Short Communication Open Access
Short Communication on Phototoxicity Assessment: Evaluation of Skin Phototoxicity Study Using SD Rats by Transdermal and Oral Administration
Yutaka Yonezawa1,2*, Kazuto Hashimoto1, Hiroaki Nejishima1 and Haruko Ogawa3
1Pharmacokinetics and Safety Department Drug Research Center Kaken Pharmaceutical Co., Ltd, Gensuke, Fujieda, Shizuoka 426-8646, Japan
2United Graduate School of Veterinary Sciences, Gifu University, 1-1 Yanagido, Gifu, Gifu 501-1193, Japan
3Diagnostic Center for Animal Health and Food Safety, Obihiro University of Agriculture and Veterinary Medicine, 2-11 Inada, Obihiro, Hokkaido 080 8555, Japan
- *Corresponding Author:
- Yutaka Yonezawa
Pharmacokinetics and Safety Department Drug Research Center
Kaken Pharmaceutical Co., Ltd. 301, Gensuke, Fujieda
Shizuoka 426-8646, Japan
Tel: +81 54 635 2939
Fax: +81 54 635 8940
E-mail: yonezawa_yutaka@kaken.co.jp
Received date: August 27, 2016; Accepted date: September 23, 2016; Published date: September 27, 2016
Citation: Yonezawa Y, Hashimoto K, Nejishima H, Ogawa H (2016) Short Communication on Phototoxicity Assessment: Evaluation of Skin Phototoxicity Study Using SD Rats by Transdermal and Oral Administration. Toxicol Open Access 2:118. doi:10.4172/2476-2067.1000118
Copyright: © 2016 Yonezawa Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
Guinea pigs are the most frequently used animals in phototoxicity studies. However, general toxicity studies most often use Sprague-Dawley (SD) rats. To shortening of the study period and to reduce the number of animals needed for drug development, we examined whether skin phototoxicity studies could be performed using SD rats. Drugs that had previously been shown to have phototoxic potential and known phototoxic compounds were administered transdermally and orally to guinea pigs and SD rats. After administration, the animals were irradiated with UV-A and UV-B. In the result, the concordance rate of guinea pigs and SD rats was 100% in the transdermal administration study and 85% in the oral administration study. This study demonstrated that phototoxicity studies using SD rats have the same potential to detect phototoxic compounds as studies using guinea pigs.
Keywords
Phototoxicity; Guinea pig; Sprague-Dawley rats
Introduction
Phototoxicity is caused by exposure to a combination of a photoreactive chemical and light. It is an acute response and can result in the formation of erythema and edema. A compound that absorbs photons at any wavelength between 290 and 700 nm has the potential to be photoreactive, and therefore, can potentially cause phototoxicity [1].
In the drug development, the phototoxicity of drugs is assessed as part of standard safety screening, and typically, guinea pigs are used as an in vivo model [2]. The guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) [3] indicate that several animal species—including guinea pigs, mice, and rats—have been used to assess drug phototoxicity, but no standardized study design has been established. The guidelines also note that phototoxicity studies must involve the collection of pharmacokinetic (PK) data because for the data to be relevant, the animals should be irradiated at approximately Tmax. In the early stage of drug development, PK data is commonly collected using mice and rats. However, if the phototoxicity study is to be carried out using guinea pigs, additional PK must be collected for guinea pigs in another trial. On the other hand, Sprague-Dawley (SD) rats are widely used in general toxicity study and PK data of SD rats is usually collected before phototoxicity assessment. Therefore it removes the need for additional PK study.
To shortening of the study period and to reduce the number of animal studies needed, we investigated the suitability of SD rats for use in phototoxicity studies [4]. In this study, drugs that had previously been shown to have phototoxic potential based on reactive oxygen species (ROS) assays [5-8] and known phototoxic compounds (8- Methoxypsoralen (8-MOP), acridin, anthracene) were administered transdermally and orally to guinea pigs and SD rats. After administration, the animals were irradiated with UV-A and UV-B. Skin reactions (erythema/eschar and edema formation) were observed and scored 24, 48, and 72 h after UV irradiation using Draize’s method [9]. The skin reaction scores of individual animals were summed for each site and the mean score was calculated according to the following equation:
Mean score = Total of erythema and edema scores / Number of animals tested.
