Review on Different Components of Solid Oxide Fuel Cells
Received: 25-Nov-2017 / Accepted Date: 30-Nov-2017 / Published Date: 05-Dec-2017 DOI: 10.4172/2168-9806.1000181
Abstract
Solid oxide fuel cell is useful other than fuel cells due to its high efficiency, fuel flexibility, less pollution and less operating temperatures. Ceria based oxides and are earth oxide doped ceria (RDC) shows outstanding properties compared with yttria stabilized zirconia (YSZ), which is conventional oxide ion conductor used as electrolyte material for SOFC. In this paper mainly concentrate on different novel materials were presented.
Keywords: Ceria; SOFC; Electrolyte; Ionic-conductivity
Introduction
Solid Oxide Fuel Cells consist of solid-electrolyte, cathode, anode and interconnectors. For proper operation of SOFCs, all of these components must have chemical, mechanical and thermal stability under operating conditions. Each of the components must have sufficient (and appropriate type i.e. ionic, mixed conductivity or electronic) conductivity. Thermal expansion coefficient (TEC) and chemical compatibility with other cell components influence the overall performance of SOFCs. Additionally, fabrication conditions/ methods employed for each component are also an important factor for the performance of the SOFC.
Literature Review
Anode
In a fuel cell, anode is also called as ‘fuel electrode’; as fuel is fed to the system through this electrode. Anode faces the reducing environment at high temperature 973-1273 K where the hydrogen fuel combines with the oxygen ion at the electrolyte interface.
The main characteristics of SOFC anodes are as follows:
• High electronic conductivity
• Sufficient electro catalytic activity for fuel oxidation reactions
• Chemically stable and thermally compatible with adjacent cell components (electrolyte/interconnector) and has sufficient porosity for efficient gas transportation
• Operate in reducing atmosphere
• Matching thermal expansion coefficient (TEC) with adjacent cell components.
Some of the metals such as Ni, Co, Cu, Ru and Pt are those that have better catalytic activity for hydrogen oxygen recombination reaction. But they have too high TEC as compared to the electrolyte material. In order to reduce TEC, anode materials are usually mixed with ceramic (electrolyte) material. Among all metals Ni is widely used as anode material in SOFC due to its low cost and high catalytic activity. NiO is generally used as precursor of Ni which undergoes reduction in the reducing condition observed at anode side of SOFC. The minimum amount of Ni for percolation in YSZ matrix is 30% [1]. Most extensively used anode materials for SOFCs are Ni-YSZ, Ni-GDC and Cu-GDC (Gadolinium doped ceria). The metallic phase in anode serves dual purpose, namely, catalyst and electrical conduction path and ceramic (electrolyte) component serve the purpose of TEC matching with that of electrolyte material. Due to this mixed phase, anode material is also referred to as ‘cermet’ (ceramic+metal). The active reaction sites are located at the triple phase boundary (TPB) where ceramic electrolyte, metal catalyst and reactant (fuel gas) meets. The TPB length directly affects the electrode performance [2]. At high temperatures, anode with high nickel content degrades fast due to coarsening of nickel particles. When natural gases as fuel are used, carbon deposition on nickel catalyst reduces the catalytic activity. An alternate anode, Tidoped YSZ was studied for SOFC. However, when used with nickel it showed improved thermal stability and better electrical conductivity as well as a lower degradation at 1000°C [3,4]. The doped ceria shows good catalytic activity for carbon oxidation than YSZ. Hence, fuels cell with nickel-ceria anode operating on hydrocarbon fuels have resulted in decreased carbon deposition at anode. Ceria has some electronic conductivity contribution along with ionic conductivity, which helps in increasing electronic conductivity contribution in anode performance [5]. La1-xSrxCrxMn1-xO3 (LSCM) material has also been established as anode for SOFC [6]. The advantage of this material is that it has good electrochemical activity at both cathode and anode environment and compatibility with many solid electrolytes. This material has very low ionic transport and low electrical conductivity which can be improved by YSZ/ceria addition. Due to dual catalytic activity (anodic and cathodic) LSCM can be used as dual-electrode in a single chamber- SOFC.
