Review Article Open Access
Rare Forms of Disorders of Sex Development (DSD) in Patients with Female Phenotype
Kristesashvili J1,2*, Chipashvili M3,4, Jorbenadze T5 and Greydanus DE61Center for Reproductive Medicine, Georgia
2Department of Obstetrics, Gynecology and Reproductology, Tbilisi State University, Georgia
3Zhordania Institute of Human Reproduction, Georgia
4Department of Pharmacology, Tbilisi State University, Georgia
5Department of Pathology, Tbilisi State University, Georgia
6Pediatrics and Human Development, Michigan State University, East Lansing & Kalamazoo, Michigan, USA
- *Corresponding Author:
- Jenara Keristesashvili
Associate Professor, Department of Obstetrics
Gynecology and Reproductology, Tbilisi State University
2 Chiaruli street, Tbilisi, 0159, Georgia
Tel: +995593306294
E-mail: jenarakrist@hotmail.com
Received date: May 26, 2016; Accepted date: June 23, 2016; Published date: June 29, 2016
Citation: Kristesashvili J, Chipashvili M, Jorbenadze T, Greydanus DE (2016) Rare Forms of Disorders of Sex Development (DSD) in Patients with Female Phenotype. J Community Med Health Educ 6:446. doi:10.4172/2161-0711.1000446
Copyright: © 2016 Kristesashvili J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Disorders of Sex development (DSD) belong to uncommon pathologies; in addition, there are especially rare forms, such are ovotesticular disorders (OT), Turner Syndrome and early malignisation of intraabdominal located gonads in the cases of androgen insensitivity syndrome. In this article we present 5 rare cases of DSD in female phenotype patients: 3 cases of ovotesticular DSD with 46,XX and 46,XY karyotypes; 1 familial case of AIS with early malignancy (19-year-old) of intraabdominally-located testicle in older sibs; and a case of spontaneous menstruation in a patient with Turner syndrome and mosaic karyotype 45,X/47,XXX. Rare cases of DSD are connected with diagnostic and management difficulties and so description of each such case and collection of data in this field is very important from a scientific as well as a practical point of view. Determination of prognosis and adequate management of each individual patient are also essential. Study of this issue is especially sensitive in the case of adolescent patients, in order to avoid physiological stress, to reduce health risks, and to improve the quality of their life.
Keywords
Case report; Sexual development
Introduction
Disorders of sex development (DSD) are rare in the common population, while their incidence in selective groups is much higher. The incidence of Turner syndrome is 1 per 2,500-3,000 births [1], while that of Androgen Insensitivity Syndrome is 1 per 99 000 births and that of Muller aplasia is 1 per 4500 [2,3]. The share of DSD in the structure of primary amenorrhea exceeds 50%. There is also a high prevalence of uterine development disorders in patients with sterility (7.3%) and repeated pregnancy loss (16.7%) [3,4]. Adolescents with DSD visit the physician mainly due to delayed sexual development in puberty and/or menarche or because of sexual development irrelevant to passport gender, while adults–for absence of menstruation and infertility. However, if sexual development retardation is not manifested, in some cases it is only when patients with primary amenorrhea become sexually active and suspect infertility that patients turn to physicians for consultation.
Although the DSD itself belongs to rare (uncommon) pathologies, there are in addition its especially rare forms, such as ovotesticular disorders (OT). In early times individuals with ovotesticular disorders were called intersex, or true hermaphrodites. In the literature this pathology is mainly described as single or few cases [4,5]. In various literary sources different authors describe several hundred (400-750) of cases of OT-DSD. Among all forms of DSD the share of OT-DSD is about 10% [4,5].
This group of patients is characterized with various degrees of irrelevance of genital development with presented sex chromosomes. Consideration of OT-DSD as a particular syndrome is impossible, as there is no typical phenotype or karyotype characteristic for this disorder. Thus, the determining factor when making a diagnosis is the presence of ovotestis that is identified only by histomorphological investigation. The phenotype may be various: ambisexual, female or male [4,6-8]. In patients with ambiguous genitalia the necessity of determining the diagnosis arises during childhood or adolescence. Patients with a male phenotype will possibly visit a health facility due to inadequate sex (genital) development in adolescence.
In patients with external female genitalia, OT-DSD may be revealed in adolescence as well as in adulthood. In such cases adult patients visit the physician due to primary amenorrhea or to problems related to sexual intercourse and/or infertility. According to some authors, a diagnosis of OT-DSD is mainly identified before adulthood; in 20% of cases this occurs when the patient is under 5 years of age, while in 75% of cases this occurs in the pubertal period [4,5]. In some cases of OTDSD pregnancies and even physiological deliveries are described, mostly in 46,XX patients; pregnancies and deliveries are also described in 46,XY patients too [9,10].
Diagnostic problems as well as difficulties in terms of management and determination of reproductive prognosis arise in cases of OT-DSD because OT-DSD is characterized by wide variations in karyotype, clinical manifestations, genital anatomy, and results of hormonal and instrumental tests/examinations. Note that karyotype 46,XX/46,XY, which might be pathogenetically related to the presence of ovotestis, is quite rare in patients of this group; therefore, the outcome of a karyotype investigation cannot be considered as a determining factor for diagnostics. In each particular case of genital ambiguity, sex assessment is difficult, as is determining the type of genital organs correction to make, especially in children. Despite the fact that in cases of OT-DSD the risk of malignancy of intraabdominally-located ovotestis/gonads is comparably low, consideration of the risk of malignancy and the time of gonadectomy is very important, especially in adolescents [11,12].
Cases of Swayer syndrome are considered as the only exception with the presence of a Y chromosome or its fragment in karyotype; in this case gonadectomy of intraabdominally-located gonads should be performed immediately after diagnostics [4,13]. In cases of AIS, performing a gonadectomy of intraabdominal gonads is recommended after completion of the pubertal period, since these gonads participate in the initiation of puberty and in the formation of secondary sexual characteristics [14]. The risk of gonadal malignancy increases after adolescence and at the age of 50, the malignancy risk reaches 22% [11,15]. The risk of malignancy in cases of Partial Androgen Insensitivity Syndrome (PAIS) is higher–15% [11,14,15] compared to in cases of Complete Androgen Insensitivity Syndrome (CAIS) where it is about 5-10% [8,11,15]. In patients with AIS, malignancy of intraabdominally located gonads during pubertal period is rare. Thus, the description of cases of early malignization is very important for revision of the current approach, as well as in terms of making possible changes in the monitoring and management of such patients.
