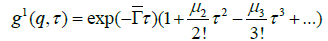 (6)
(6)
where is the average decay rate and is the second order polydispersity index (or an indication of the variance). A third-order polydispersity index may also be derived but this is necessary only if the particles of the system are highly polydisperse.
The z-averaged translational diffusion coefficient Dz may be derived at a single angle or at a range of angles depending on the wave vector q.
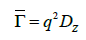 (7)
(7)
One must note that the cumulant method is valid for small and sufficiently narrow G(Γ).
One should seldom use parameters beyond μ3, because overfitting data with many parameters in a power-series expansion will render all the parameters including and μ2, less precise [75-80].
Process: A 0.1% w / v solution of Cy was prepared and then centrifuged at 10000 rpm for 5 minutes to remove the larger aggregates. It was placed in the cell of the equipment (Delsa Nano C, Beckman Coulter) and a monochromatic light beam of a laser diode with a wavelength of 658 nm and a dispersion angle of 165° was made [81,82].
Viscosimetry: Viscosity is one of the most important properties of polymer solutions. The viscosity depends on the chemical structure of the polymer, the interactions with the solvent and the molecular weight. Normally, a molecule of high molecular weight in a solvent acquires a large hydrodynamic volume and the viscosity of the solution increases [83,84].
The viscosimetry of diluted solutions is related to the measurement of the intrinsic ability of a polymer to increase the viscosity of a solvent at a given temperature and is useful for obtaining information related to the size and shape of the polymer molecules in solution and the polymer-solvent interactions. The viscosity of a diluted polymer solution is determined relative to the viscosity of the solvent. In these cases the following terms are defined:
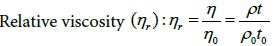 (8)
(8)
Where η is the viscosity of the polymer solution, ρ is the density and t the runoff time.
The subscript “0” indicates that the parameters correspond to the pure solvent.
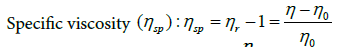 (9)
(9)
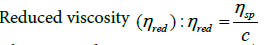 (10)
(10)
where c is polymer concentration (g/cm3).
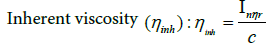 (11)
(11)
Even in highly diluted solutions, polymer molecules are capable of generating intermolecular interactions. The two contributions to reduced viscosity are the movement of the isolated molecules in the solvent and the interaction between the polymer molecules and the solution. To eliminate the interactions it is necessary to extrapolate to zero concentration, in this way the intrinsic viscosity is obtained [η].
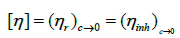 (12)
(12)
Intrinsic viscosity has units of volume/mass. It is a measure of the ability of a polymer molecule to increase the viscosity of a solvent in the absence of intermolecular interactions.
To evaluate the intrinsic viscosity, the equations of Huggins (equation 13) and Kraemer (equation 14) are usually used.
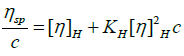 (13)
(13)
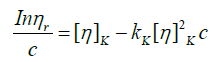 (14)
(14)
Where KH and KK are the constants of Huggins and Kraemer respectively.
However, the most usual procedure for determining the intrinsic viscosity is to determine the specific viscosity for different concentrations of polymer, represent the data ηsp/c versus c and then calculate the value at zero concentration (equation 12).
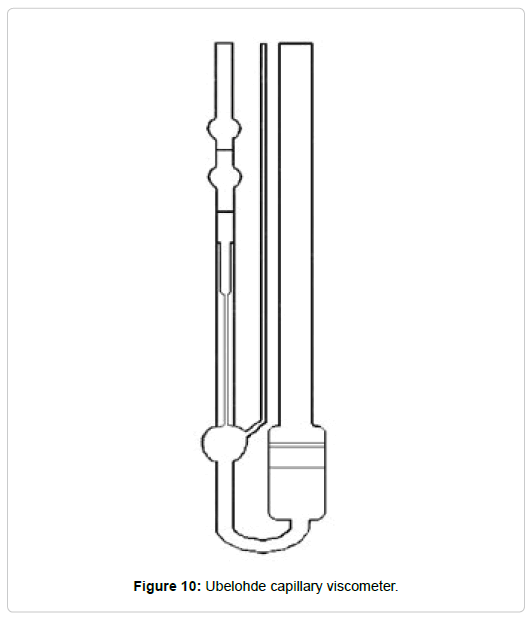
The relative viscosity measurements of diluted polymer solutions can be carried out in a variety of ways including capillary viscometers, where the time required to flow between two marks in a capillary is recorded. Alternatively, coaxial cylinder viscometers [84] can also be used.
Knowing the intrinsic viscosity, the approximate molecular weight of the macromolecules can be calculated from the Einstein equation (equation 15).
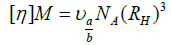 (15)
(15)
where [η] is the intrinsic viscosity, M is the molecular weight, is the number of Simha, NA is the number of Avogadro and RH is the hydrodynamic radius.
Viscometer: A Ubelohde glass capillary viscometer (Figure 10) with a 30-second run-off time was used, partially submerged in an Ultraterm S-383 thermostatic bath at 25°C. A digital chronometer was used to measure the runoff time of the solutions. An Anton Paar DMA 35 N densimeter was also used to determine the density of water and solutions.
Process: Diluted solutions of Cy of 0.1%, 0.2%, 0.4% and 0.6% concentrations were prepared. For this, 10 g of Cy was weighed and placed in 250 ml of distilled water, heated at 70°C for two hours. It was allowed to settle and the supernatant was separated, this was filtered and then ethanol precipitated repeatedly until a clear solution was obtained. The precipitate obtained in this process was dried in an oven at 60°C for 24 hours. Finally, 0.1 g of this precipitate was dissolved in a 0.1 M NaCl solution, which acts as a stabilizer. The solutions were allowed to stand for 24 hours.
Before starting the viscosity measurement, the solutions were immersed in the thermostatic bath until they reached 25°C. The same was done with distilled water. Using the viscometer, the runoff time of the water and of the solutions was measured in triplicate.
The intrinsic viscosity was obtained by applying equations 8, 9 and 10 and plotting ηsp/c versus c. The intrinsic viscosity is obtained at the intersection of the line with the axis of the ordinates, that is, when the concentration approaches zero.
Rheology: Viscosity information and knowledge of rheological behavior are fundamental for process engineering and need to be described for pipe design, pump selection, design and operation of heat exchangers, agitation systems, etc. It is also necessary to know the effect of temperature and concentration on the rheological behavior for a good understanding and dimensioning of unit operations [85].
The viscosity (η) constitutes a resistance to deformation and is defined in Newton’s law of viscosity (equation 16), which states that when the layers of a liquid slide together, the the resistance to movement depends on the gradient of the fluid. velocity 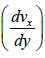 and area (A).
and area (A).
