Research Article Open Access
Remediation of Escravous Crude Oil Contaminated Soil Using Activated Carbon from Coconut Shell
MD Ibrahim1*, Rilwan Shuaibu2, Sirajudeen Abdulsalam1 and SO Giwa1
1Faculty of Engineering and Engineering Technology, Department of Chemical Engineering, Abubakar Tafawa-Balewa University, Yelwa Campus, Bauchi State, Nigeria
2Petroleum Pricing Marketing Company (PPMC) Deport, Plateau State, Nigeria
- *Corresponding Author:
- MD Ibrahim
Faculty of Engineering and Engineering
Technology, Department of Chemical Engineering
Abubakar Tafawa-Balewa University
Yelwa Campus, Bauchi State, Nigeria
Tel: +2347037785356
E-mail: imdanladi@atbu.edu.ng
Received date: April 14, 2016; Accepted date: August 05, 2016; Published date: August 08, 2016
Citation: Ibrahim MD, Shuaibu R, Abdulsalam S, Giwa SO (2016) Remediation of Escravous Crude Oil Contaminated Soil Using Activated Carbon from Coconut Shell. J Bioremediat Biodegrad 7:365. doi:10.4172/2155-6199.1000365
Copyright: © 2016 Ibrahim MD, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Environmental pollution is one of the major hazards facing humanity in the quest for energy today. In Nigeria, the activities of oil exploration and exploitation have repeatedly exposed the environment to the effect of hydrocarbon spill. This research work was carried out to investigate the potential of activated carbon produced from coconut shell to treat Escravous crude oil contaminated soil, by varying pollutant dosage, adsorbent dosage and time. The present study has clearly demonstrated that activated carbon from coconut shell (ACCS) can be used to significantly enhance the rate of degradation of petroleum hydrocarbon in the soil when homogenized with water in a ratio of 20 w/w and studied under an atmospheric temperature. A significant degradation was achieved after 32 days of the remediation process. When the soil to ACCS ratio and crude oil to ACCS were 48 w/w, and 1 mL/g respectively, (AX1) sample code had a ratio of crude to ACCS as 1:1, which reduces the total petroleum hydrocarbon (TPH) from 43.56 to 18.78 residue (mg/L). TPH was also observed to reduce to 2.83 mg/L from initial concentration of 28.92 mg/L when ratio of crude oil to ACCS was 1:1.5 (BX1) sample code, while reduction in THP of 13.63 mL was achieved with 0.5:1.5 ratio of crude oil to ACCS (CX1) sample code. The three (3) results had their rate and percentage of remediation for AX1, BX1, and CX1 as 0.77, 0.82 and 0.43 (mg/L)/day and 56.88%, 90.22% and 92.97% respectively. BX1 approach with the ratio of 1:1.5 has the high rate and percentage remediation more promising compared to AX1 and CX1. Lead which is a big treat to both plants and animals was almost reduced to a Zero (0) percentage.
Keywords
Activated carbon; Adsorption; Agro waste; Contaminated soil; Crude oil; Remediation; Total petroleum hydrocarbon
Introduction
Many major oil spill incidents have occurred in various parts, at different times in Nigeria. Some of these major reported spills include the Chevron’s Escravos spill of 1978 that involve about 300,000 barrels; Shell Petroleum Development Company’s Forcados Terminal tank failure also in 1978 with about 580,000 barrels and the Texaco Funiwa-5 blow-out of 1980 with about 400,000 barrels. Other major oil spill incidents include the Jesse Fire Incident which claimed about a thousand lives in Jesse, Delta State in 1998 and the most recent Chevron Funiwa blow out leading to two fatalities and the KS Endeavour rig being burnt to the ground. Considering the high volume of oil spilled as a result of exploitation and exploration activities and the negative impact on the ecosystem necessitating, there is the need for urgent cleaning of the polluted sites immediately the spillage occur. The most effective methods through which this can be achieved is by using remediation techniques [1].