Cathode
The cathode operates at 1273 K in an oxidizing environment (air/ oxygen) and also contributes in the oxygen reduction reaction. At cathode and electrolyte interface oxygen/air is reduced to oxygen ions with the help of cathode catalyst and two electrons arriving from the external circuit.
The main characteristics of SOFC cathodes are as follows:
• It should have sufficient electronic conductivity
• Thermal and chemical stability during cell operation and cell fabrication
• Thermal expansion coefficient should matches with cell components
• Compatibility and minimum reactivity with adjacent cell components
• Low cost.
Doped lanthanum manganites’, and doped lanthanum cobaltite’s are widely used as cathode materials.
Manganites: (Ln1-xAxMnO3) (Ln-lanthanides, A=Ca, Sr, Ba, Pb) (LaMnO3) works well at operating temperature above 1073 K due to its high electronic conductivity, equivalent thermal expansion coefficient with many electrolyte materials (e.g. YSZ, GDC etc.), and good catalytic activity is for oxygen reduction reaction at the cathodeelectrolyte interface [7]. Lanthanum strontium manganite (LSM) is generally used as a cathode material for fuel cells operating at 1073- 1273 K [8]. Electrochemical reactions can only occur at the triple-phase boundaries (TPBs), which are defined as the confluence of sites where the oxygen ion conductor, electronic conductor, and the gas phase come in contact.
Some of the alternative perovskite structured ceramic electrode materials for lower temperature operation are listed below [9].
➢ Lanthanum strontium ferrite (LSF), (LaSr)(Fe)O3
➢ Lanthanum strontium cobaltite (LSC), (LaSr)CoO3
➢ Samarium strontium cobaltite (SSC), (SmSr)CoO3.
Electrolyte
In a solid oxide fuel cell, electrolyte is the main component. Therefore, the property of the oxide electrolyte material defines the whole structure of the fuel cell [10].
The main characteristics SOFC of electrolytes are as follows:
• Electrolyte should have high ionic and negligible electronic conductivity
• Chemically stable at elevated temperature
• Gas tight/free of porosity
• Low ohmic losses
• Matching TEC with adjacent cell components (anode/cathode).
Oxygen ionic conduction mechanism
Conduction is the process in which migration of an ion or electron from one lattice site to neighboring crystal lattice in solid. In SOFCs diffusion is a fundamental phenomenon where the oxygen ions are transferred from cathode to anode through oxide ion conducting electrolyte. In oxide materials, diffusion of ion is restricted due to existence of anion and cation in its own sub lattice [11]. In oxides, the oxygen self-diffusion is faster than cation diffusion [12]. Diffusion mechanism’s significant role is to transport atoms away from their equilibrium positions in crystalline materials. Therefore, the role of point defect is most important for the oxygen diffusion, and the defect– defect interactions are significant in this motion [13].
Oxygen ion conductors
In case of oxygen ion conductors, current transports due to movement of oxide ions through the crystal lattice. Oxide ion movement is controlled by oxygen ions vacancies, due to thermally activated hopping of the oxygen ions within crystal lattice. The ionic conductivity is temperature dependent, and at high temperatures it can approach values close to 1 Scm−1 due to the crystal that contains unoccupied sites equivalent to those occupied by the lattice oxygen ions and the energy involved in the process of migration from one site to the unoccupied equivalent site must be small, certainly less than 1 eV. Gadolinium and samarium are the commonly used rare earth do pants. The ionic conductivity value of gadolinium doped ceria is approximately three times higher than the equivalent quantities of samarium doping [14]. The ionic conductivity depends on do pant type and concentration. For In rare earth doped ceria Gd0.2Ce0.8O2 (GDC), shows the maximum oxide-ion conductivity [15]. The electrical conductivity of various types of fluorite oxides can be arranged in an order like Bi2O3>CeO2>ZrO2>ThO2>HfO2. Among these Bi2O3 shows the highest oxygen-ion conductivity as reported so far for the solid electrolytes [16].