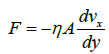 (16)
(16)
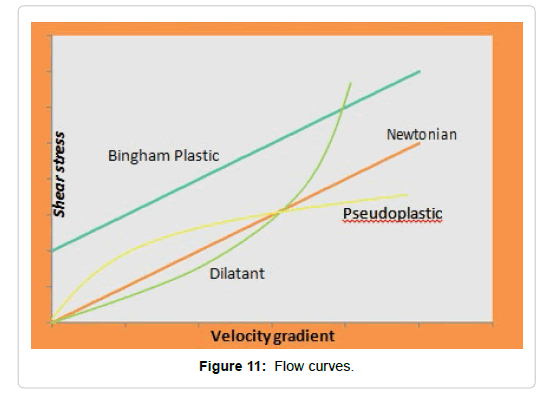
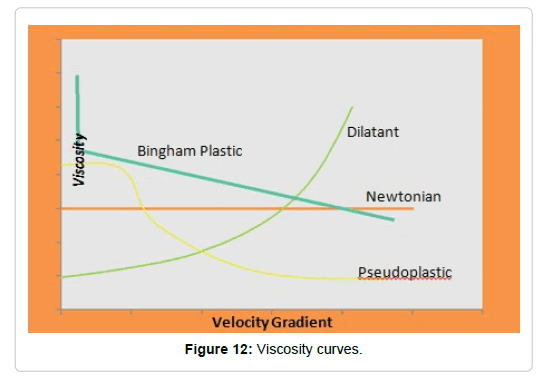
When treating molten materials, liquids and dispersions, it is important to describe the resistance to flow under the action of an applied stress. This relationship describes the fluidity of a liquid that graphically represented is called the flow curves (Figures 11 and 12) [86,87].
According to its rheological behavior, fluids are classified into three groups:
1. Newtonian fluids: they behave according to Newton’s law of viscosity, that is, their viscosity is independent of the velocity gradient.
Non-Newtonian fluids: they do not behave according to Newton’s Law. They can be classified as:
Pseudoplastics: show a strong decrease in viscosity when the velocity gradient is raised.
Dilatants: show an increase in viscosity when increasing the speed gradient.
Bingham plastics: behave like Newtonian or pseudoplastic fluids but have a flow limit. They are cohesive dispersions, which in a state of rest give the substance a solid character. When the applied forces are large, they overcome the joining forces and the structure flows.
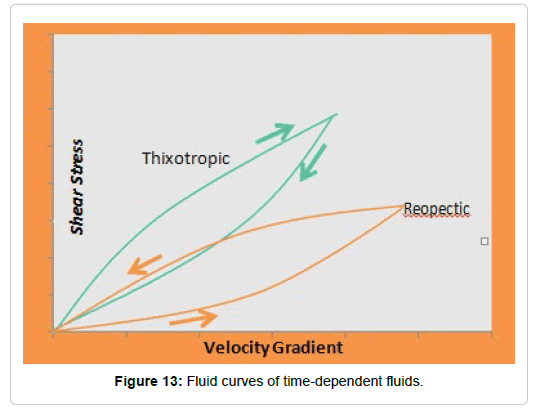
Time-dependent fluids: within these are the thixotropic and the rheopectics
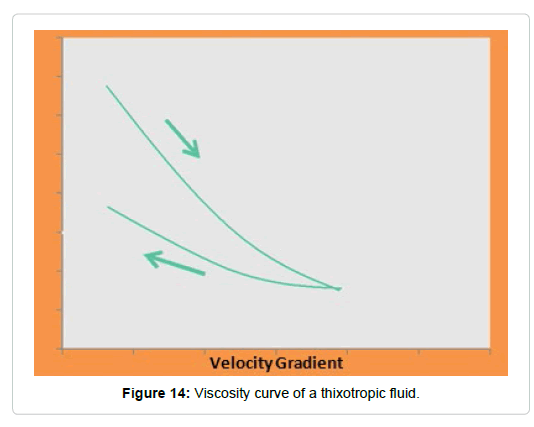
Thixotropic: The thixotropy occurs in non-Newtonian liquids in which at the end of the cutting effort they only recover their initial viscosity after a lapse of time (Figure 13). These liquids may have a flow limit. The typical viscosity curve for these fluids is shown in Figure 14.
Rheopectics: They show a behavior in which the viscosity increases with the duration of the cutting effort and the original viscosity can be recovered only after a lapse of time after the end of the cutting effort. Reopexy is the opposite of thixotropy. This is observed in the sense of the direction in which the ascending curve meets the descending curve, showing a counter-clockwise direction: the descending curve passes over the ascending curve (Figure 13).
Viscoelastic: They are characterized by both viscous and elastic properties. This mixture of properties may be due to the existence in the liquid of very long and flexible molecules or also to the presence of liquid particles or dispersed solids [86].
Equipment: To determine the rheological behavior of the solutions, a Brookfield viscometer model DVIII was used. Its operation is based on the rotation of a cylinder (spindle) inside the test material. The equipment is powered by a low speed and high torque synchronous motor. The mechanism of the gear train allows different increases of shear which results in the measurement of a wide range of viscosities using the same instrument. Newtonian and non-Newtonian fluids can be measured at different shear values by changing the spindle, speed, or both. The equipment presents a control display, a thermal jacket where the sample holder cylinder connected to a thermostatic bath is placed by means of a recirculation hoses system in order to maintain the sample at the desired working temperature. In addition, the equipment has several spindles of different diameter to operate in a wide range of viscosities [88].
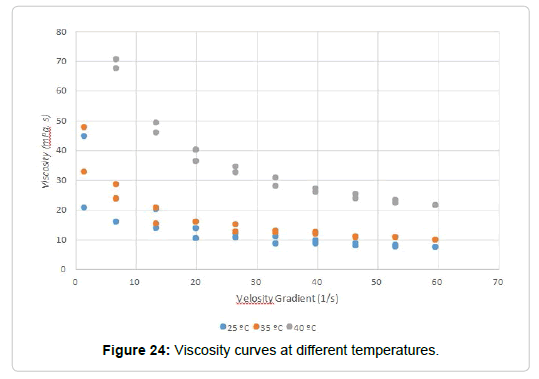
Process: Cy dispersions of different concentrations were prepared: 2, 3, 4, 5, and 6% in distilled water, which were designated Cy(d). Some of the prepared dispersions were hydrolysed, these were called CyOH(d). The dispersion was placed in the sample cylinder and this in the heating jacket, which is connected to the thermostatic bath at a temperature of 25°C. The selected spindle was placed totally submerged in the dispersion. The measurement program was selected, which consists of a series of stages of different speed gradients (rpm) and time (min) in which the spindle is kept turning inside the solution. Then, the trial began. Once completed, the viscosity data (mPa.s), shear stress (dyn/cm2) and velocity gradient (s-1) were obtained for each of the stages of the selected program. With these data, graphs of shear stress versus velocity gradient and viscosity versus velocity gradient were constructed. The test was repeated by adding 2, 4 and 5% glycerin to some of the hydrolysed dispersions (CyOH(d)) and then increasing the temperature of the thermostatic bath at 35 and 40°C.
Results and Discussion
It is always intended to relate the behavior with the structure to try to establish generalizations that facilitate the understanding and prediction of the behavior of the materials. The properties of polymeric materials are responsible for their use instead of other materials and in some cases they have unique properties that make them irreplaceable for certain applications [89].
In the evaluation of the behavior of the material, it is intended to understand the structure-property relationships, as well as to compare the performance and characteristics of one product with another. In the case of the development of new products, the understanding of the structure-property relationship allows controlling their behavior and making decisions related to the development process that will be followed. This knowledge is crucial to facilitate the design of the material and to predict how the product will behave in real service conditions. The comparison between materials is essential in the exploration of opportunities to replace existing products [83].
Next, the results obtained are presented both in the filmogenic dispersions and in the films. The films obtained from 3% dispersions of Cy turned out to be very fragile so all the tests were carried out on the films obtained with the 6% dispersions.