Biological remediation is defined as a process that uses the microorganisms or plants to detoxify or remove organic or inorganic xenobiotic compounds from the environment, is a remediation option that offers green technology solution to the problem of environmental degradation [2]. The attendant negative consequences of physicochemical methods of oil degradation make biological alternative or biodegradation more attractive. It is cost-effective and does not involve high skilled technology, making it acceptable and suitable for all countries of the world [3]. Despite the great advantages of bioremediation, this approach is now used in only about 5% of all soil treatments. One of the reasons for the low effectiveness of soil bioremediation is the high toxicity of chemical contaminants to microbes and plants. This phenomenon often restricts use of this method for highly contaminated soils. Amendment of soil with natural adsorbents can help to overcome this problem. We suggested applying AC for accelerated bioremediation of highly contaminated soil and successfully used this approach for cleaning soil after an accidental leakage of 17 tons of the herbicide propanil (3',4'-dichloropropionanilide) in the Krasnodar region of Russia. A demonstrated accelerated degradation and/or detoxification of TNT (2,4,6-trinitrotoluene) and PCB in soil in the presence of AC [4].
Many techniques of remediation of contaminated soil have been developed, such as physical, chemical degradation, photodegredation. However, most methods have some drawbacks in completely remediating the contaminated soil. Some of these methods leave behind daughter compound which are more toxic to the environment than their parent compounds. Biological method offers the best environmentally friendly technique for remediating hydrocarbon and heavy metals contaminated soil because it utilized the capabilities of the indigenous microorganisms in the soli environment to break down the hydrocarbon and the heavy metals into innocuous substances [2].
Activated carbon from Agro wastes such as barks of trees, saw dust, bran (wheat, rice), husks (wheat, rice, black gram), shells (groundnut, coconut, hazelnut, walnut, cotton seed), waste leaves (tea, Cassia fistula), stalk (cotton, grape, sunflower), peels (apple, banana, orange), and others (coffee beans, biochar, water hyacinth, soybean hulls, sugarcane bagasse, jatropha deoiled cakes, maize corn cob, sugar beet pulp, etc.) can be used as sorbents to remove the contaminants, especially heavy metals [5].
The common natural precursors for activated carbon synthesis include coal, petroleum coke, pitch, wood, nutshells, peat, lignite, and more exotic ones include starch, sucrose, corn grain, leaves, coffee grounds, and straw. More advanced Activated Carbons (AC’s) with better developed porosity, reproducible properties and more uniform microstructure and pores are produced from synthetic polymers, such as polyacrylonitrile (PAN), polyvinylidene chloride (PVDC), polyfurfuryl alcohol (PFA), polyvinyl chloride (PVC), polypyrrole (PPy), polyaniline (PANI), polydivinylbenzene (PDVB), to mention a few [6].
In fact, most organic materials rich in carbon that do not fuse upon thermal decomposition can be used as precursors. The activation process is generally divided into two categories: (1) thermal (also called physical) and (2) chemical. Production of AC’s by physical activation involves carbonization of precursor (removal of non-carbon species by thermal decomposition in inert atmosphere) and gasification (development of porosity by partial etching of carbon during the annealing with an oxidizing agent, such as CO2, H2O, or a mixture of both) [6].
Factors considered in selection of raw materials for the preparation of activated carbon are carbon content, inorganic content (ash), density and volatile content, stability of supply in the country, potential extent of activation, cost of material and degradation upon storage. A good activated material must be rich in carbon content, have low ash and sufficient volatile content. It must also be readily available and inexpensive.
For every organic compound produced by a living organism, there is an enzyme in the ecosystem that makes that substance biodegradable. The enzyme will break the substance down and recycle its components in the biochemistry of life. The biosphere evolved as a self-consistent but limited array of substances and reactions that are internally harmonious.
The objective of this research is to investigate the possibility of application of ACCS for the removal of TPH from crude oil, rate and percentage remediation as a promising alternative to overcome the current industrial procedures’ technological problems. The difficulty settles on finding an adsorbent that selectively adsorbs the metal compounds, but does not adsorb (or only weakly adsorb) the coexisting aromatic hydrocarbons and olefins. The adsorption capacity of activated carbons obtained from different precursors has been investigated for the removal of hydrocarbon compounds from liquid hydrocarbon solutions.