A test compound was judged to be phototoxic if the mean score of the UV-irradiated group or area was higher than that of the nonirradiated group or area at any observation period.
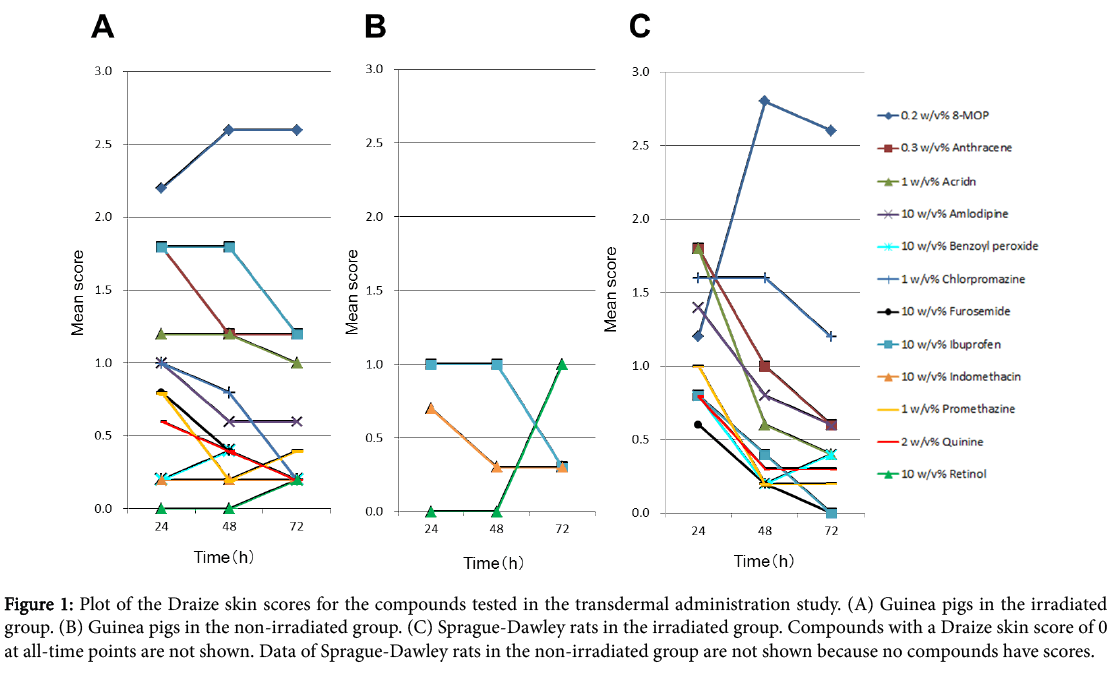
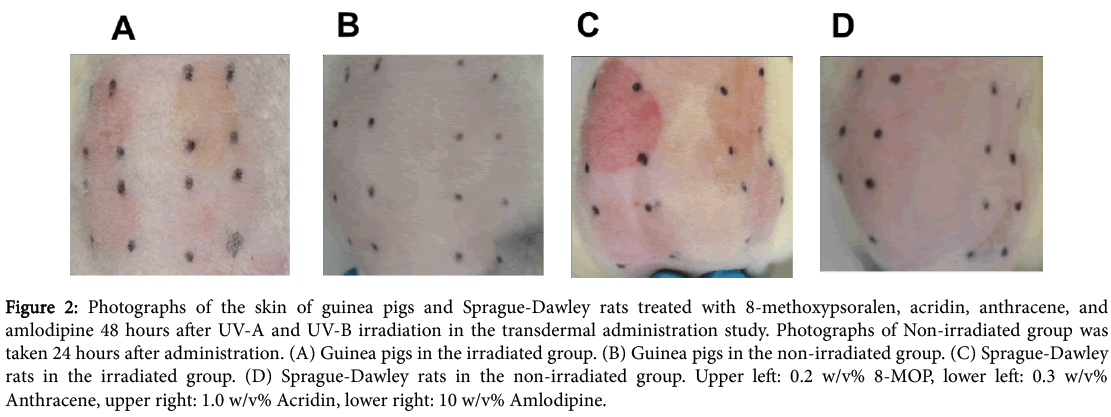
The skin scores recorded in the transdermal administration study are shown in Figure 1. Representative photographs of irradiated skin that had been treated with 8-MOP, acridin, anthracene, or amlodipine are shown in Figure 2. Three positive controls (8-MOP, acridine, and anthracene) were found to be phototoxic in both guinea pigs and SD rats. Aside from the positive controls, seven compounds (amlodipine, benzoyl peroxide, chlorpromazine, furosemide, ibuprofen, promethazine, and quinine) were found to be phototoxic in both guinea pigs and SD rats. A summary of the transdermal administration study is shown in Table 1. The concordance rate of guinea pigs and SD rats was 100%.