Dynamic light scattering
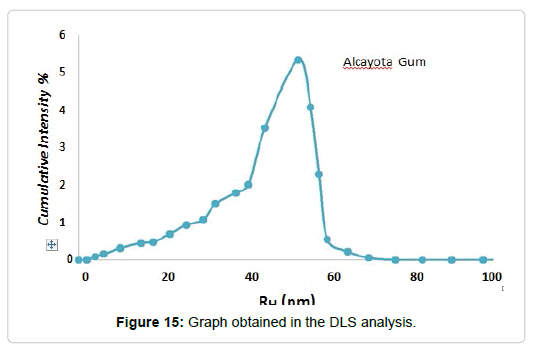
In order to know the approximate size of the particles forming the polysaccharide, the dynamic light scattering test was carried out. From a solution of Cy to 0.1% wt and then centrifuged to remove larger aggregates. The sample was placed in the cell of the equipment and a monochromatic light beam was incised, obtaining (Figure 15).
As can be seen in Figure 15, the hydrodynamic radius (RH) of the polysaccharide present in the solution is 53 ± 7 nm. The size of the macromolecule is similar to that obtained for other polysaccharides, as presented in (Table 2).
| Polisaccharides | RH (nm) |
|---|---|
| Alginate | 27-55 |
| Chitosan | 11.2-24.5 |
| Galactomannan | 22-47 |
| Glycogen | 07-65 |
| Methyl cellulose | 05-30 |
| Pectin | 12-55 |
Table 2: Hydrodynamic radius of some polysaccharides [80].
Viscosity
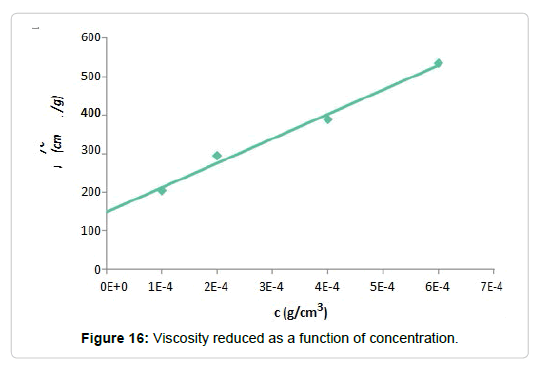
The viscosity of a liquid is noticeably altered by the presence of small amounts of macromolecules. The effect that these molecules generate on the viscosity depends on a large number of factors such as chemical structures, molecular interactions, molecular weight, etc. Using a Ubelohde capillary viscometer, the runoff times of the diluted solutions and the distilled water were measured. Applying equations 1-3, the reduced viscosity was calculated and plotted as a function of the concentration. Next, the graph obtained is shown Figure 16, from which the intrinsic viscosity of the solution was obtained.
The ordinate at the origin of the trend line corresponds to the intrinsic viscosity obtained, in this case, 149.83 cm3/g. This value represents the ability of the molecules to increase the viscosity of the solvent, in this case distilled water, in the absence of intermolecular interactions.
Table 3 shows the intrinsic viscosity values for some polymers for comparative purposes. It is observed that the intrinsic viscosity of carboxymethyl cellulose is of the same order as that of the polysaccharide under study.
| Polymer | Intrinsic Viscosity (cm3/g) |
|---|---|
| Carboxymethyl cellulose | 64 |
| Alginate | 106 |
| κ- Carragenaan | 603 |
Table 3: Intrinsic viscosity of some polysaccharides [90].
Molecular weight and Mark-Houwink parameters: Mark- Houwink correlated the intrinsic viscosity with molecular weight:
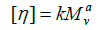 (17)
(17)
where k and a both are constants. The Mark-Houwink equation is applicable to many polymers and is extensively used to determine molecular weight. The constants k and a both vary with polymers and solvents. Equation 17 describes the relationship between intrinsic viscosity and molecular weight. Since molecular weight is related to the size of the polymer chain.
The calculation of Mark-Houwink (M-H) parameters is carried out by the graphic representation of the following equation:
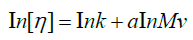 (18)
(18)
Where k and a are M-H constants, these constant depend of the type of polymer, solvent, and temperature of viscometric determinations. For a values are from 0-0.5 rigid sphere in ideal solvent, from 0.5- 0.8 random coil in good solvent, and from 0.8-2 rod like. The Mark- Houwink “a” constant is close to 0.75 or higher for these “good” solvents [90-95].
From the Huggins method data is calculated of Mark-Houwink parameters for Alcayota gum solution used in this work, where a=0.7583 and k=0.00263 cm3/g are obtained. Comparing these data with those of literature [96-103], the Mark-Houwink parameters are very similar. The calculated molecular weight is of 1867 kDa, greater than of other authors [96-103], where this increase may be due to the high viscosity of the solutions and water interaction with Alcayota gum. This is evidenced by the high value of intrinsic viscosity and its difficult Alcayota gum solution in water. As to the value of “a” this shows that Alcayota gum for this system behaves as a random-coil coonformation, although others place it as an ellipsoid (random-coil) where “a” is 0.70 to 0.747 for galactomannans, the latter boundary between random-coil and rod-like is, although closer to the ramdom-coil conformation. This high molecular weight and intrinsic viscosity the ranks as an ideal and excellent food thickener.
The hydrodynamic radius obtained in the DLS test of 53 nm was used for determinate of diffusion constant with following equation:
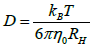 (19)
(19)
where kB is Boltzmann constat, T is work temperature, η0 is solution viscosity.
With D is calculated molecular weight using Mark-Houwink equation for diffusion,
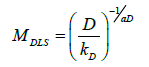 (20)
(20)
Finally, Mark-Houwink parameters were calculated, with following values: aD=0,5998 and kD=0,002543, with a molecular weight of 1722 kDa.
Hydration Values. It is well known that biopolymers adsorbed water during dry storage and its quality depends on water content. For example, the length of keratin depends on water content and therefore it is used as a hygrometer. The amount of adsorbed water depends on temperature and pressure of water vapor.
Hydration consists of the binding of water dipoles to ions or ionic groups, to dipoles, or polar groups. Hydration takes place in solid substances as well as in solution.
Since the components of a compound are linked to each other in such a way that they have lost some of their free translational mobility, the volume of hydrated molecule is always smaller than the sum of the volumes of its components, the hydration is accompanied by a decrease of the total volume.
The amount of water bound to the proteins and polysaccharides depends primarily on the ratio of water to the biopolymer in the investigated system. The two extreme cases are the dry biopolymer (water content tend to zero) and highly diluted aqueous solutions of the biopolymers. The dry biopolymer undergoes hydration if is exposed to the water vapor of increased vapor pressure. The extent of hydration can be determined and measuring the increment in weight. It is much more difficult to determine the extent of hydration in aqueous solutions of biopolymers. Although hydration is accompanied by a volume contraction of the solute and the solvent, this change in volume is very small and difficult to measure directly. It is customary to measure the density of biopolymer solution.
The amount of hydrated biopolymer and of free water in the biopolymer-water system, the thermodynamic motion of partial specific volume has been introduced and is frequently determined.
However is not to measure solution, density at each concentration since can be applied:
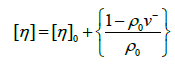 (21)
(21)
Of course if this latter is not known for the solvent conditions being used, or cannot be calculated from the chemical composition of the macromolecule then solution density measurements are required:
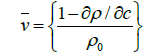 (22)
(22)
0 and are density of solvent and solution, respectively, and can be measured using densimeter or picnometer.