Most patients with Turner syndrome have primary amenorrhea [1,16,17], though spontaneous menstruations in these patients have been observed in particular cases [16,18]. The presence of spontaneous menstruation and even delivery in 45, X patients, inexplicable in earlier times, has been anecdotally described [18]. Improvements in methods of genetic investigations (Fluorescent In-situ Hibridisation) now make it possible to reveal previously undetected mosaicism. An analysis of existent material revealed that 5% of patients with Turner syndrome may have spontaneous menstruation [18]. Current data show that spontaneous menarche most frequently occurred in patients with mosaic karyotype 45,X with 47,XXX cellular clone, compared to 45,X/46,XX mosaic patients (70% and 34%) [18]. The phenomenon of such a mechanism is not yet fully identified. A description of each particular case is very valuable in determining the frequency of such forms, in identifying the phenotype variability and phenotypekaryotype correlation, and in improving the management of these patients.
Rare cases of DSD are connected with diagnostic and management difficulties and so description of each such forms and collection of data in this field is very important from a scientific as well as a practical point of view. Determination of prognosis and adequate management of each individual patient are also essential. Below we present 5 rare cases of DSD in female phenotype adolescents: three cases of ovotesticular DSD with 46,XX and 46,XY karyotypes; 1 familial case of AIS with early malignancy (19-year-old) of intraabdominally-located testicle in older sibs and a case of spontaneous menstruation in a patient with Turner syndrome and mosaic karyotype 45,X/47,XXX.
Case Report 1
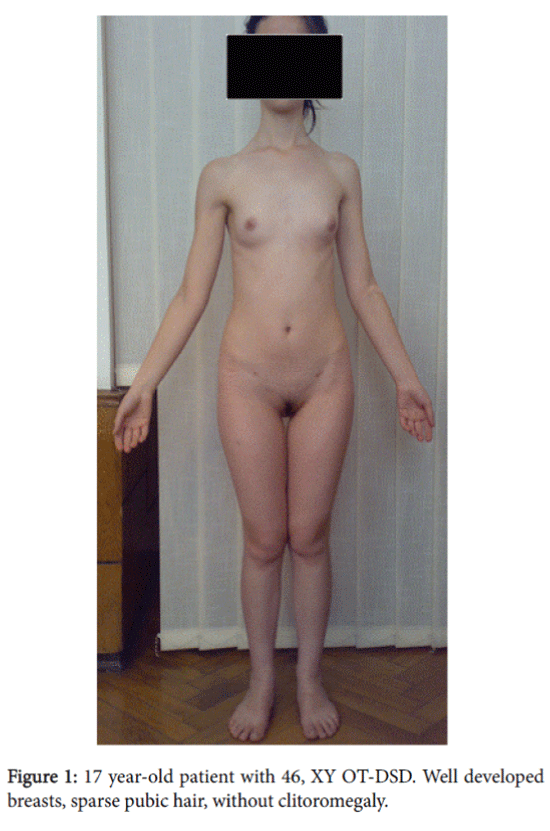
A 17 year-old patient with female phenotype and psychosexual disposition was admitted to our clinic in Tbilisi, Georgia complaining about absence of menstruation. The height was 164 cm, the weight–54 kg, and the body mass index–20.1. The patient had clean skin without acne and hair on the face; subcutaneous fatty tissue was normally distributed on the body and sexual development by Tanner’s scale: Ma4P2Ax1Me0. External genitalia was the female type without clitoromegaly, the labia majora and minora were well developed, and there was a blind ending vagina with a length of 4 cm. By rectoabdominal examination cord-like formation was palpated in the uterus area.
The ultrasonography examination revealed cord-like uterus; visualization of cervix uteri was impossible. The right gonad sized 24 × 14 mm and was located above the uterus, with homogenic structure; the left gonad was located in the normal ovary projection area: the size–34 × 18 × 18 mm and volume–5.7 cm3. Two follicles were visualized and sized at 18.5 × 14 mm and 14 × 6 mm.
Hormonal testing data: FSH–4,2 IU/l (normal for female–3-12, for male–1-14); LH -13 IU/l (norm for female –0.8-10.5, for male–0.7-7.4); estradiol–45 pg/ml (norm for female –30-200, for male<55); and testosterone–0,8 ng/ml (norm for female–0.07-0.65, for male-3,.5-8.6). Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72 hour cultures. Patient’s karyotype corresponded to 46, XY.
The preliminary diagnosis was probable Complete Androgen Insensitivity Syndrome (CAIS)-46,XY ovotesticular (DSD). The basis for making a preliminary diagnosis of CAIS was the karyotype 46, XY, female phenotype, and psychosexual disposition, sparse pubic hair, and well developed breasts, female external genitalia, and blind-ending vagina. The basis for making a preliminary diagnosis of OT-DSD presumable ultrasonography detected presence of Muller’s derivatives and in the left gonad, the presence of structure characteristic for the ovary, presumably in the form of follicles with low testosterone levels for the male range.
After obtaining informed consent and in order to verify the diagnosis, a diagnostic laparoscopy was performed that detected: 4 cm length and 0.3 cm in diameter cord-like formation in the projection area of the uterus. The right side –gonad had a smooth surface and hard consistency, size 3.5 × 2.5 × 2.5 cm; there was a rudiment fallopian tube 3 cm length with expressed fimbria. In the left side a gonad was detected sized at 5.0 × 3.0 × 2.5 cm, with smooth surface and with a few small follicles; there was a rudiment fallopian tube 3.5 cm length with expressed fimbria. Bilateral gonadectomy was performed with derivatives of mullerian duct.
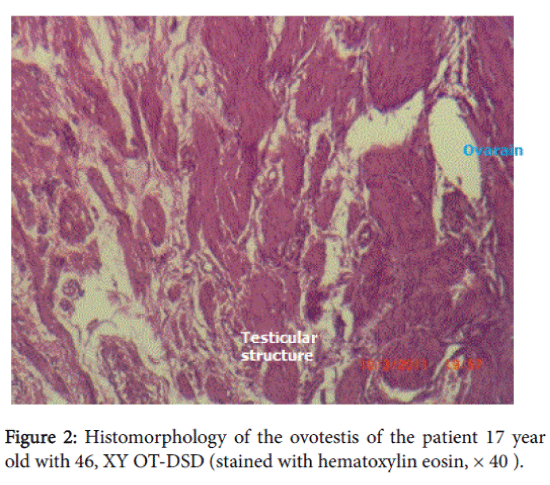
The histomorphological data: in the right gonad there was hypoplastic testicular tissue with sharply expressed fibrosis. Left gonad-hypoplastic testicular tissue with structural elements of ovary, fibrosis. There were Mullerian duct remnants present with a tube structure but without epithelium. Cellular atypia was not detected. The histomorphological diagnosis was 46, XY ovotesticular DSD. After the surgery replacement hormonal therapy was prescribed with estrogens and calcium-containing preparations due to osteopenia detected by densometry (Figure 1 and 2).