| Compounds | Judgement | References | ||
| Guinea pigs | SD rats | ROS assay | Clinical reports | |
| Phototoxic compounds | ||||
| 8-MOP | Phototoxic | Phototoxic | Phototoxic1) | Phototoxic 2) |
| Anthracene | Phototoxic | Phototoxic | N.D. | Phototoxic 3) |
| Acridn | Phototoxic | Phototoxic | N.D. | Phototoxic 3) |
| Drugs | ||||
| Amlodipine | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Benzoyl peroxide | Phototoxic | Phototoxic | Phototoxic1) | Phototoxic5) |
| Bufexamac | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Chlorpromazine | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Diclofenac | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Furosemide | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Haloperidol | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Ibuprofen | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Indomethacin | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Ketoprofen | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Naproxen | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Nifedipine | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Nimodipine | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Nitrendipine | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Piroxicam | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Promethazine | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Quinine | Phototoxic | Phototoxic | Phototoxic1) | N.D.4) |
| Retinol | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Tamoxifen | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.4) |
| Concordance rate* | 100%ï¼Â?22/22 compoundsï¼Â? | |||
1) Data from Onoue et al., 2008 [6]; 2) Adverse drug reaction reporting, noted in the drug package insert; 3) Noted in the material safety data sheet; 4) No data with transdermal administration; 5) Data from Jeanmougin and Civatte, 1987 [12]. *Concordance rate was calculated according to the following formula: the number of compounds judged phototoxic or non-phototoxic in both guinea pigs and SD rats/total number of compounds × 100.
Table 1: Summary of the transdermal administration study.
Figure 1: Plot of the Draize skin scores for the compounds tested in the transdermal administration study. (A) Guinea pigs in the irradiated group. (B) Guinea pigs in the non-irradiated group. (C) Sprague-Dawley rats in the irradiated group. Compounds with a Draize skin score of 0 at all-time points are not shown. Data of Sprague-Dawley rats in the non-irradiated group are not shown because no compounds have scores. Figure
Figure 2: Photographs of the skin of guinea pigs and Sprague-Dawley rats treated with 8-methoxypsoralen, acridin, anthracene, and amlodipine 48 hours after UV-A and UV-B irradiation in the transdermal administration study. Photographs of Non-irradiated group was taken 24 hours after administration. (A) Guinea pigs in the irradiated group. (B) Guinea pigs in the non-irradiated group. (C) Sprague-Dawley rats in the irradiated group. (D) Sprague-Dawley rats in the non-irradiated group. Upper left: 0.2 w/v% 8-MOP, lower left: 0.3 w/v% Anthracene, upper right: 1.0 w/v% Acridin, lower right: 10 w/v% Amlodipine.