The swollen specific volume vsp (cm3/g) is defined when an anhydrous biomacromolecules essentially expand in suspended or dissolved in solution because of solvent association, and
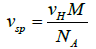 (23)
(23)
where vH is swollen or hydrodynamic volume (cm3), M the molecular weight (Da or g/mol), and NA is Avogadro’s number. This associated solvent which we consider in more detail below can be regarded as which is either chemically attached or physically entrained by the biopolymers. vsp can be related to a popular term called the hydration value by the relation
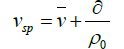 (24)
(24)
The corresponding of ‘hydration value’of the molecule, δ defined by
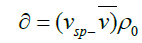 (25)
(25)
where vsp is specific volume (cm3/g). Although, because of the approximations we have made, the actual numerical value must be treated with very great caution, this treatment does however suggest that polysaccharide is highly expanded, but perhaps not to the same extent as found for coil-like polysaccharide structures to is ~50 g/g, are important in food hydrocolloids [104,105], although they can be higher than this value [106,107].
Perrin Number Combination of the Perrin function, P often referred as the ‘frictional ratio due to shape’ with the frictional ratio (f/f0) enables the degree of expansion of the molecule (vH/ v ) to be estimated, where vH, (cm3/g) is the volume of the swollen molecule (polysaccharide or protein + associated solvent) per unit mass of polysaccharide and v is the partial specific volume (essentially the anhydrous molecule):
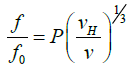 (26)
(26)
When the biopolymer is contracted, term of expansion is negligible [98].
Viscosity Increment There are two molecular contributions to the intrinsic viscosity: one from shape, the other from size or volume, as summarized by the relation
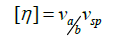 (27)
(27)
Where νa/b is a molecular shape parameter known as viscosity increment
The viscosity increment a/b is referred to as a universal shape function or Simha number (Table 4); it can be directly related to the shape of a particle independent of volume. For its experimental measurement it does however require measurement of sp, v, 0, as well as of course [h].
| P | a/b | ̅cm3/g | Vs | (g/g) |
|---|---|---|---|---|
| 1.4231 | 3.12 | 0.4061 | 48.02 | 47.63 |
Table 4: Hydrodynamic data of Alcayota gum solution.
In a study of the viscosity of a solution of suspension of spherical particles (colloids), suggested that the specific viscosity hsp is related to a shape factor νa/b in the following way:
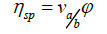 (28)
(28)
where θ is the volume fraction;
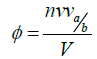 (29)
(29)
where n is the number of no interacting identical particles, ν is the volume of each particle, and V is the volume of the solution or suspension. Assume that the molecules are of a spherical shape, rigid and large relative to the size of the solvent molecules, and that the particles are small enough to exhibit Brownian motion but large enough to obey the laws of macroscopic hydrodynamics [108,109]. Then a/ b=2.5 for spherical particle [108]. The Einstein equation is now used as a reference to estimate the shape of macromolecules. Any deviation can be interpreted as the fact that the molecules are not a sphere [75].
As seen in Table 4 the values of P and a/b are far away from the spherical form as dextrans [108], and are accurate to form rod-like, as pectin [84] and alginate [109]. The hydration value accounts for the high water adsorption capacity for this polysaccharide and its great industrial potential application in highly viscous and thick solutions [110-115].
Rheology
Due to the importance of rheological behavior in fluid mechanics and in process engineering, viscosity measurements were made based on the velocity gradient in polymer solutions of different concentrations. The influence of the addition of a plasticizing agent (glycerol) as well as the effect generated by the increase in temperature on the behavior of these solutions was also analyzed. The measurements were made using a Brookfield viscometer model DVIII. At the end of this section, a brief description of the behavior of thixotropic fluids in the industry is presented.
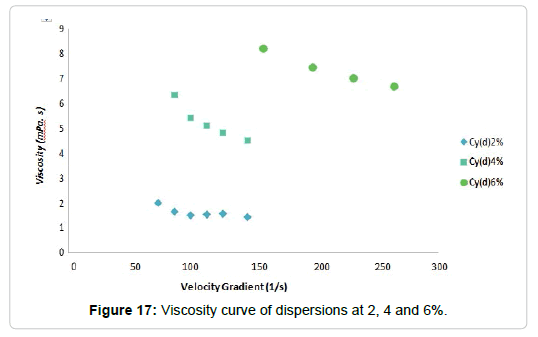
Dispersions of polysaccharides: The rheological tests were performed on dispersions of Cy at 2%, 4% and 6%, registering the viscosity and the velocity gradient. From these data, the viscosity curves shown in Figure 17 were obtained. As can be observed, the dispersions of polysaccharides analyzed show pseudoplastic behavior since, with the increase of the speed gradient, the viscosity decreases.
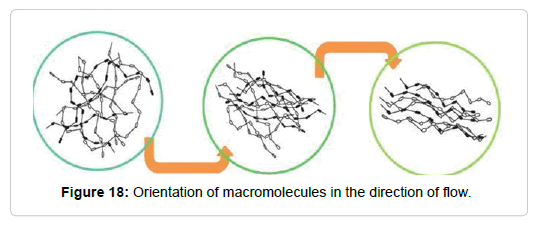
This decrease in viscosity can be explained through structural changes. Viscous fluids contain particles of irregular shapes, small droplets or long branched or tangled molecular chains. At rest, the entropy is high, that is, the particles, droplets and molecules are chaotically distributed in the structure of the viscous material. The system tries to maintain this state, but if the shear stress is increased, the components of the structure are aligned in the direction of the flow (Figure 18). The tangled molecular chains unravel and the spherical rolls of macromolecules deform into ellipsoids. Also the drops in the emulsions take an ellipsoid shape and the aggregates disarm. Clearly, the system will flow more easily in a state where its components can be aligned in the direction of the flow [116]. It is also observed that, increasing the concentration of the solutions, they increase their apparent viscosity. When the suspensions are concentrated, the flow lines are remarkably modified and the particles interact with others increasing even more the viscosity of the suspension [117].
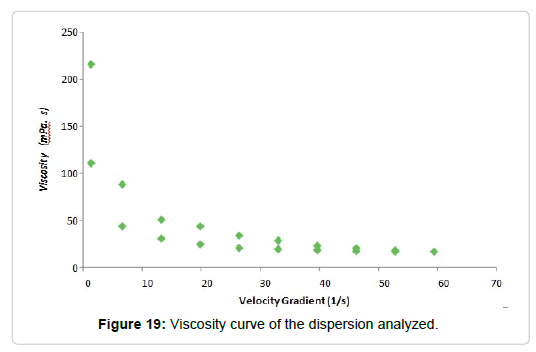
Hydrolyzed dispersions of polysaccharides: A 3% hydrolysed dispersion was obtained in Cy, on which the rheological tests were carried out, obtaining the curve shown in Figure 19.
It was observed that the dispersions behaved like thixotropic fluids (Figure 19). The molecular associations in some polysaccharide solutions affect its rheological behavior, justifying the appearance of thixotropy [118]. In many polysaccharide solutions, this behavior is the result of strong intermolecular associations through hydrogen bridge junctions [119]. Some authors explain the thixotropy through the temporary changes that occur in the microstructure of the material when subjected to cutting efforts, these changes occur not only in the number of intermolecular associations but also in the size of the aggregates [120].
In our case, the change in rheological behavior occurs after carrying out the basic hydrolysis. This process significantly increases the amount of oxydryl groups in the polysaccharide chains allowing the formation of hydrogen bonding between the molecules, which leads to the appearance of thixotropy in the dispersions of hydrolyzed alcayota.