Yttria stabilized zirconia (YSZ): Doping with 8 mol% Y2O3 stabilizes cubic zirconia gives the highest ionic conductivity (~0.18 S/ cm at 1273 K) while doping with 9-10 mol% Y2O3-ZrO2 conductivity decreases slightly. Main drawback of YSZ is that ionic conductivity decreases when temperature of SOFC reduces below 1000 K due to increasing resistance of the cell. Ex. Ionic conductivity at 1273 K is 0.1 S/cm, when temperature is reduced up to 1000 K, its ionic conductivity is 0.02 S/cm [17]. The defect formation reaction in yttrium stabilized zirconia is given by the Kroger-Vink notation:
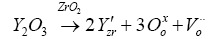
Scandia stabilized zirconia: Scandia stabilized zirconia shows high ionic conductivity than YSZ due to the smaller mismatch in size between Zr4+(0.84 Å) and Sc3+(0.87 Å), as compared to that between Zr4+(0.84 Å) and Y3+, leading to a smaller energy for defect association, which increases mobility and thus conductivity. The defect formation reaction in Sc2ZrO3 stabilized zirconia is given by the Kroger-Vink notation.
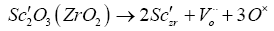
9 mol % Sc2O3 has the highest conductivity 0.343 S/cm at 1273 K which is much higher than that of 8YSZ at the same temperature, 0.164 S/cm [18].
Doped lanthanum gallate (LSGM): A perovskite oxide, La0.8Sr0.2Ga0.8Mg0.2O3 (LSGM), has oxide ion conductivity higher than that of YSZ at intermediate temperatures, 823-1073 K and also shows good chemical stability, negligible electronic conductivity over a large range of oxygen partial pressures [19].
Stabilized δ-Bi2O3: Bi2O3 is predominantly an electronic conductor at room temperature. However, at high-temperature δ-Bi2O3 phase with fluorite-related structure has the maximum known oxide-ion conductivity σ0≈2.3 S/cm at 1063 K [20].
Some other oxide ion conducting solid electrolyte systems which can be used in SOFC are mentioned below [21]:
➢ Cerium oxide doped with gadolinium (GDC)
➢ Cerium oxide doped with samarium (SDC)
➢ Cerium oxide doped with yttrium (YDC)
➢ Cerium doped with calcium (CDC).
Doped cerium dioxide [CE1-x(M)xO2-Δ]
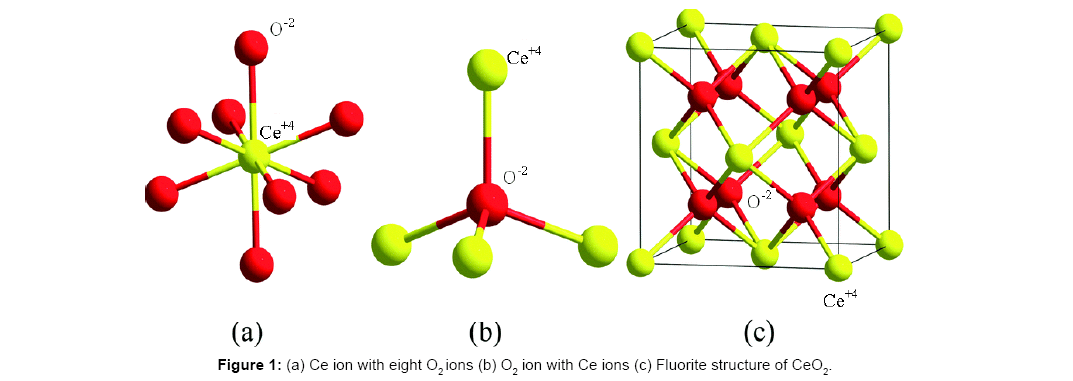
Structure of CeO2: Ceria has fluorite type crystal structure. The structure can be viewed as an FCC array of Ce ions with the oxygen ions residing in the tetrahedral holes. Unit cell consists of 4Ce, 8 oxygen ions. The structure of ceria is shown in Figure 1. Fluorite structure is a cubic structure with a space group Fm 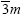 . Each Ce is surrounded by eight equivalent O2- ions and each O2- ion is surrounded by tetrahedron of four equivalent Ce ions. In this structure, each unit cell contains eight coordinate Ce4+ ions and four coordinate O2- ions [22].
. Each Ce is surrounded by eight equivalent O2- ions and each O2- ion is surrounded by tetrahedron of four equivalent Ce ions. In this structure, each unit cell contains eight coordinate Ce4+ ions and four coordinate O2- ions [22].