Case Report 2
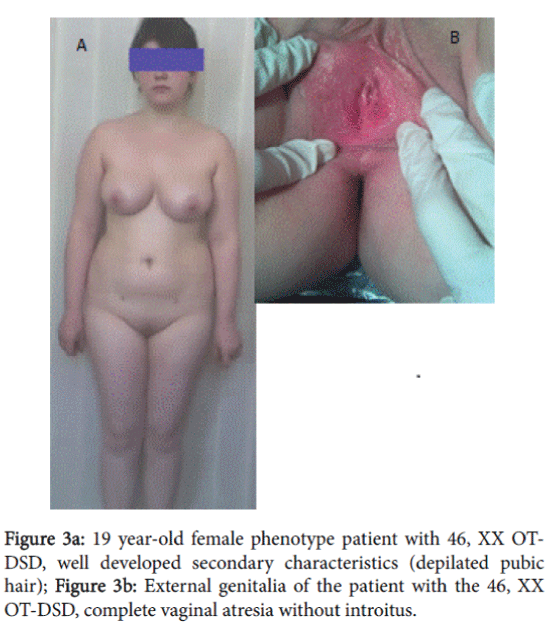
A 19 year-old patient with female phenotype was admitted in the clinic complaining of the absence of menstruation and of difficulties with sexual intercourse. The patient was married 1 year ago. During this period normal sexual intercourse was impossible. Height–168 cm, weight–80 kg, body mass index: 28.3 Skin–clean, without acne and hair on the face; fatty tissue was distributed in female type. Sexual development status by Tanner’s was assessed: Ma5P5Ax5Me0.
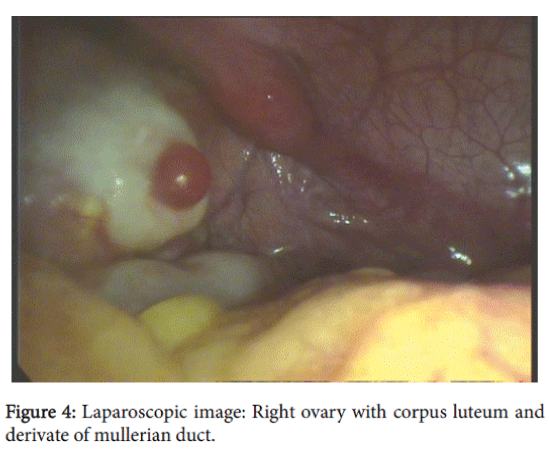
External genitalia was the female type without clitoromegaly; there was well developed labia majora and minora, without vaginal introitus. During the recto-abdominal examination of uterine area, a cord-like formation was palpated (Figure 3a, 3b and 4).
The ultrasonography examination revealed a uterus sized at 21 × 14 × 18 mm; the volume was 2.7 cm3 and the endometrium was linear. Visualization of cervix uteri was impossible. The right gonad was 18.4 × 9.9 × 9.9 mm with a volume of 0.9 cm3. The left gonad was 14 × 10 × 8.6 mm, with a volume of 0.8 cm3. In both gonads small folicules (2-4 m diameter) were expressed.
Laboratory data revealed a prolactin–16.5 ng/ml (norm for female– 1.2-19.5, norm for male–1.8-17); FSH–8.7 IU/l (norm for female-3-12, for female-1-14); LH -4.5 IU/l (norm for female–0.8-10.5, for male– 0.7-7.4); estradiol–45 pg/ml (norm for female-30-200, for male<55); and testosterone–0.3 ng/ml (norm for female–0.07-0.65, for male-3.5-8.6). Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72-hour cultures. Patient’s karyotype corresponded to 46, XX.
The preliminary diagnosis was Partial Mullerian aplasia—probable 46,XX-OT-DSD. This condition was explained to the patient. In order to clarify the diagnosis and to form a neovagina, the patient was offered diagnostic laparoscopy and the formation of a neovagina. During the patient consultation, the reproductive prognosis for both possible pathologies as well as the risk of malignancy in the case of detection of ovotestis were discussed. At this stage the patient refused a gonadectomy, because for improvement of reproductive prognosis by assisting reproductive technologies in case of presence of ovotestis, she wanted to use her own reproductive materials and if necessary to have gonadectomy at a later time.
After obtaining informed consent diagnostic laparoscopy was performed. A uterus was present in the center in the form of a 2 cm cord that was passing in Mullerian duct remnants in both sides: 2.5 cm length and 1 cm diameter on the right side and 2 cm length and 1.5 cm diameter on the left side. The right gonad had an atypical location; visually it had an ovary-like appearance with a size-2,5 × 1,0 × 1,0 cm, with a yellow body (0.4-0.5 cm) and small follicles –0.2-0.5 cm. Left adnexa: hypoplastic fallopian tube with established ampulla and fibria and a gonad (visually an ovary) sized at 2.0 × 1.0 × 1.0 cm, with small follicles (about 5 mm). Extended biopsy of both gonads and left Mullelrian duct remnants were performed, as was the formation of a neovagina. On both sides Mullerian duct remnants were presented with tube-like remnants with ampula. Fimbria was presented only on one side.
Histomorphological data revealed the following: in the samples taken from left Mullerian duct remnants clasts of fibroid tissue with muscular fibers were detected; a few small fragments of endometrium which is characteristic for proliferation phase of menstruation. The right gonad had a typical ovarian structure with stromal hyperplasia, luteal cyst, small follicles on different stage of development, in size not over 5 mm. The left gonad was present with ovarian tissue, with a few small follicles (0.2-0.5 cm), stromal hyperplasia, small testicular fragments with few tubular structures, and testicular stroma polymorphosis. Cellular atypia was not detected.
Based on these additional investigations, a final diagnosis was: ovotesticular 46, XX DSD. During final consultation the patient was once again told about the risk of malignancy of intraabdominally located ovotestis and of the necessity of performing gonadectomy in the nearest future.