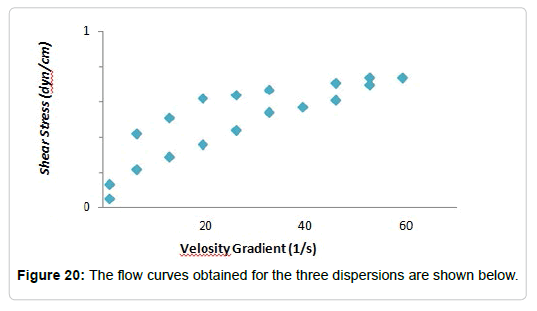
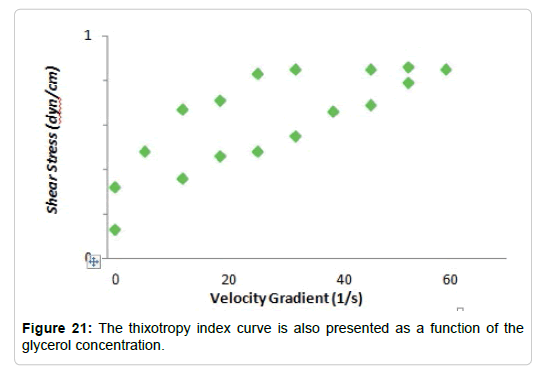
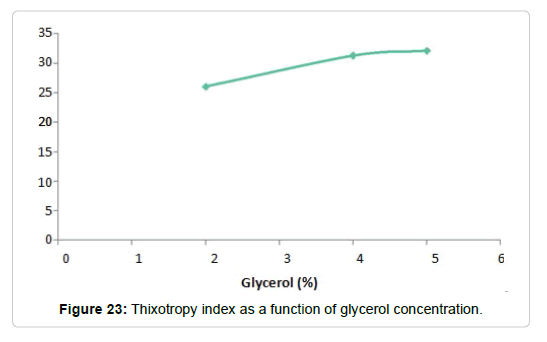
Effect of the addition of glycerol to hydrolysed dispersions: In the synthesis process of the films, glycerol is added after the hydrolysis as a plasticizing agent. These substances, as discussed above, are intended to increase the flexibility of the film. They act interposing between the polymer chains, improving the mobility of them. The solutions used to carry out the measurements were three dispersions of Cy at 4%, which were hydrolyzed and to which, respectively, 2, 4 and 5% of glycerin were added. The flow curves obtained thixotropy index curve is (Figure 21) for the three dispersions are shown below (Figure 20). The also presented as a function of the glycerol concentration
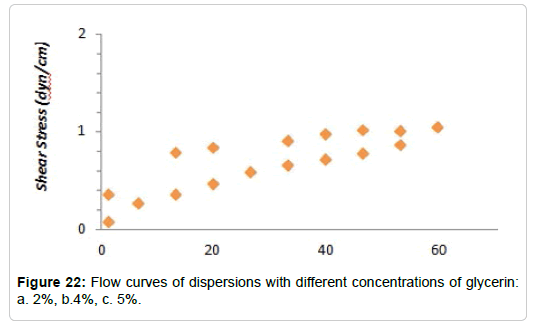
Temperature efect: The behavior of solutions hydrolyzed with 5% glycerol was analyzed against the increase in temperature. The analysis was carried out at 25, 35 and 40°C, obtaining the curves shown in Figures 20 and 21. There it can be seen that, as the temperature increases, the thixotropy index decreases. At 40°C the dispersion presents a behavior similar to a pseudoplastic fluid
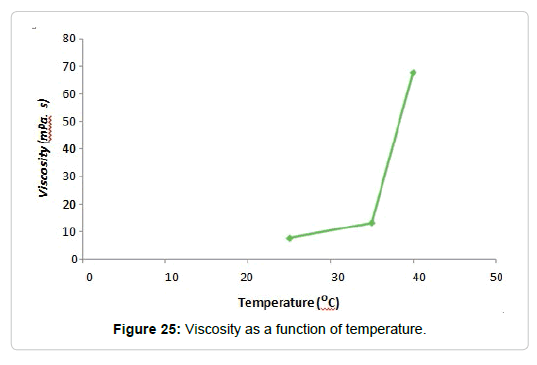
A more interesting conclusion is the increase in viscosity with increasing temperature (Figure 22). This behavior is contrary to that of most liquids, which decrease their viscosity by increasing the temperature, mainly because the molecules are closely spaced with strong cohesive forces between them. The dependence of viscosity with temperature can be explained through these cohesive forces. As the temperature increases, the cohesive forces present between the molecules diminish and begin to flow. As a result, the viscosity of liquids decreases with increasing temperature [121].
However, the behavior observed in the dispersions studied, can be justified considering that the polysaccharides in question are macromolecules of great absorbent capacity that do not form ideal solutions, but dispersions. These macromolecules are not completely soluble at room temperature, so when the temperature increases the solubilization of the polysaccharides occurs, with the consequent increase in viscosity [122,123]. However, this behavior occurs only in the temperature range in which the macromolecules are solubilized (Figure 23). A rise in temperature above this range would produce a decrease in viscosity [124,125].
Similar results have been obtained in galactomannan dispersions from guar gum (GG) and Locust Bean gum (LBG) [122]. This phenomenon has also been observed in xanthan gum and in polyvinylalcohol-co-vinylacetate, see reference [110]. Many processes involve liquids of complex behavior. This frequently happens in the food, pharmaceutical, paper and polymer packaging industries. One of the most complex phenomena that can occur is the development of thixotropy [126-129].
Conclusions
From this flour, a laboratory-scale synthesis process was proposed, consisting of the liquid-liquid extraction of undesirable compounds, followed by a purification by ethanolic precipitation. Next, a basic hydrolysis is carried out whose objective is to generate oxydryl groups and obtain wáter soluble polysaccharides. The alcayota gum solutions were characterized by three techniques: dynamic light scattering (DLS), viscosimetry and rheological characteristics analysis. Through the DLS test, the hydrodynamic radius of the macromolecules of 53 nm. The measurements of intrinsic viscosity and molecular weight were obtained (1867 kDa [130]) and can be applied as film former biopolymer [131].
The dispersions of polysaccharides showed pseudoplastic behavior before hydrolysis,with an increase in viscosity observed when increasing the concentration of thedispersions. However, after the basic hydrolysis, the behavior changes to thixotropic, a characteristic that is maintained with the subsequent addition of glycerin, where theincrease of the thixotropy index was observed when increasing the concentration of this plasticizing agent. In view of the increase in temperature, there was a decrease in the index of thixotropy and it was found that, in the range of temperatures analyzed, the viscosity of the dispersions increases, as a result of the solubilization of the polysaccharides.
Aknowlegment
The authors thank Universidad Nacional de San Luis, Instituto de Física Aplicada (INFAP-CONICET). The UNSL projects 2-1712, 2-2414 and 2-1916 for their financial support. Dr. Rolando Curvale for their valuable contributions.
References
- Morales OA, Carvajal ME, López FY, Rascón CA, Lizardi MJ, et al. (2013) Characterization of water extractable arabinoxylans from a spring wheat flour: Rheological Properties and Microstructure. Molecules 18: 8417-8428.
- Vinod VTP, Sashidhar RB, ÄŒernÃk M (2013) Morphology and metal binding characteristics of a natural polymer-kondagogu ( Cochlospermum gossypium) Gum Molecules 18: 8264-8274.