Conclusion
Ceria has both electronic and ionic conductivity by nature. Pure ceria shows high electronic conductivity than ionic conductivity [23,24]. Rare-earth doped ceria solid solutions have more oxygen conductivity than conventional YSZ because Ce4+ radius (0.97 Å) is greater than Zr+4 (0.72 Å). As a result oxygen ions easily migrate through Ce4+ in the operation temperature range 773-973 K, e.g. Gd3+ or Sm3+ can create the oxygen vacancies which will increase the ionic conductivity by hopping mechanism [24].
Advantages of Ceria Based Electrolytes
Ceria based electrolytes have higher ionic conductivity than YSZ at lower operating temperature, chemical inertness and thermal expansion match with high performing cathode materials such as LSCF and LSM. Finally it shows enhanced performance when used in composite electrodes.
Drawbacks of Ceria Based Electrolytes
In case of ceria-based electrolyte some of the ions of Ce4+ reduce to Ce3+ at high temperatures (above 700°C) and low oxygen partial pressures (P02), and these results in mixed ionic/electronic conductivity, which may lead to a decreased open-circuit voltage and internal short circuiting. Thus pure ceria has a serious problem in degradation in performance with time at elevated temperature [25].
References
- Skarmoutsos D, Nikolopoulos P, Tietz F, Vinke IC (2004) Physical characterization of Y0.25 Zr0.60 Ti0.15 O2-x and its performance as a Ni/Y0.25 Zr0.60 Ti0.15 O2-x anode cermet in an SOFC. Solid state ionics 170: 153-158.
- Martins RF, Brant MC, Domingues RZ, Paniago RM, Sapag K, et al. (2009) Synthesis and characterization of NiO-YSZ for SOFCs. Materials Research Bulletin 44: 451-456.
- Chebotin VN, Perfiliev MV, Electrochemistry of solid electrolytes (1978) Technical Information Center, US Department of Energy, Oak Ridge.
- Liu J, Madsen B, Barnett S (2002) Electrochem, Solid State Lett 5: 122-128.
- Wang Y (2015) Gum-like nanocomposites as interfacial energy materials for energy storage devices. Washington State University.
- Ralph JM, Kilner JA, Steele BCH (2001) Material Reserch Soc Symp Proc 575: 309.
- Stambouli AB, Traversa E (2002) Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renewable and sustainable energy reviews 6: 433-455.
- Minh NQ, Takahashi T (1995) Science and technology of ceramic fuel cells. Elsevier.
- Mehre H (2007) “DiffusioninSolidsâ€, Springer, NewYork, ISBN-13:9783540714866.
- Zhu B, Fan L, Lund P (2013) Breakthrough fuel cell technology using ceria-based multi-functional nanocomposites. Applied energy 106: 163-175.
- Shaula AL, Kharton VV, Marques FMB (2005) Oxygen ionic and electronic transport in apatite-type La10-x (Si, Al) 6O26±δ. Journal of Solid State Chemistry 178: 2050-2061.
- Rock NL (2009) Synthesis and characterization of novel electrocatalysts and supports for use in polymer electrolyte membrane fuel cells (Doctoral dissertation, Carnegie Mellon University)
- Ksapabutr B, Chalermkiti T, Wongkasemjit S (2013), Thin Solid Films 546-549.
- Ishihara T (2006) Development of new fast oxide ion conductor and application for intermediate temperature solid oxide fuel cells. Bulletin of the Chemical Society of Japan 79: 1155-1166.
- Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. Journal of computational physics 117: 1-19.
- Fuentes RO, Baker RT (2008) Synthesis and properties of Gadolinium-doped ceria solid solutions for IT-SOFC electrolytes. International Journal of Hydrogen Energy 33: 3480-3484.
- Inaba H, Tagawa H (1996) Ceria-based solid electrolytes. Solid state ionics 83: 1-16.
Citation: Koteswararao P, Suresh BM, Wani BN, Rao PVB (2017) Review on Different Components of Solid Oxide Fuel Cells. J Powder Metall Min 6: 181. DOI: 10.4172/2168-9806.1000181
Copyright: © 2017 Koteswararao P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 10584
- [From(publication date): 0-2017 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 9271
- PDF downloads: 1313