Materials and Methods
Materials
The major raw materials used for this work were Escravous crude oil which was obtained from Kaduna Refining and Petrochemical Company (KRPC), and coconut shells gotten from Muda Lawan market, Bauchi Nigeria. The shells were collected form a waste bag of one the coconut sellers in the market. Also, the soil samples used were obtained from Abubakar Tafawa Balewa University Farm Bauchi.
Methodology
The study area is located at Abubakar Tafawa Balewa University farm house in Bauchi, Bauchi State, Nigeria with the coordinate: 10°18’57’’N, 09°50’39’’E with a population of 2,369,266 male and 2,283,800 female which amount to a total of 4,653,066 as at 2006 Census [7]. The environment is moderately dry with average temperature of 25 - 29°C between the months of April-June, 2015.
The soil and coconut shell analysis
Physicochemical analysis of both soil and coconut shell was carried out at the National Metallurgical development Centre, Jos Nigeria before the experimental set up to determine the quintiles of the elements present in both the soil and coconut shell samples and also to determine whether or not the project area (soil) is pristine. The low concentration of elements present in the soil was an indication that the environment is pristine and has not received any significant hydrocarbon pollution in the past. The pH of the soil was measured with a portable water proof pH meter (Jenway, 3150, USA), while temperature was measured using portable thermometer (Hanana H1-93510, USA) [8].
Raw material preparation
The coconut shell used as the raw material for the production of activated carbon was collected from Kasuwan Gwari market, Bauchi State Nigeria. It was washed several times using tap water and finally with distilled water. The coconut shell samples was dried at 120°C for 24 hrs in an Electric oven (Iron box type) to remove excess water content and some volatile component until constant weight. Then, the dried samples was crushed into smaller size and stored at room temperature for impregnation.
A required mass of the crushed coconut shell sample was soaked in water for 24 hrs, there after it was decanted and followed by addition of diluted Tetraoxosulphate (IV) acid (H2SO4) solution of 30% concentrations to be sucked for 24 hrs. The coconut shell sample was dried using electric oven at temperature of 120°C in the presences of oxygen for 12 hrs. The Impregnated coconut shell sample was heated in a muffle furnace at the temperatures of 700°C for one hour in the absence of oxygen. The activated carbons produced were allowed to cool; thereafter it was labeled and stored in air tight closed plastic containers.
The activators were removed by washing the carbonized coconut shell. A hot dilute potassium hydroxide (KOH) was used to produce an activated carbon such that the solid to liquid ratio is 1:10 in g/cm3. The mixture was then left overnight at room temperature in a conical flask. After, 24 hrs the supernatant liquid was then decanted in a filter paper followed by three successive washings and decantation using water. During the last washing, the whole activated carbon of each sample was transferred to the filter paper and washed using distilled water and the washing continued until pH Value was approximately 7 (neutral stage). And the Activated carbon was dried at temperature of 110°C for 2 hrs and kept in tight closed plastic bottles and carefully labeled.
Experimental design
In the experiment, twenty seven (27) field cells (plastic containers) were use as treatment options (i.e., options AX1, AX2, AX3, AY1, AY2, AY3, AZ1, AZ2, AZ3), BX1 to BZ3 and CX1 to CZ3 were made into beds of 25 cm × 25 cm × 10 cm each container with 7.2 Kg of soil sample. This was done in order that the depth, exposed surface area of the soil, the temperature, nutrient concentration and oxygen availability could be controlled. The beds also served as run off control to nearby land or cell; because the design was made in such a way that fluid movement from one place to another was totally restricted since the experiments were conducted amidst the rains (April to June, 2015). The nine (9) field cells (plastic containers) AX1, AX2, AX3, BX1, BX2, BX3, CX1, CX2 and CX3 were most considered for this report, which were equally filled with 7.2 Kg garden soil from Abubakar Tafawa Balewa University farm in Bauchi. The field cells were then polluted with 150 mL, 100 mL and 50 mL of crude oil (Escravos light) respectively; obtained from Kaduna Refining and Petrochemical Company (KRPC). The setup was wetted with the ratio of soil to water 20 (w/w) per cell, mixed and was allowed to stand undisturbed for 24 hr. The objective was to simulate conditions of a major spill. Then the soil sample was collected for analysis from each field cell. All treatment applications commenced after the 48 hrs period. Thereafter 150 g, 100 g and 50 g of granular activated carbon was also applied to all the samples respectively by varying each dosage. There was intermittent mixing of the soil samples by tilling to increase aeration in the field cell. The experimental set up was allowed to standard at an interval of five (5) days; samples were collected and analyzed for residual total petroleum hydrocarbon. The detailed description of the experimental conditions and sample code or treatment options are as shown in the Table 1.