- Yao Y, Shi Z, Ren G (2014) Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd). Int J Mol Sci 15: 19307-19318.
- Fimbres OD, López EJA, Carvajal ME, Márquez EJA, MartÃnez CLR, et al. (2016) Sulfated polysaccharide gels induced by Fe(III): Rheology and Microstructure. Int J Mol Sci 17: 1238.
- Chen G, Zhang S, Ran C, Wang L, Kan J (2016) Extraction, characterization and antioxidant activity of water-soluble polysaccharides from Tuber huidongense. Int J Bio Macromol 91: 431–442.
- Zhao J, Yang J, Song S, Zhou D, Qiao W, et al. (2016) Anticoagulant activity and structural characterization of polysaccharide from abalone (Haliotis discus hannai Ino) Gonad. Molecules 21: 697.
- Huang KLiY, Tao S, Wei G, Huang Y, Chen D, et al. (2016) Purification, characterization and biological activity of polysaccharides from dendrobium officinale. Molecules 21: 701.
- Liu H, Jiang N, Liu L, Sheng X, Shi A, et al. (2016) Extraction, purification and primary characterization of polysaccharides from defatted peanut (Arachis hypogaea) cakes. Molecules 21: 716
- Li H, Yuan Q, Zhou, X, Zeng F, Lu X. (2016) Extraction of Opuntia dillenii haw. polysaccharides and their antioxidant activities. Molecules 21: 1612
- Liu Y, Xu Z, Zhang W, Duan J, Jiang J, et al. (2016) Characterization of fractional polysaccharides from gleditsia sinensis and gleditsia microphylla gums. Molecules 21: 1745
- Hammi KM, Hammami M, Rihouey C, Le CD, Ksouri R, et al. (2016) Optimization extraction of polysaccharide from tunisian Zizyphus lotus fruit by response surface methodology: Composition and antioxidant activity. Food Chemistry 212: 476-484
- Qu YLiC, Zhang C, Zeng R, Fu C. (2016) Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carboh Polym 148: 345-353
- Vasile FE, Martinez MJ, Pizones RH, Judis VMMA, Mazzobre MF (2016) Physicochemical, interfacial and emulsifying properties of a nonconventional exudate gum (Prosopis alba) in comparison with gum arabic. Food Hydrocolloids 56: 245-253
- Chen Y, Yao F, Ming K, Wang D, Hu Y (2016) Polysaccharides from traditional Chinese medicines: extraction, purification, modification, and biological activity. Molecules 21: 1705.
- Romdhane MB, Haddar A, Ghazala I, Jeddou KB, Boisset HC, et al. (2017) Optimization of polysaccharides extraction from watermelon rinds: Structure, functional and biological activities. Food Chem 216: 355-364.
- Varma CAKK, Kumar J. (2017) Structural, functional and pH sensitive release characteristics of water-soluble polysaccharide from the seeds of Albizia lebbeck L. Carboh Poly 175: 502–508.
- Zou YF, Fua YP, Chen XF, Austarheim I, Inngjerdingen KT, et al. (2017) Polysaccharides with immunomodulating activity from roots of Gentiana crassicaulis. Carboh Poly 172: 306-314.
- Yu Y, Shen M, Song Q, Xie J (2018) Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carboh Poly 183: 91-101
- Gao, Z, Zhang C, Tian C, Ren Z, Song X (2018) Characterization, antioxidation, anti-inflammation and renoprotection effects of selenized mycelia polysaccharides from Oudemansiella radicata. Carbohy Polym 181: 1224-1234.
- Chi YLi, Y Zhang, G GY, Ye H, Gao J, Wang P (2018) Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carboh Poly 181: 616-623
- Nie C, Zhu P, Ma S, Wang M, Hud Y (2018) Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carboh Poly 188: 236-242
- Fu X, Cao C, Ren B, Zhang B, Huang QLiC (2018) Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carboh Poly 183: 230-239
- Cai L, Zou S, Liang D, Luan L (2018) Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carboh Poly 184: 354-365
- Yi Y, Lamikanra O, Sun J, Wang LM, Min T, et al. (2017) Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carboh Poly
- Chen Y, Jiang X, Xie H, Li X, Shi L (2018) Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr Polym
- Thomas B, Webb J (1977) Multiple forms of α-galactosidase in mature leaves of Cucurbita pepo. Phytochemistry 16: 203-206
- Sakurai N, Tanaka S, Kuraishi S (1987) Changes in wall polysaccharides of squash (cucurbita maxima duch.) hypocotyls under water stress condition i. wall sugar composition and growth as affected by water stress. Plant Cell Physiol 28: 1051-1058
- Wakabayashi K, Sakurai N, Kuraishi S (1991) Differential effect of auxin on molecular weight distributions of xyloglucans in cell walls of outer and inner tissues from segments of dark grown squash. (Cucurbita maxima duch) hypocotyls. Plant Physiol 95: 1070-1076.
- Wakabayashi K, Yamaura K, Sakurai N, Kuraishi S (1993) Unchanged molecular-weight distribution of xyloglucans in outer tissue cell walls along intact growing hypocotyls of squash (cucurbita maxima duch) seedlings. Plant Cell Physiol 34:143-149.
- Ptitchkina NM, Danilova IA, Doxastakis G, Kasapis S, Morris ER (1994) Pumpkin pectin: gel formation at unusually low concentration. Carboh Poly 23: 265-273.
- Shkodina OG, Zeltser OA, Selivanov NY, Ignatov VV (1998) Enzymic extraction of pectin preparations from pumpkin. Food Hydrocolloids 12: 313-316
- Jun HI, Lee CH, Song GS, Kim YS (2006) Characterization of the pectic polysaccharides from pumpkin peel. LWT 39: 554-561.
- Yang X, Zhao Y, Lv Y (2007) Chemical composition and antioxidant activity of an acidic polysaccharide extracted from cucurbita moschata duchesne ex poiret. j. agric. food chem. 55: 4684-4690
- Kurz C, Carle R, Schieber A (2008) Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), peaches (Prunus persica L) and pumpkins (Cucurbita sp.) for the evaluation of fruit product authenticity. Food Chem 106: 421-430.
- Kostalova Z, Hromadkova Z, Ebringerova A (2009) Chemical evaluation of seeded fruit biomass of oil pumpkin (Cucurbita pepo L. var. Styriaka). Chemical Papers 63: 406-413.
- Yadav M, Jain S, Tomar R, Prasad GBKS, Yadav H (2010) Medicinal and biological potential of pumpkin: An updated review. Nutrition Research Reviews 23: 184-190.
- Kostalova Z, Hromadková Z, Ebringerova A (2010) Isolation and characterization of pectic polysaccharides from the seeded fruit of oil pumpkin (Cucurbita pepo L. var. Styriaca). Industrial Crops and Products 31: 370-377.
- Nosaľova G, Prisenznakova LKZ, Ebringerova A, Hromadkova Z (2011) Suppressive effect of pectic polysaccharides from Cucurbita pepo L. var. Styriaca on citric acid-induced cough reflex in guinea pigs. Fitoterapia 82: 357-364.