Case Report 3
Patient, 35 years old, with female phenotype and psychosexual disposition was admitted to our clinic complaining about infertility. Patient was married for 4 years, she hasn’t problems with coitus. The height was 167 cm, the weight–64 kg, and the body mass index is 22.9. The patient had clean skin without acne and hair on the face; subcutaneous fatty tissue was normally distributed on the body and sexual development by Tanner’s scale: Ma4P2Ax1Me0. External genitalia was the female type without clitoromegaly, the labia majora and minora were well developed, and there was a blind ending vagina with a length of 6 cm. By recto-abdominal examination cord-like formation was palpated in the uterus area.
The ultrasonography examination revealed cord-like uterus; visualization of cervix uteri was impossible. The left gonad sized 23 × 14 mm and was located above the uterus, with homogenic structure; the right gonad was located in the normal ovary projection area: the size–32 × 19 × 17 mm. Two follicles were visualized and sized at 15 × 12 mm and 13 × 7 mm.
Hormonal testing data: FSH–5,6 IU/l (normal for female–3-12, for male–1-14); LH -15 IU/l (norm for female –0,8-10,5, for male–0.7-7.4); estradiol–40 pg/ml (norm for female –30-200, for male<55); and testosterone–1.0 ng/ml (norm for female–0.07-0.65, for male-3,5-8,6). Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72 hour cultures. Patient’s karyotype corresponded to 46, XY.
The preliminary diagnosis was probable Complete Androgen Insensitivity Syndrome (CAIS)-46,XY ovotesticular (DSD). The basis for making a preliminary diagnosis of CAIS was the karyotype 46, XY, female phenotype, and psychosexual disposition, sparse pubic hair, and well developed breasts, female external genitalia, and blind-ending vagina. The basis for making a preliminary diagnosis of OT-DSD presumable ultrasonography detected presence of Muller’s derivatives and in the right gonad, the presence of structure characteristic for the ovary, presumably in the form of follicles with low testosterone levels for the male range.
After obtaining informed consent and in order to verify the diagnosis, a diagnostic laparoscopy was performed that detected: 3.8 cm length and 0.3 cm in diameter cord-like formation in the projection area of the uterus. The left side –gonad had a smooth surface and hard consistency, size 3.2 × 2.4 × 2.3 cm; there was a rudiment fallopian tube 2.8 cm length with expressed fimbria. In the right side a gonad was detected sized at 4.8 × 3.1 × 2.4 cm, with smooth surface and with a few small follicles; there was a rudiment fallopian tube 3.6 cm length with expressed fimbria. Bilateral gonadectomy was performed with derivatives of mullerian duct.
The histomorphological data: in the left gonad there was hypoplastic testicular tissue with sharply expressed fibrosis. Right gonad – hypoplastic testicular tissue with structural elements of ovary, fibrosis. There were Mullerian duct remnants present with a tube structure but without epithelium. Cellular atypia was not detected. The histomorphological diagnosis was 46, XY ovotesticular DSD. After the surgery replacement hormonal therapy was prescribed with estrogens and calcium-containing preparations for prevention of osteopororisis (Figure 5).
Case Report 4
A 17 year-old patient with female phenotype and psychosexual disposition was evaluated at the clinic complaining of the absence of menstruation. Her height was 165 cm, weight–53 kg, and body mass index was 19.5. Her skin was clean, without acne and hair on the face. The secondary sexual characteristics by Tanners was assessed as- Ma4P1Ax0Me0. External genitalia–female type, sparse pubic hair, hypoplastic labia majora and minora, and without clitoromegaly. She had a blind-ending vagina with a length of 4 cm. By recto-abdominal examination the uterus was not palpated.
On the pelvic ultrasonography a uterus not visualized and gonads were located laterally in pelvic area. The right gonad was 2.1 × 1.6 cm, left–2.0 × 1.7 cm; the gonads had a homogenic structure. Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72 hour cultures. This patient’s kariotype corresponded to 46, XY. A preliminary diagnosis was made–Complete Androgen Insensitivity Syndrome.
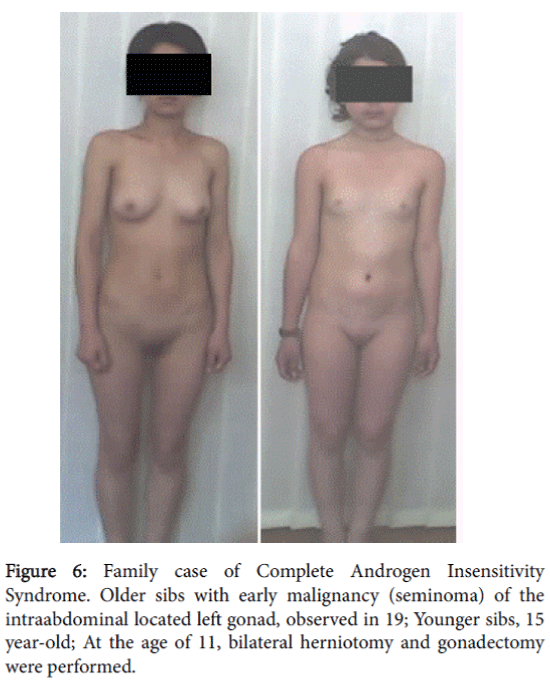
The patient did not come for further examination and treatment. Two years later the mother visited our clinic to have her younger daughter (15 years) examined because of the absence of menstruation. The mother informed us that the older sibling, who had not come to the clinic for further examination, was hospitalized at the age of 19 with acute abdominal pain. She was diagnosed with the presence of a tumor in the left small pelvic area. Histomorphological study performed after surgery detected malignancy of left gonad-a seminoma.
The 15 year-old attended our clinic for examination and had a female phenotype. At the age 11 years and 10 months, a bilateral herniotomy and bilateral gonadectomy were performed. In the clinic her height was 152 cm, weight–46 kg, and body mass index–19.9. Her Skin was clear, without acne and hairs on the face. She had nderdeveloped breasts. It should be mentioned that breast development and growth retardation was expressed after gonadectomy. She had sparse pubic hear, absence of axillary hair and hersSexual development status by Tanner’s-Ma2P1Ax0Me0. Examination of the external genitalia revealed a female type with sparse pubic hair, hypoplastic labia majora and minora and without clitoromegaly. There was a blind-ending vagina with a length of 3.5 cm. By rectoabdominal examination a uterus was not palpated.
Visualization of a uterus and gonads was impossible on pelvic ultrasonography. Data of hormonal investigations revealed: FSH- 14 IU/l (norm for female-3-12, norm for male-1-14); LH -17 IU/l (norm for female–0.8-10.5, for male–0.7-7.4); estradiol-15 pg/ml (norm for female-30-200, for male<55); testosterone -0.02 ng/ml (norm for female–0.07-0.65, for male -3.5-8.6).
Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72-hour cultures. Patient’s karyotype corresponded to 46, XY. Clinico-genealogical analysis detected that the patient’s mother had only the above-mentioned two children and that no cases of DSD were recorded among her relatives. It should be mentioned that mother had late menarche at the age 16.
A diagnosis was made–Complete Androgen Insensitivity Syndrome. X-ray examination of the younger sibling’s hand showed that growth zones were behind age norm and were not closed. The patient was prescribed hormone replacement therapy with estrogens. Considering the osteopenia detected through densitometry, calcium-containing medications was also prescribed. After 2 years from treatment initiation, the patient’s height increased by 6 cm and breasts development reached Ma4.
Case Report 5
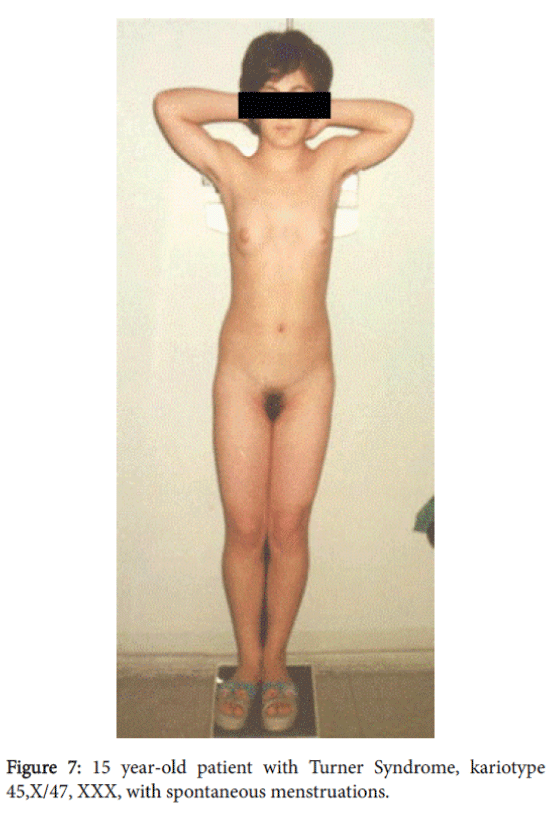
A 15-year-old patient with female phenotype was seen at our clinic complaining of a menstrual disorder. At the moment of evaluation the patient had moderate uterine bleeding. The patient had menarche at age 13 and since then she had oligomenorrhea; after being delayed, menstrual bleeding was excessive and prolonged. She had a female phenotype with a height of 140 cm, weight of 50 kg, and body mass index–25.5. The skin was clear and she had a short, wide neck, with low line of hair on it. She had a disproportion of body parts with the shoulder girdle wider than pelvic girdle, the extremities were shorter compared to the trunk; she also had cubitus valgus and short fourth metacarpals. The sexual development status by Tanner’s was assessed as-Ma3P4Ax3, (Figure 6). A gynecological examination revealed pubic hair of female type, hypoplastic labia minora without clitoromegaly. The vaginal length was 8 cm and there moderate vaginal bleeding. Rectoabdominal examination detected that uterus was bigger for age norm. Adnexa uteri were painless by palpation (Figure 7).
Pelvic ultrasonography noted a uterine size of 6.0 × 3.4 × 4.5 cm with a volume of 47.7 cm3 and an endometrial thickness of 12 mm. The right ovary was 3.3 × 1.6 × 3.2 cm with a volume of 8.8 cm3; the left ovary was 3.1 × 1.8 × 3.3 cm with a volume of 9.6 cm3 and 8-10 small follicles were found in both ovaries with maximal diameter of 6 mm.
Renal ultrasonography detected no pathology. No changes were found in the cardio-vascular system and X-ray inspection of hands detected closed growth zones. Hormonal investigation data revealed the following: FSH- 2 ME (N- 1.8-10.5); LH- 2.1 ME (N-0.5-5.0); estradiol- 52 pg/ml (N- 30-200); testosterone–0.4 ng/ml (N-0.07-0.65). A blood count showed mild anemia.
Cytogenetic analysis was done on peripheral blood using standard techniques. Karyotyping was done on G-banded metaphases obtained from 72-hour cultures. This patient’s karyotype corresponded to 45, X. Spontaneous menarche and menstruations at 45,X karyotype made us suspect the presence of occult mosaicism. By molecular cytogenetic method (FISH) at Zurich (Switzerland) Institute of Human Genetics, a mosaicism of 45, X/47, XXX was detected. The final diagnosiswas Turner syndrome. Treatment was prescribed for menstrual cycle regulation and correction of anemia.
Discussion
OT-DSD is characterized with sharply expressed phenotype and genetic variability. Phenotype varies from female to male, including ambisexual. From the point of pathogenesis the most relevant karyotype for OT-DSD is 46,XY/46,XX, which, according to literary data, is detected only in a small percentage of patients. The 46,XX karyotype is expressed in 60% of ovotesticular DSD cases. In contrast, the 46, XY karyotype has a lower frequency (15%) while different types of mosaicism (46,XX/46,XY, 46,XY/47,XXY, 45,X/46,XY) are detected in 20%. According to recent data, at the presence of 46,XX/46,XY karyotype application of genetic markers shows chimerism in than 1% of OT-DSDs.
Recently the problem of identification of the mechanisms of ovotestis development at presence of 46,XX and 46,XY karyotypes or various mosaicisms, has attracted the special interest of scientists. Methods of molecular genetics are successfully used in testing of various tissues aimed at detection of occult mosaicism. The SRY gene (located on the chromosome Yp11.3) in 46,XX ovotesticular patients presents in approximately one-third of cases. It should be mentioned that most individuals with ovotesticular disorders of sexual development with 46, XX are SRY negative. The mechanism of testicular tissue development in SRY negative patients is still unclear. It is proposed that perhaps autosomal or X-linked mutations of unknown genes control testicular tissue development or there may have been an early presence of SRY sufficient to differentiate testicular tissue but vanishing later on. Another possibility is undetected mosaicism with a Y chromosome containing line and expression of SRY gene in the cases of 46, XX OT-DSD [9,19]. It is possible, that in the cases of 46, XY OT-DSD, the presence of occult mosaicism or the development of ovarian tissue contributes to autosomal genes [12,19].