| Experimentalconstants | ExperimentalVariables | ||
|---|---|---|---|
| Samples | Activatedcarbondosage(g) | Samplecodes | |
| Samplecollection5days | 150 | AX1 | |
| Averagetemperature28°C | A(150mLofcrudeoil) | 100 | AX2 |
| KEYS | 50 | AX3 | |
| A=150mLofcrudeoil | 150 | BX1 | |
| B=100mLofcrudeoil | B(100mLofcrudeoil) | 100 | BX2 |
| C=50mLofcrudeoil | 50 | BX3 | |
| 1=150gActivatedcarbondosage | 150 | CX1 | |
| 2=100gActivatedcarbondosage | C(50mLofcrudeoil) | 100 | CX2 |
| 3=50gActivatedcarbondosage | 50 | CX3 | |
| X=Soil | |||
Table 1: Experimental Conditions and Samples Codes.
Solvent extraction of residual TPH
Twenty gram (20 g) of the soil sample of each field cell was introduced into a separating funnel containing 50 mL of hexane, this was followed by vigorous shaking for 10 mins and filtration using Watman No.1 filter paper and the filtrate was collected in a clean conical flask. There after transferred to vertex for analysis using photometer (Wagtech 7100). This was done by simply inserting the vertex bottle into the photometer and readings taken.
Results and Discussion
Physicochemical analysis of both the soil and coconut shell was carried out before the experimental set up to determine the quintiles of the elements present. The detailed results of the physicochemical properties of the soil study area and the coconut shell samples before the commencement of the experiment are shown in Table 2.
| Elements | Mg | Al | Si | P | S | K | Ca | Fe | Mn | Zn | Rb | Sr | Pb |
| mg/g | 2.42 | 43.71 | 249.84 | 1.54 | 0.76 | 8.81 | 16.53 | 19.84 | 365.62 | 4973.96 | 20.89 | 38.05 | 398.73 |
Table 2: The XRF Analysis of soil before the commencement of the experiment.
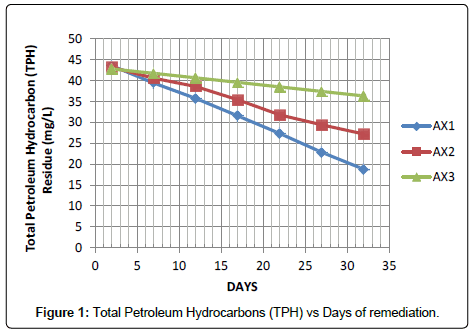
Figure 1 represent TPH remediation experimental results carried out at a constant crude oil volume; while the activated carbon dosage was varied in each of the Sample field Cells (AX1, AX2 and AX3) at 150 g, 100 g and 50 g respectively. However, it shows that remediation increased with increase in activated carbon (150 g) dosage. The best remediation was obtained at AX1.
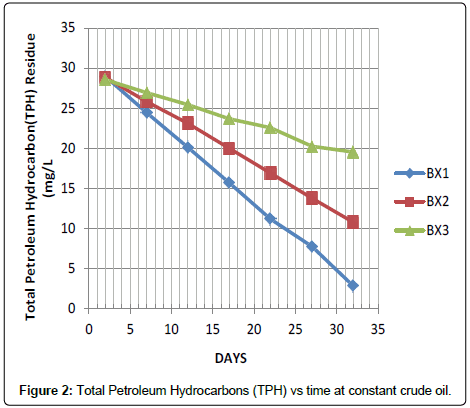
The data represents the result in Figure 2 for the remediation experiment carried out at a constant Crude Oil 100 mL, while Activated Carbon dosage was varied in each of the sample field Cells (BX1, BX2 and BX3) at 150 g, 100 g and 50 g respectively. From Figure 2, it indicates that the remediation increases with increase in Activated Carbon (150 g) dosage. The best remediation was obtained at BX1.