- Huang G, Chen Y, Wang X (2011) Extraction and deproteinization of pumpkin polysaccharide. IJFSN 62: 568-571
- Song Y, Li J, Hu X, Ni Y, Li Q (2011) Structural Characterization of a polysaccharide isolated from lady godiva pumpkins (Cucurbita pepo lady godiva). Macromolecular Research 19: 1172-1178
- Sedigheh A, Jamal MS, Mahbubeh S, Somayeh K, Mahmoud Rk, et al. (2011) Hypoglycaemic and hypolipidemic effects of pumpkin (Cucurbita pepo L.) on alloxan-induced diabetic rats. African Journal of Pharmacy and Pharmacology 5: 2620-2626
- Kostálová Z, Hromádková Z, Ebringerová A, Polovka M, Michaelsen TE, et al. (2013) Polysaccharides from the Styrian oil-pumpkin with antioxidant and complement-fixing activity. Industrial Crops and Products 41:127-133.
- Kostálová Z, Hromádková Z, Ebringerová A (2013) Structural diversity of pectins isolated from the Styrian oil-pumpkin (Cucurbita pepo var. styriaca) fruit. Carbohy Poly 93: 163-171.
- Song Y, Yang Y, Zhang Y, Duan L, Zhou C, et al. (2013) Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carboh Poly 98: 686-691.
- Song YNiY, Hu X, Li Q (2015) Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. IJBM 81: 41-48.
- Kostálová Z, Aguedo M, Hromádková Z (2016) Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chemical Engineering and Processing 102: 9-15.
- Wang S, Lu A, Zhang L, Shen M, Xu T, et al. (2017) Extraction and purification of pumpkin polysaccharides and their hypoglycemic effect. IJBM 98: 182-187.
- Wang L, Liu F, Wang A, Yu Z, Xu Y, et al. (2017) Purification, characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds. Food Hydrocolloids 66: 357-364.
- Li H, Ding F, Xiao L, Shi R, Wang H, et al. (2017) Food-derived antioxidant polysaccharides and their pharmacological potential in neurodegenerative diseases. Nutrients. 9: 778.
- Alarcon-AFJ, Hernandez GE, Campos SAE, Xolalpa MS, Rivas VJF, et al. (2002) Evaluation of the hypoglycemic effect of Cucurbita ficifolia Bouche´ (Cucurbitaceae) in different experimental models. J Ethnopharmacology 82: 185-189.
- Xia T, Wang Q (2006) Antihyperglycemic effect of Cucurbita ficifolia fruit extract in streptozotocin-induced diabetic rats. Fitoterapia 77: 530-533.
- Simpson R, Morris GA (2014). The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioactive Carbohydrates and Dietary Fibre 3: 106-114.
- Garcia GJ, Garcia LM, Zamilpa A, Almanza PJC, Jasso VEI, et al. (2017) Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Tradit. Complement Altern. Med. 14: 218-230
- León J (2000) Botany of tropical crops. 3rd edtn. San Jose Costa Rica. ISBN 92: 9039-9395.
- Lira R, Rodriguez C, Alvarado J (1998) Diversity and importance of the Cucurbitaceae family in Mexico. Mexican Botanical Act. 77: 42-43
- Della GP (2013) Manual of the cultivation of the ancilla squash (C. moschata duch.). National Institute of Agricultural Technology. Mendoza, 978-987-521-465-1.
- Della GP, Revista R (2007) National Institute of Agricultural Technology. 9 ISSN 1668-5083.
- Arevalo, JAG (2008) Physical-chemical characterization of the zambo ( Cucurbita ficifolia B.) and elaboration of two products from the pulp. National polytechnic school. Ecuador
- GEF-CIBIOGEM Biosecurity Project. Fig leaf squash ( Cucurbita ficifolia). Information System of Modified Living Organisms (SIOVM), 2007.
- Arriaga L, Huerta E, Lira SR, Moreno E, Alarcon J. Assessing the risk of releasing transgenic Cucurbita spp. in Mexico. Agriculture, Ecosystems and Environment 112: 291-299
- Gomez DD, Navaza JM (2003) Rheology of aqueous solutions of food additives Effect of concentration, temperature and blending. Journal of Food Engineering 56: 387-392
- Marchese J (1995). Processes with membranes. University Editorial, National University of San Luis, San Luis, Argentina.
- Ortiz B (2003) Drying with heat pump for dehydration of fruits. México.
- Brennan J (1998) The operations of food engineering. Editorial Acribia.
- Geankopolis C (1998) Transport processes and unit operations. México ISBN 968-26-1316-7.
- Cerqueira M, Pinheiro A, Souza B, Lima A, Ribeiro C, et al. (2009) Extraction, purification and characterization of galactomannans from non-traditional sources. Carboh Poly 75: 408-414
- Whistler R, Bemiller J (1958) Alkaline degradation of polysaccharides. Advances in Carbohydrate Chemistry 13:289-329.
- Uçar G, Balaban M. (2003) Hydrolysis of Polysaccharides with 77% Sulfuric Acid for Quantitative Saccharification. Turk J. Agric. For. 27: 361-365.
- Glass JE (1989) Polymers in aqueous media. American Chemical Society. Advs chem 223 USA,
- Cerqueira M, Souza B, Teixeira VA (2012) Effect of glycerol and corn oil on physicochemical properties of polysaccharide films: A comparative study. Food Hydrocolloids 27: 175-184.
- Yang L, Paulson AT (2000) Mechanical and water vapour barrier properties of edible gellan films. Food Research International 33: 563-570.
- Albacete C, Chiralt A, González MC (2008) Effect of moisture and glycerol on phase transitions of biopolymers: pea protein and chitosan. Polytechnic university of Valencia. Spain.
- Andreuccetti C, Carvalho R, Grosso C (2009) Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Research International 42: 1113-1121.
- Harding SE, Johnson P (1985) The concentration-dependence of macromolecular parameters. Biochem J, 231: 543-547.
- Porsch B, Sundelof LO (1994) Size-exclusion chromatography and dynamic light scattering of dextrans in water: explanation of ion-exclusion behavior. J Chromatogra 669: 21-30.
- Harding SE (1999) Photon correlation spectroscopy. In TE. Creighton (Ed.), Encyclopaedia of molecular biology (pp. 1847-1849) New York: John Wiley and Sons.
- Berne BJ, Pecora R (2000) Dynamic Light Scattering. Courier Dover Publications ISBN 0-486-41155-9.
- Gordon AM, Gary GA, Stephen EH (2014) On hydrodynamic methods for the analysis of the sizes and shapes of polysaccharides in dilute solution: A short review. Food Hydrocolloids 42: 318-334.
- Rojas R, Giacomelli C (2013) Effect of structure and bonding on the interfacial properties and the reactivity of layered double hydroxides and Zn hydroxide salts. Eng. Aspects 419: 166-173
- Shastri R (2003) Handbook of plastics technologies. The Dow chemical company. USA.
- Masuelli MA (2011) Viscometric Study of Pectin. Effect of Temperature on the Hydrodynamic properties. IJBM 48: 286-291
- Chasquibol SN, Morales GJ (2010) Contribution to the study of the gelation process of the pectin of the loquat. Industrial engineer 28: 157-176.
- Fundamentals of Food Process Engineering. 3th Ed. Romeo Toledo, Springer Sci., 2007.
- Ramirez J (2006) Basics of food rheology. Ed. Acribia, Colombia, Colombia,
- Gonzalez M (1997) Structural elements of polymeric materials. pp. 65-67. ISBN 84-89694-35-4.
- Quintans RL (2010) Rheology of food products. Doctoral thesis University of Santiago de Compostela, Spain.
- Vega ED, Vasquez E, Diaz JRA, Masuelli MA (2015) Influence of the Ionic Strength in the Intrinsic Viscosity of Xanthan Gum. An Experimental Review. J Polym Biopoly Phy Chem 3: 12-18.