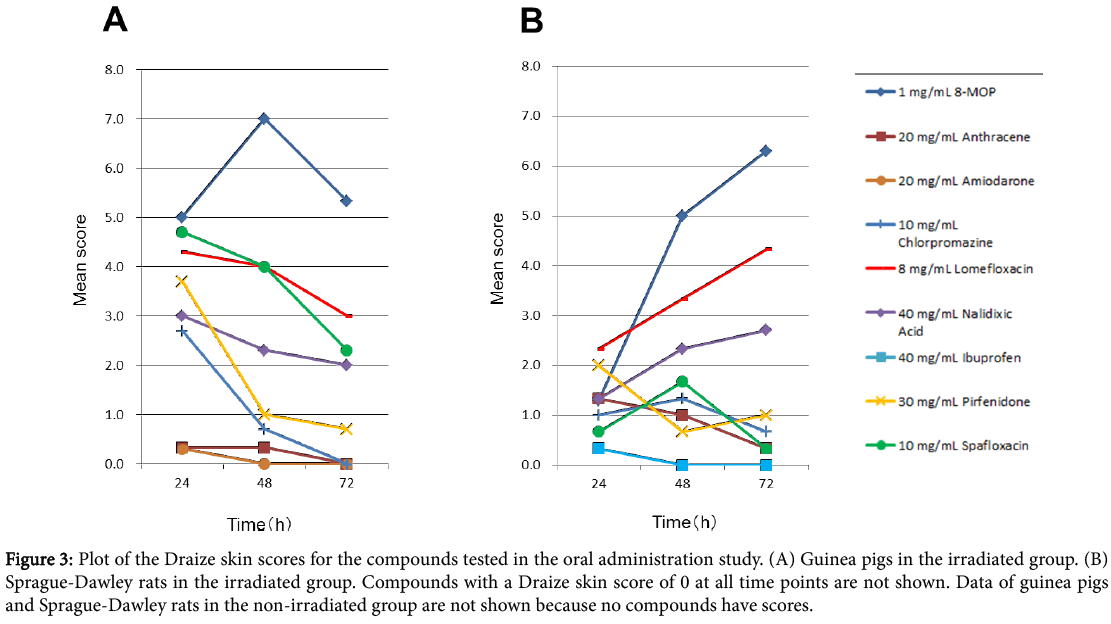
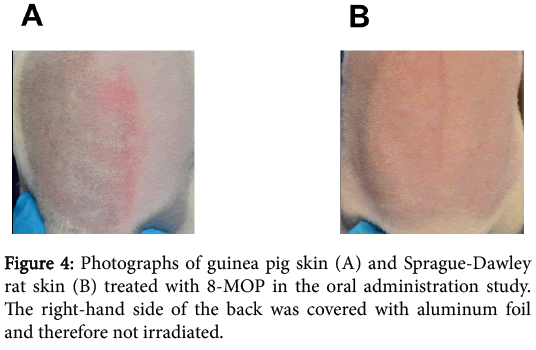
The skin scores recorded in the oral administration study are shown in Figure 3. Representative photographs of irradiated skin of animals treated with 8-MOP is shown in Figure 4.
Figure 3: Plot of the Draize skin scores for the compounds tested in the oral administration study. (A) Guinea pigs in the irradiated group. (B) Sprague-Dawley rats in the irradiated group. Compounds with a Draize skin score of 0 at all time points are not shown. Data of guinea pigs and Sprague-Dawley rats in the non-irradiated group are not shown because no compounds have scores.
Two positive controls (8-MOP and anthracene) were found to be phototoxic in both guinea pigs and SD rats. Apart from the positive controls, chlorpromazine, lomefloxacin, nalidixic acid, pirfenidone, and sparfloxacin were found to be phototoxic in both types of animals. Amiodarone was only phototoxic in guinea pigs, and ibuprofen was only phototoxic in SD rats.
A summary of the oral administration study is shown in Table 2. The concordance rate of guinea pigs and SD rats was 85%.