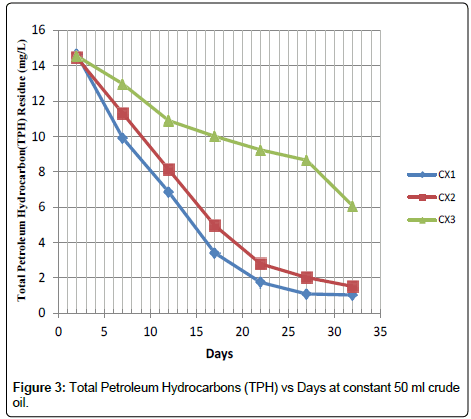
The data represents the graph in Figure 3 on remediation experiment carried out at a constant crude oil of 50 mL and activated carbon dosage was varied in each of the Sample field Cells (CX1, CX2 and CX3) at 150 g, 100 g and 50 g respectively. The Figure 3 clearly shows that the remediation increased with an increase in Activated Carbon (150 g) dosage. The best remediation was obtained at CX1.
At this juncture it is necessary to highlight the observation that after thirty-two days of remediation the TPH level dropped from 43.56 to 18.78 gm/L considering sample AX1 which had the highest rate of remediation, while sample CX3 has the lowest TPH degradation rate. Comparing the results obtained from Figures 1-3 indicates the adsorption process by the activated carbon. The trend changes as the subsequent sample were tested or determined for the TPH, it shows that field cell with higher dosage of activated carbon has a better remediation.
In the present study, application of lowest Activated Carbon (50 g) dosage as demonstrated in Figure 5 indicates the removal of about 15.13%, 31.61% and 58.28% of the TPH in sample AX3, BX3 and CX3 after thirty-two days of application but when high dosage of activated carbon (150 g) was added, it facilitate the remediation of 56.88%, 90.22% and 92.97% of the TPH in the soil sample codes AX1, BX1 and CX1 within the same period and the result was better when compared with a similar study conducted by Ref. [3], that the impact of hydrocarbon on the acidity of soil as it prevents leaching of basic salts as well as leads to production of organic acids. There was a distinct increase in soil pH when samples were taken five days after contamination, [9] had previously reported that such variability in soil pH which could arise during organic decay processes which may result in the formation of both acid and base forming chemicals. However, as remediation treatments were applied the mean pH value for the options dropped from 5.21 to 4.08 at the end of the thirty second day period in Table 3. The pH range observed during the study highlighted the view that soils with pH on the acid side of neutrality are best suited for agriculture.
| Elements | %Al2O3 | %CaO | %Fe2O3 | %K2O | %MgO | %Na2O | %SiO2 | %MnO | %ZnO |
| mg/g | 15.6 | 0.57 | 12.4 | 0.52 | 0.76 | 8.81 | 16.53 | 19.84 | 0.3 |
Table 3: The XRF Analysis of the Coconut shell before the commencement of the experiment.
However, it is evident in Table 4 that the rate of remediation is faster at sample code AX1, BX1 and CX1 with 0.77, 0.82 and 0.43 mg/L/ day while the percentage remediation is at 56.88, 90.22 and 92.97% respectively.