- Ma X, Pawlik M (2007) Intrinsic viscosities and Huggins constants of guar gum in alkali metal chloride solutions. Carboh Poly 70: 15-24.
- Masuelli MA, Takara A, Acosta A (2013) Hydrodynamic properties of tragacanthin. Study of temperature influence. Journal of the Argentine Chemical Society 100: 25-34.
- Masuelli MA (2013). Hydrodynamic properties of whole arabic gum. American Journal of Food Science and Technology 1: 60-66.
- Masuelli MA, Sansone MG (2012) Hydrodynamic properties of Gelatin. Studies from intrinsic viscosity measurements. Book: Products and Applications of Biopolymers.
- Masuelli MA (2013) Study of Bovine Serum albumin solubility in aqueous solutions by intrinsic viscosity measurements. Advances in Physical Chemistry. Volume 2013, Article ID 360239, 8 pages.
- Curvale R, Masuelli M, Perez PA (2008) Intrinsic Viscosity of bovine serum albumin conformers. IJ Biological Macromolecules 42: 133-137.
- Sharman WR, Richards EL, Malcom GN (1978) Hydrodynamic properties of aqueous solutions of galactomannans. Biopolymers 17: 2817-2833
- Doublier JL, Lounay B (1976) Proc. Inter. Congr. Rheology, Gorhorbarg, 6: 532-533.
- Doublier JL, Launay B (1981) Rheology of galactomannan solutions: Comparative study of guar gum and locust bean gum. Journal of Texture Studies 12: 151-172.
- Robinson G, Ross MSB, Morris ER (1982) Viscosity molecular-weight relationships, intrinsic chain flexibility, and dynamic solution properties of guar galactomannan. Carboh Research 107: 17-32
- Petkowicz CLO, Reicher F, Mazeau K (1998) Conformational analysis of galactomannans: from oligomeric segments to polymeric chains. Carboh Poly 37: 25-39.
- Beer MU, Wood PJ, Weisz J (1999) A simple and rapid method for evaluation of Mark– Houwink–Sakurada constants of linear random coil polysaccharides using molecular weight and intrinsic viscosity determined by high performance size exclusion chromatography: application to guar galactomannan. Carbohy Poly 39: 377-380.
- Morris GA, Patel TR, Picout DR, Ross MSB, Ortega A et.al. (2008) Global hydrodynamic analysis of the molecular flexibility of galactomannans. Carbohydrate Polymers 72: 356-360.
- Mazeau K, Rinaudo M (2004). The prediction of the characteristics of some polysaccharides from molecular modeling. Comparison with effective behavior. Food Hydrocolloids 18: 885-898
- Sittikijyothin W, Sampaio P, Goncalves MP (2007) Heat-induced gelation of - lactoglobulin at varying pH: Effect of tara gum on the rheological and structural properties of the gels. Food Hydrocolloids 21: 1046-1055.
- Parvathy KS, Susheelamma NS, Tharanathan RN (2007) Hydration characteristics of guar gum samples and their fractions. Food Hydrocolloids 21: 630–637.
- Masuelli, MA. (2013). Dextrans in Aqueous Solution. experimental review on intrinsic viscosity measurements and temperature effect. J P Biopoly Phy Chem 1: 13-21
- Masuelli MA, Illanes CI (2014) Review of the characterization of sodium alginate by intrinsic viscosity measurements. Comparative analysis between conventional and single point methods. International JBSE1: 1-11.
- Masuelli MA (2014) Mark-Houwink Parameters for Aqueous-Soluble Polymers and Biopolymers at Various Temperatures. Journal of Polymer and Biopolymer Physics Chemistry 2: 37-43.
- Richardson PH, Juliette WJ, Foster TJ (1998) Dilute solution properties of guar and locust bean gum in sucrose solutions. Food Hydrocolloids 12: 339-348.
- Duxenneuner MR, FischerP, Windhab EJ, Cooper WJJ (2008) Extensional properties of hydroxypropyl ether guar gum solutions. Biomacromolecules, 9: 2989-2996
- Ganter JLMS, Milas M, Correa JBC, Reicher F, Rinaudo M (1992) Study of solution properties of galactomannan from the seeds of Mimosa scabrella. Carboh Poly 17: 171-175.
- Kapoor VP, Milas M, Franqois R, Taravel FR, Rinaudo M (1994) Rheological properties of seed galactomannan from Cassia nodosa buch-hem. Carbohydrate Polymers 25: 79-84
- Wu Y, Li W, Cui W, Eskin NAM, Goff HD (2012) A molecular modeling approach to understand conformation functionality relationships of galactomannans with different mannose/galactose ratios. Food Hydrocolloids 26: 359-364.
- Brummer R (2006) Rheology essentials of cosmetic and food emulsions. Alemania
- GarcÃa Q( 2008) ReologÃa J of multiphase systems. University of Alicante. University of Alicante.
- Li X, Fang Y, Zhang H, Nishinari K, Al-Assaf S, Phillips, O (2011) Rheological properties of gum arabic solution From Newtonianism to thixotropy. Food Hydrocolloids. 25: 293-298.
- Wang Q, Cui S (2005) Food carbohydrates Chemistry, Physical Properties and Applications. Taylor & Francis
- Wang Q, Cui S (2005) Food carbohydrates Chemistry, Physical Properties and Applications. Taylor & Francis
- Maestro A, Gonzalez C, Gutierrez J (2002) Shear thinning and thixotropy of HMHEC and HEC water solutions. J. Rheol. 46: 1445-1457.
- Gerham Schcramm (1998) A Practial Approach to Rheology and Rheometry. 2th Ed. Thermo Haake Reology,
- Sahin S, Sumnu S (2006) Physical Properties of Foods. TurquÃa. Springer Science.
- Masuelli MA (2015) Tara Gum the new biopolymer for different applications. Hydrodynamic Properties. Handbook of Sustainable Polymers Processing and Applications. Edited by Vijay Kumar Thakur, Manju Kumari Thakur. Pan Stanford Publishing,
- Kok MHS, Mitchell J (1999) Viscosity of galactomannans during high temperature processing: influence of degradation and solubilisation. Food Hydrocolloids 13: 535-542.
- Masuelli MA, Gassmann J (2017) Intrinsic viscosity bovine serum albumin in aqueous solutions. Temperature Influence in Mark-Houwink Parameters. Advances in Physicochemical Properties of Biopolymers Part 1, pp. 46-75. Edited by Martin Masuelli, Denis Renard. Editorial Bentham Publishing.
- Derksen JJ (2009) Simulations of complex flow of thixotropic liquids. J. Non-Newtonian Fluid Mech. 160: 65-75
- Cheng D (1979) Reports LR 155 (MH) and 317 (MH), Warren Spring Laboratory, UK.
- Howard AB (1997) Thixotropy: a review. J. Non-Newtonian Fluid Mech, 70: 1-33.
- Kemblowski Z, Petera J (1981) Memory effects during the flow of thixotropic fluids in pipes. Rheol. Acta 20: 311-323.
- Masuelli MA (2016) Alcayota films: Effect of crosslinking. Journal of Advances Chemical Engineering 6, 3 49.
Citation: Zanon M, Masuelli M (2018) Purification and Characterization of Alcayota Gum. Experimental Reviews. Biopolymers Res 2: 105.
Copyright: © 2018 Zanon M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4416
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 3457
- PDF downloads: 959