| Compounds | Judgement | References | ||
| Guinea pigs | SD rats | ROS assay | Clinical reports | |
| Phototoxic compounds | ||||
| 8-MOP | Phototoxic | Phototoxic | Phototoxic1) | Phototoxic 5) |
| Anthracene | Phototoxic | Phototoxic | N.D. | Phototoxic 6) |
| Drugs | ||||
| Amiodarone | Phototoxic | Non-phototoxic | Phototoxic2) | Phototoxic 5) |
| Benzoyl Peroxide | Non-phototoxic | Non-phototoxic | Phototoxic1) | N.D.7) |
| Chlorpromazine | Phototoxic | Phototoxic | Phototoxic1) | Phototoxic5) |
| Furosemide | Non-phototoxic | Non-phototoxic | Phototoxic1) | Phototoxic5) |
| Gatifloxacin | Non-phototoxic | Non-phototoxic | Weak phototoxic3) | Non-phototoxic |
| Ibuprofen | Non-phototoxic | Phototoxic | Phototoxic1) | Phototoxic5) |
| Ketoprofen | Non-phototoxic | Non-phototoxic | Phototoxic1) | Photoallergic5) |
| Lomefloxacin | Phototoxic | Phototoxic | Phototoxic3) | Phototoxic5) |
| Nalidixic Acid | Phototoxic | Phototoxic | Phototoxic1) | Phototoxic5) |
| Pirfenidone | Phototoxic | Phototoxic | Phototoxic4) | Phototoxic5) |
| Spafloxacin | Phototoxic | Phototoxic | Phototoxic3) | Phototoxic5) |
| Concordance rate* | 85%ï¼Â?11/13 compoundsï¼Â? | |||
1) Data from Onoue et al., 2008 [6]; 2) Data from Onoue and Tsuda, 2006 [5]; 3) Data from Seto et al., 2011 [7]; 4) Data from Seto et al., 2013 [8]; 5) Adverse drug reaction reporting, noted in the drug package insert; 6)Noted in the material safety data sheet; 7) No data with oral administration. *Concordance rate was calculated according to the following formula: the number of compounds judged phototoxic or non-phototoxic in both guinea pigs and SD rats/total number of compounds × 100.
Table 2: Summary of the oral administration study.
The present study aimed to examine whether skin phototoxicity studies could be performed using SD rats instead of guinea pigs. The results confirm that phototoxicity studies using SD rats are as sensitive and specific as studies using guinea pigs.
The use of SD rats in phototoxicity studies contributes to shortening of the study period as well as 3R principles in animal experiments because it removes the need for additional PK study. Moreover, SD rats are widely used in general toxicity study, so it may be possible to incorporate phototoxicity assessments to general toxicity study. Incorporating a study into the general toxicity study is recommended by the ICH guidelines [10-12]. In a general toxicity study using SD rats, a satellite group for toxicokinetics might be used for the phototoxicity study after final blood sampling is completed. If this study can be validated, the number of animals used for the phototoxicity study can be reduced. In conclusion, the use of SD rats in phototoxicity testing is in line with the 3R principles and can speed up drug development.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
References
- Diffey B L, Daymond T J, Fairgreaves H (1983) Phototoxic reactions to piroxicam, naproxen and tiaprofenic acid. Rheumatology 22: 239-242.
- Morikawa F, Nakayama Y, Fukuda M, Hamano M, Yokoyama Y, et al. (1974) Techniques for evaluation of phototoxicity and photoallergy in laboratory animals and man. Sunlight and Man 529-557.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2013). Photosafety Evaluation of Pharmaceuticals S10.
- Yonezawa Y, Ohsumi T, Miyashita T, Kataoka A, Hashimoto K, et al. (2015) Evaluation of skin phototoxicity study using SD rats by transdermal and oral administration. The Journal of toxicological sciences 40: 667-683.
- Onoue S, Tsuda Y (2006) Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharmaceutical research23: 156-164.
- Onoue S, Igarashi N, Yamada S, Tsuda Y (2008) High-throughput reactive oxygen species (ROS) assay: an enabling technology for screening the phototoxic potential of pharmaceutical substances. Journal of pharmaceutical and biomedical analysis 46: 187-193.
- Seto Y, Inoue R, Ochi M, Gandy G, Yamada S, et al. (2011) Combined use of in vitro phototoxic assessments and cassette dosing pharmacokinetic study for phototoxicity characterization of fluoroquinolones. The AAPS journal 13: 482-492.
- Seto Y, Inoue R, Kato M, Yamada S, Onoue S (2013) Photosafety assessments on pirfenidone: photochemical, photbiological, and pharmacokinetic characterization. Journal of Photochemistry and Photobiology B: Biology 120: 44-51.
- Draize JH (1959) Dermal toxicity. In Appraisal of the safety of chemicals in foods, drugs and cosmetics. The Association of Food and Drug Officials of the United States, Texas State Department of Health, Austin 46-59.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2008). Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2 (R1).
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2009) Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals M3 (R2). In International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use.
- Jeanmougin M, Civatte J (1987) Prediction of benzoyl peroxide phototoxicity by photoepidermotests after repeated applications. Preventive value of a UVB filter. Archives of dermatological research 280: S90-3.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 13870
- [From(publication date):
October-2016 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 12869
- PDF downloads : 1001