All above mentioned patients with OT-DSD had female phenotype and female external genitalia. In two cases the visit of the patients to the specialized clinic during adolescence, with the resulting clarification of the diagnosis, were caused by primary amenorrhea. Only one with 46, XY OT-DSD attended in adulthood. In the case of the patient with 46, XX OT- DSD, difficulties of sexual intercourse also played a part. In three cases diagnostic problems were faced. Particularly, in the cases of 46, XY OT-DSD, despite the fact that the patients had almost the complete range of clinical symptoms characteristic for CAIS (46, XY karyotype, female phenotype and psychosexual disposition, well-developed breasts, sparse pubic hear, female external genitalia, unvirilized clitoris; short, blind-ending vagina), detection by ultrasonography of structures similar to Mullerian duct remnants and follicle structure in the left gonad (in the right- for another patient), as well as a low level of plasma testosterone for male that is not characteristic for AIS, was uncommon for this diagnosis. Exactly this data aroused suspicions about the presence of ovotestis. After diagnosing OT-DSD it was quite difficult to explain the AIS-characteristic sparse pubic hair in the 46, XY patients.
In the above-described OT-DSD patients, we detected some relation between the testosterone secretion level and the type of tissue found in their gonads. In the 46,XX OT-DSD patient, the testosterone secretion level was within female characteristic norms on the background of normal secretion of gonadotropins, and in the gonads was detected mainly development of ovarian tissue (only ovarian tissue at right side, and in left ovotestis mainly with ovarian tissue). In the case of the patients with 46, XY OT-DSD, the patients’ testosterone secretion level was slightly increased for females, but sharply decreased for males. In these patients, ovotestis with testicular tissue development was detected on one side, with only testicular tissue on the other side. There is almost equal estradiol secretion levels within female norm, in both 46, XY OT-DSD, and 46, XX OT-DSD patients, with different development of ovarian and testicular tissues. Both our own data and that of the medical literature show that OT-DSD are not characterized with specific hormonal indicators and accordingly, its determination has no diagnostic value [7,5].
In the case of 46, XX OT-DSD, diagnostic difficulties were encountered when at the presence of symptoms characteristic for Mullerian aplasia, it was atypical to find the presence of comparatively large size (21 × 14 × 18 mm), Mullerian remnants with linear endometrium, small sizes and structure of gonads with weakly expressed follicular apparatus; also creating diagnostic difficulty was the absence of a vaginal introitus with normal development of external genitalia, which is normally present in Mullerian aplasia [3]. It is well known that by gynecological examination in the case of Mullerian aplasia, a vaginal introitus is present, though vaginal length does not exceed 0.5-1 cm. In such patients with even small Mullerian remnants usually an endometrium was not detected by ultrasonography; however, the ovaries had normal size and structure. In some cases of Mullerian aplasia, polycystic ovaries were detected by ultrasonography [3].
Suspicion for OT-DSD in the above-described 46, XX patient was based on irrelevance to typical forms of Mullerian aplasia. It should be mentioned that there are cases of incomplete aplasia of Mullerian ducts described in the literature, though in such cases normally functioning or polycystic ovaries are present [3,10,20,21]. Thus, in atypical cases of Mullerian aplasia, diagnostic laparoscopy and histomorphological investigation of gonads is quite expedient for clarifying the diagnosis. Our own as well as data in the literature indicate that in clinical polymorphism of OT-DSD, it should be noted that diagnostic difficulties occur especially in patients with female external genitalia [13,22].
As is well-known, ovulation is present in 50% of OT-DSD. In such patients, cases of pregnancies and even deliveries are described that indicate that there might be normal development of external as well as internal genitalia of female type even in 46, XY OT- DSD cases [12]. According to literary data pregnancies and deliveries more frequently take place in cases of 46, XX OT-DSD (in patients with female phenotype)–in total 11 cases are described [9]. We can identify only one described case of fertility in a 46, XY patient [5,9]. It is known that OT-DSD is characterized by genetic and ethnic peculiarities; in particular, such cases are more frequent in the South African black population, although literature analysis shows that such cases are not rare in the Asian population either [4,12,23]. At our clinic, among 243 patients with female phenotype and different forms of DSD, OT-DSD was detected in four cases, including one case of 46, XX karyotype, and three cases with 46, XY karyotype. It should be mentioned that among them 2 cases were detected in the patients’ adolescence and 2 cases were diagnosed when the patients were over 30 years old. Possibly, an important fact in late admission of patients with OT-DSD at our clinic is that they had female phenotype and no sexual development retardation.
According to the literature, ovarian tissue mostly is found on the left side in the form of ovary or ovotestis [9]. In our cases of 46, XY ovotesticular DSD, as expected, the ovotestis was identified at the left/on the right side and the testis at the right/left side. It is interesting that in the case of 46, XX ovotesticular DSD typical ovarian tissue was at the right side, and ovotestis at the left.
The literature indicates that commonly, Mullerian remnants are detected on the side where ovarian tissue is present [4,9,13]. In this regard it should be noted that in our cases of 46, XY OT-DSD, Mullerian duct remnants were detected on both sides (testis and ovotestis sides). It was interesting that in the case of 46, XX OT- DSD, typical ampulla of fallopian tube and fimbria were present at left, on the side of ovotestis, and on the right side were gonad was identified as anovary-Mullerian duct remnants were presented only with tube without ampulla and fimbria.
According to data published in the literature, in the case of ovotesticular DSD, different abnormalities of Mullerian ducts development (absence of cervix, incomplete development of vagina) often cause hematometra and hematocolpos with manifestation of DSD at the initial stage of puberty. It might be assumed that in our patient with 46,XX OT-DSD and poorly developed follicle apparatus was sufficient for provision of normal estradiol secretion indicated by normal level of gonadotropins and normal pubertal development.
However, disorders of Mullerian duct development in embriogenesis stipulated just fragmented development of endometrium in Mullerian duct remnant. In turn, incomplete development of endometrium caused the patient with normal serum level of estrogens to have no menstrual reaction and no occurrence of hematometra, which was expected in our patients without a cervix. It is worth mentioning that in our 46, XX OT-DSD patient, the presence of a luteal cyst indicates on ovulation on the background of poorly developed follicular apparatus was less expected. In the 46, XY OTDSD patient, Mullerian duct remnants were presented with tube without epithelium that might be explained by the presence of scarce ovarian tissue on the one side and the testicle on the other side. Thus, in this case, there was a poorly developed Mullerian duct remnant with appropriate structural elements.