| Totalpetroleumhydrocarbon(TPH)residue(mg/L)(Crudeoil) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | 2 | 7 | 12 | 17 | 22 | 27 | 32 | âÂ?Â?inTPH | Rate(mg/L)/day | %Remediation |
| Samplecodes | ||||||||||
| AX1 | 43.56 | 39.42 | 35.73 | 31.57 | 27.34 | 22.83 | 18.78 | 24.78 | 0.77 | 56.88 |
| AX2 | 43.21 | 40.61 | 38.61 | 35.41 | 31.81 | 29.41 | 27.21 | 16 | 0.5 | 37.03 |
| AX3 | 42.83 | 41.75 | 40.67 | 39.59 | 38.51 | 37.43 | 36.35 | 6.48 | 0.2 | 15.13 |
| BX1 | 28.95 | 24.43 | 20.11 | 15.69 | 11.17 | 7.75 | 2.83 | 26.12 | 0.82 | 90.22 |
| BX2 | 28.83 | 25.82 | 23.11 | 19.98 | 16.89 | 13.78 | 10.77 | 18.06 | 0.56 | 62.64 |
| BX3 | 28.54 | 26.92 | 25.43 | 23.68 | 22.56 | 20.24 | 19.52 | 9.02 | 0.28 | 31.61 |
| CX1 | 14.66 | 9.91 | 6.86 | 3.41 | 1.74 | 1.09 | 1.03 | 13.63 | 0.43 | 92.97 |
| CX2 | 14.47 | 11.3 | 8.13 | 4.96 | 2.79 | 2.02 | 1.51 | 12.96 | 0.41 | 89.57 |
| CX3 | 14.55 | 12.97 | 10.89 | 10.01 | 9.23 | 8.65 | 6.07 | 8.48 | 0.27 | 58.28 |
| AveragepH | 5.56 | 5.79 | 5.41 | 5.34 | 5.3 | 5.17 | 5.13 | |||
Table 4: Total Petroleum Hydrocarbons (TPH), rate and percentage remediation for A’s, B’s and C’s Samples.
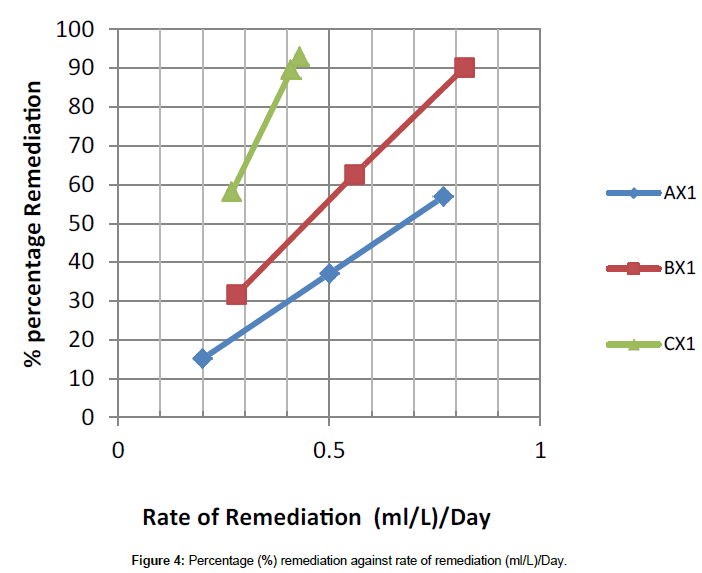
The data from Figure 4 also deduced that the rate of remediation and the percentage remediation is promising at 0.82 mg/L/day and 90.22% respectively. Therefore, the ratio combination of sample Code BX1 is highly recommended for such a remediation exercise (1:1.5).
The Tables 5 and 6 revealed from the XRF analysis after the remediation study reduction in the elemental compositions of Magnesium, Aluminum, Phosphorous, Sulfur, Calcium, Iron, Manganese, Zinc Strontium and Lead. However, a remediation in the following elements such as Manganese, zinc and lead. Especially, while an incrementing of about 50% after remediation for both Silicon and Potassium was observed in the Tables 5-7 as most promising three (3) samples AX1, BX1 and CX1 respectively. All samples are almost commensurate to the pristine soil samples after the soil remediation. However, maize sowed survived on the treated (setup) soil samples.
| Elements | Mg | Al | Si | P | S | K | Ca | Fe | Mn | Zn | Rb | Sr | Pb |
| mg/g | 1.62 | 20.76 | 387.3 | 0.21 | 0.46 | 16.41 | 2.22 | 2.58 | 1.41 | 1.45 | 20.89 | 0.13 | 1.17 |
Table 5: The XRF Analysis of soil sample AX1 after the 32 days remediation.