Malignancy of ovotestis into dysgerminoma, seminoma, gonadoblastoma and yolk sac carcinomas has been described in the literature. According to the literature, the risk of malignancy in case of OT-DSD is comparatively low, about 2.6% [11,15]. The reproductive prognosis changes significantly with the development of assisting reproductive technologies. Determination of reproductive prognosis is especially crucial in adolescents, who often wish to preserve and use their reproductive opportunities. In these cases, there arises the issue of the physician’s responsibility to establish appropriate communication with the patient and to adequately identify the risks of malignancy and the application of these opportunities, as well as the appropriate sequence of medical interventions.
Determination of the risk of intraabdominally-located gonads malignancy is also crucial in the cases of androgen insensitivity syndrome. Regardless of already established comparably trustworthy approaches, considering even the comparatively low risk of gonadoblastoma development in adolescence, some authors describe malignancy of intraabdominally-located gonads in patients with AIS in early ages also [8,14]. That is why it is very important to describe our familial case of AIS, with early malignization of intraabdominallylocated gonads (by the type of seminoma) at adolescence. The issue requires further study, as each individual patient may have the risk of fatal outcome [15].
Androgen insensitivity is an X chromosome-linked recessive disease with its gene located in the long arm of the X chromosome-Xq11- q12. In cases of complete androgen insensitivity and in some cases of partial androgen insensitivity, psychosexual disposition and passport gender are female. It is recognized that secondary sexual characteristics were formed with female type: normal development of breasts and sparse pubic hair. Feminization of these patients develops partly due to testicular estrogens and partly due to peripheral conversion of androgens into estrogens [2,6,13]. The role of the testicles in the initiation of puberty and in the formation of secondary sexual characteristics in cases of AIS is indicated in our familial case also, where delay or blunting in height growth and secondary sexual characteristics were observed in a younger sibling after gonadectomy at the age of 11 years. Further progress in this regard was achieved through hormone replacement therapy with estrogens.
Usually, patients with CAIS visit the physicians in adolescence due to the delay of menarche. Such patients have fewer psychological problems even after detection of the 46, XY karyotype for identification of gender belonging, because they have female psychosocial disposition, phenotype, sexual development and passport gender. The problem in these cases is explaining to the adolescent that because of the presence of the male karyotype, they may live as females, but they will not menstruate and the reproductive prognosis will be limited or absent. The assistance of a psychologist in such cases is very important.
Here arises another essential issue of determining the time for gonadectomy. It is important to assess the risk of intraabdominallylocated gonads malignancy and to explain this to the patient. The approach is different in partial forms, when a phenotype varies and may be primarily of female type, intersexual and primarily male. In these cases it becomes more difficult to determine gender belonging and the sequence of medical interventions [7,24]. If ambiguous genitalia are not present in the female phenotype patient, this issue arises not in childhood, but during adolescence, after the onset of puberty and the manifestation of masculinization. It is generally accepted that in PAIS patients with female phenotype, gonadectomy should be performed as early as possible at a very initial stage of puberty regardless of the location of gonads, in order to avoid masculinization and related psychological problems [8,14].
The risk of malignancy at PAIS is higher–15%, compared to CAIS– 5-10%, which might be explained by the fact that in the case of CAIS rapid and complete atrophy of germinal cells in the testis take place from 1 year of age, while in the case of PAIS the number of germinal cells which are necessary for normal puberty equals to 2/3 of cells [11,15]. In any form of AIS the risk of malignancy increases in the post-pubertal period and at the age 50 reaches 33% [15]. Because initiation of puberty and development is effectively achieved through hormone replacement therapy with estrogen and because in such patients with CAIS there is a low but possible risk of malignancy of intraabdominally-located gonads during puberty, the issue of time performing a gonadectomy may require further reconsideration for minimizing the risk of malignancy.
There are many familial cases of AIS decribed in the literature [14]. Literature sources indicate that while making a genealogical scheme of a patient it is important to determine the age of menarche of the mother and grandmother from the mother’s side, because late menarche indicates that the mother is a carrier of a X-chromosome linked gene that is very important in terms of determining the genetic prognosis. In our above described familial case of AIS, the patients’ mother had a late menarche and presumably she transmitted the mutant gene to both of her children.
Thus, diagnosing CAIS in adolescence is very important in cases of delayed menarche and/or primary amenorrhea with the history of secondary sexual characteristics development, because these patients require intensive monitoring from pubertal period. Performing replacement therapy with estrogens after gonadectomy in adolescents has a positive outcome in terms of puberty formation, as well as in the prevention of osteopenia and osteoporosis. In such cases it is important to perform densitometry and in the case of osteopenia to prescribe calcium-containing preparations together with estrogens, since these preparations are essential for adequate formation of bone mass in puberty period [8,14].
Usually, evaluaton of patients with classic Turner syndrome (45, X karyotype) at a specialized gynecological clinic is quite rare, because when sharp growth retardation occurs (ie., when sexual development retadation is not yet manifested), patients in childhood are referred to endocrinologists. Patients initially attending specialized gynecological clinics are referred with manifested sexual development retardation, when growth retardation is not sharply expressed. These patients have either complete gonadal dysgenesia, or Turner syndrome with mosaic karyotype and nontypical Turner phenotype. At our clinic among patients with Turner syndrome and mosaic karyotypes (45,X/46,XX, 45,X/47,XXX) only one case of spontaneous menarche was observed in a patient with 45, X/47, XXX karyotype [18]. In the described patient with typical manifestations of Turner syndrome and spontaneous menstruations, mosaicism was detected only by using the FISH method. As is generally known, patients with XXX cellular clone more frequently have spontaneous menarche compared to 45,X/46,XX patients (70% and 34%). Most patients with 47,XXX cellular clone may have normal menstrual cycle and then secondary amenorrhea or early menopause [17,18]. Usually in the case of Turner syndrome, gonads are presented in the form of connective tissue cords and do not contain follicles.
Based on the study of fetuses with Turner syndrome, it was detected that initially there are a normal number of follicles, which progressively decreases in the middle of gestational period, particularly, from 13 week of gestation and by week 20, apoptosis of about 70% of oocytes take place. Thus, at birth follicles are completely absent and in the postnatal period, the gonad is presented by cord-like fibrotic tissue only (18). Studies showed that in the ovaries of 45,X karyotype individuals, there is a high risk of degeneration of germinal cells in the process of folliculogenesis (meiosis prophase), and after completion of follicle formation the risk is minimal. It was also identified that on the 20th week of pregnancy, not all oocytes are apoptosised; some normal oocytes may develop as primordial follicles, This explains the presence of poor follicular apparatus in some patients with Turner syndrome. At present study of the molecular basis of apoptosis is not complete (14,17). A possible cause of germinal cell degeneration in 45,X individuals may be a disorder of chromosome conjugation during meiosis, that in turn is caused by the absence of a second sexual chromosome [16].