| Elements | Mg | Al | Si | P | S | K | Ca | Fe | Mn | Zn | Rb | Sr | Pb |
| mg/g | 1.44 | 21.57 | 379.5 | 0.22 | 0.49 | 15.41 | 2.29 | 2.8 | 1.18 | 1.5 | 20.89 | 0.15 | 1.21 |
Table 6: The XRF Analysis of soil sample BX1 after the 32 days remediation.
| Elements | Mg | Al | Si | P | S | K | Ca | Fe | Mn | Zn | Rb | Sr | Pb |
| mg/g | 1.42 | 21.66 | 383.2 | 0.2 | 0.53 | 16.23 | 2.12 | 2.84 | 1.14 | 1.49 | 20.89 | 0.14 | 1.19 |
Table 7: The XRF Analysis of soil sample CX1 after the 32 days remediation.
Conclusions
Oil spills are caused by human errors and carelessness, but sometimes by natural disasters such as hurricanes or earthquakes. Deliberate acts by terrorists, countries at war, sabotage and bunkering, or illegal dumpers however, prove that oil spills are not always accidental. The present study has clearly demonstrated that an activated carbon from coconut shell can be used to significantly enhance the rate of biodegradation of petroleum hydrocarbon in the soil when used in combination with water and proper aeration. A significant biodegradation which was achieved after 32 days of application is an indication that the remediation approach used in this study was economically justified by the application of Agro waste, effective in term of day(s) it took and efficient in the ratio of (1:1) in terms of AX1, (1:1.5) in terms of BX1 and (1/2: 1.5) in terms of CX1. In conclusion Code BX1 with the rate and percentage remediation is the best at 0.82 mg/L/day and 90.22% respectively. Therefore, the ratio combination of sample Code BX1 his recommended for such a remediation exercise i.e., (1:1.5).
Recommendations
A series of trial should also be encouraged using other agricultural by products to open up opportunity for local materials at no cost.
A simulation of the granulated ACCS and the remediation of contaminated soil should be optimized; to save cost and maximize profit.
References
- Ameh CU,Jimoh A, Abdulkareem AS, Otaru AJ (2013) Remediation of Petroleum Polluted Site in Nigeria Using Granulated Activated Carbon (Gac). IOSR J Environ Sci Toxicol Food Technol 3:100-109
- Abioye OP (2011) Biological Remediation of Hydrocarbon and Heavy Metals Contaminated Soil. In: Soil Contamination. Simone Puscucci (Editor). InTech Web, Croatia, pp: 127-142.
- Yakubu MB(2007) Biological approach to oil spills remediation in the soil. African Journal of Biotechnology 6: 2735-2739
- Vasilyeva GK, Strijakova ER, Shea PJ (2006) Use of activated carbon for soil bioremediation. In: Soil and Water Pollution Monitoring, Protection and Remediation. Springer, Netherlands, pp: 309-322.
- YenenehAM, Maitra S, Usama E (2011) Study on Biosorption of Heavy Metals by Modified Lignocellulosic Waste. Journal of Applied Sciences 11: 3555-3562.
- Wentian G,Gleb Y (2013)Review of nanostructured carbon materials for electrochemical capacitor applications: advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and grapheme.Wiley Interdisciplinary Reviews: Energy and Environment 3: 424-473
- http://www.population.gov.ng/
- Conlette OC (2014) Aerobic Degradation of Paraffin and Olefin Synthetic Based Drilling Mud Base Fluids by Gulf of Guinea Sediments under Natural Environmental Conditions. Academia Arena 6: 53-59.
- Iqbal J (2003) Effect of Temperature on Efficiency of in Situ Bioremediation Technology: A Laboratory Microcosm and Field Study (Doctoral dissertation, Louisiana State University).
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14755
- [From(publication date):
September-2016 - Sep 04, 2025] - Breakdown by view type
- HTML page views : 13521
- PDF downloads : 1234