On the basis of all the above-mentioned data it can be explained that, although sexual infantilism is one of the most prevalent characteristics of Turner syndrome and 90% of patients have gonadal dysfunction, in 30% of patients with Turner syndrome initiation of puberty may be spontaneous, while in 2-5% of patients spontaneous menstruations and delivery without medical interventions may take place [3,16] .
Summary
Special attention should be paid to the provision of reproductive materials preservation based on the willingness of the patient with disorders of sex development (DSD), especially adolescents. In these cases due to possibility of atrophy of ovarian function, the issue of reproductive material preservation arises at an early age, especially as currently there are many technical possibilities available [16-18]. Thus, description of rare DSD cases has not only scientific but also practical value. In this regard accumulation of important material is very important for improvement of diagnosis and management of these pathologies. Study of this issue is essential and especially sensitive in the cases not for adolescent patients, but for adult patients too, in order to avoid psychological stress, to reduce health risks, and to improve the quality of life. Five cases of DSD are presented from our specialized gynecologic clinic in Tbilisi, Georgia to contribute important observations to the literature on DSD.
References
- Omar HA, Greydanus DE, Tsitsika AK, Patel DR, Merrick J (2010) Pediatric and adolescent sexuality and gynecology. Principles for the primary care clinician. Nova Science, New York.
- Kousta E, Papathanasiou A, Skordis N (2010)Sex determination and disorders of sex development according to the revised nomenclature and classification in 46,XX individuals. Hormones 9: 218-231.
- Speroff L, Glass RH, Kase NG (2005) Clinical gynecologic endocrinology and infertility. Lippincott Williams Wilkins, Baltimore, MD.
- DSD (2006) Consortium on the Management of Disorders of Sex Development. Clinical guidelines for the management of disorders of sex development in childhood. Intersex Society of North America, Rohnert Park, CA.
- Damiani D, Fellous M, McElreavey K, Barbaux S, Barreto ES, et al. (1997) True hermaphroditism: clinical aspects and molecular studies in 16 cases. Eur J Endocrinol 136: 201-204.
- Chen CP, Chern SR, Sheu JC, Lin SP, Hsu CY, et al. (2005) Prenatal diagnosis, sonographic findings and molecular genetic analysis of a 46,XX/46,XY true hermaphrodite chimera. PrenatDiagn 25: 502-506.
- Denes FT, Cocuzza AS, Schneider-Monteiro ED, Silva F, Costa E, et al. (2005) The Laparoscopic management of intersex patients: the preferred approach. BJU Int 95: 863-867.
- Wisniewsky A, Migeon C, Meyer-Bahlburg H, Gearhart J, Berkovitz G, et al. (2000) Complete androgen insensitivity syndrome: long-term medical, surgical and psychosexual outcome. J ClinEndocrinolMetab 85: 2664-2669.
- Dumic M, Lin-Su K, Leibel N, Giglar S, Vinci G, et al. (2008) Report of fertility in a woman with predominantly 46,XY karyotype in a family with multiple disorders of sexual development, J ClinEndocrinolMetab 93: 182-189.
- Schultz BA, Roberts S, Rodrigers A, Ataya K (2009) Pregnancy in true hermaphrodites and all male offspring to date. J ObstetGynecol 113: 534-536.
- Cools M, Drop S, Wolffenbuttel K, Oosterhuis J, Looijenga L (2006) Germ cell tumors in the intersex gonad: Old paths, new directions, moving frontiers. Endocrine Rev 27: 468-484.
- Verkauskas G, Jaubert F, Lortat-Jacob S, Malan V, Thibaud E, et al. (2007) The long-term follow-up of 33 cases of true hermaphroditism: A 40-yer experience with conservative gonadal surgery. J Urology 177: 726-731.
- Hughes IA, Houk C, Ahmed SF, Lee PA, LWPES Consensus Group, et al. (2006) Consensus statement on management of intersex disorders. Arch Dis Child 91: 554-563.
- Boehmer ALM, Brüggenwirth H, Assendelft C, Otten BJ, Verleun-Mooijman MCT, et al. (2001) Genotype versus Phenotype in families with androgen insensitivity syndrome versus genotype. J ClinEndocrinolMetab 86: 4151-4160.
- Cools M, Stoop H, Kersemaekers AF, Drop S, Wolffenbuttel K, et al. (2006) Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J ClinEndocrinolMetab 91: 2404-2413.
- Bondy CA, Turner Syndrome Consensus Study Group (2007) Care of girls and women with Turner syndrome: A guideline of the Turner syndrome study group. J ClinEndocrinolMetab 92: 10-25.
- Loscalzo M (2008) Turner syndrome. Pediatr Rev 29: 219-227.
- Hreinsson JG, Otala M, Fridstrom M, Borgstrom B, Rasmussen C, et al. (2002)Follicles are found in the ovaries of adolescent girls with Turner syndrome. J ClinEndocrinolMetab 87: 3618-3623.
- Berkovitz GD, Fechner PY, Marcantonio SM, et al. (1992) The role of the sex determining region of the X chromosome (SRY) in the etiology of 46, XX true hermaphroditism. Hum Genet 88: 411-416.
- Chavhan GB, Parra DA, Oudjhane K, Miller SF, Babym P, et al. (2008) Imaging of ambiguous genitalia: classification and diagnostic approach. RadioGraphics 28: 1891-1904.
- Saravelos S, Cocksedge K, Li TC (2008) Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: A critical appraisal. Hum Reprod Update 14: 415-429.
- Halder A (2010) Mullerian duct remnants in the 46,XY disorder of sex development. J ClinDiagn Res 2: 2169-2175.
- Sugawara N, Tokunaga Y, Maeda M, et al. (2005) A successful pregnancy outcome using frozen testicular sperm from a chimeric infertile male with a 46,XX/46,XY kariotype: Case report. Hum Reprod 20: 147-148.
- Tekgül S, Riedmiller H, Gerharz E, Hoebeke P, Kocvara R, et al. (2009) Guideline on pediatric urology. Eur Society Paediatr Urology, EurAssoc Urology, Arnhem, Netherlands.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 24312
- [From(publication date):
June-2016 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 22996
- PDF downloads : 1316