Tannic Acid Selectively Extracting Titanium from Ilmenite: Experimental and Theoretical Investigation
Received: 15-Oct-2017 / Accepted Date: 20-Oct-2017 / Published Date: 27-Oct-2017 DOI: 10.4172/2168-9806.1000183
Abstract
A novel route of extracting titanium using tannic acid from ilmenite was investigated. The strong affinity of tannic acid towards metal ions having high oxidation states was explored to extract titanium selectively from a multi-element solution prepared by dissolving ilmenite in sulphuric acid and ammonium sulphate. Titanium-tannic acid complex was selectively precipitated from the multi-element solution at pH 4.5. The precipitate was separated by vacuum filtration and the filter cake was washed with 2% hydrochloric acid solution, deionized water and isopropyl alcohol after filtration. The washed filter cake was dried at room temperature and ground into powder on drying. The titaniumtannic acid complex so obtained was composed of 98.6% of titanium, 0.2% of iron and 1.2% of other impurities. The density functional theory simulation at B3LYP method with a basis of 6-31G was also conducted to verify optimized structure and its stability. The result showed energy gap of 2.43 eV between two frontier orbitals (highest occupied molecular orbitals and lowest unoccupied molecular orbitals), which is large enough to indicate energetic feasibility of the complex formation.
Keywords: Tannic acid; Titanium; Ilmenite; Digallic acid; Density functional theory simulation
Introduction
The merits of a metal production process have to be judged not only by the metallurgical quality of the product obtained but also by its impact on environmental and health. It has been reported that abnormalities in pulmonary function and pleural disease among people associated with titanium manufacturing can be correlated to exposure to titanium tetrachloride, titanium dioxide and duration of work in titanium production [1]. At the same time, titanium and its alloys have remarkable stability at high temperature, corrosion resistance and high strength to weight ratio [2]. This creates a demand for titanium and its alloys in a variety of applications including aerospace [3], biomedical [4] and nuclear waste storage [5].
In general, the extractions of titanium dioxide (TiO2) and titania pigment are performed using mainly two routes, sulfate and chloride processes. In the sulfate process, low grade ilmenite (FeTiO3) can be used as a feed stock which is treated by concentrated sulfuric acid (H2SO4). In the next step, impurities are precipitated by cooling down the sulfuric acid solution and titanium dioxide can be obtained by performing hydrolysis on titanyl sulfate (TiOSO4) at 95-110°C and calcination at 1,000°C [6]. On the other hand, chloride process requires a high grade feed stock such as rutile (TiO2). The oxygen in the feed material rutile is removed by reducing agent, usually carbon, and titanium tetrachloride (TiCl4) is synthesized using chlorine gas. After that, pure titanium dioxide can be obtained by oxidizing titanium tetrachloride at 1200-1700°C [7]. However, greenhouse gases are generated in this process and an upgraded starting material is required, which increases the cost of production [8]. So alternate routes are still required to produce low cost environmentally friendly titanium from its naturally occurring ores such as ilmenite, by reducing prior treatments.
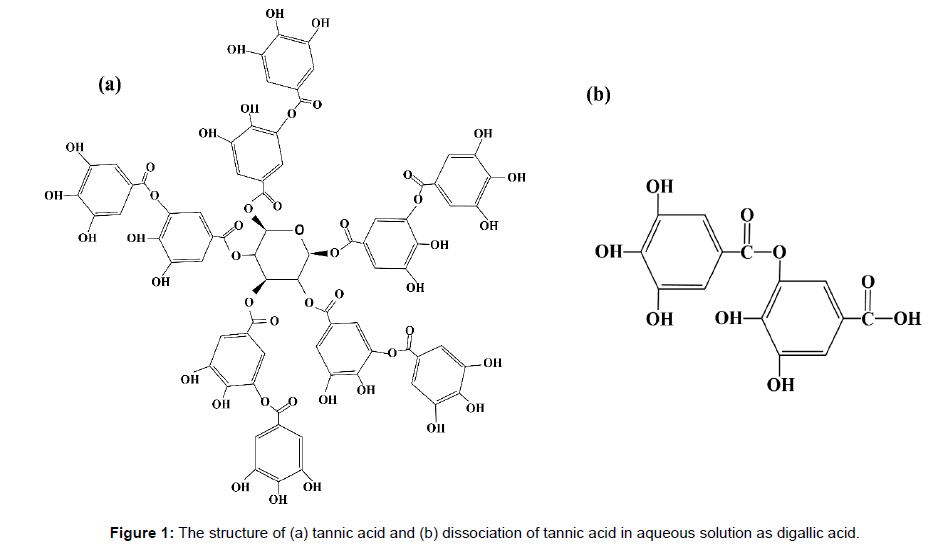
In this study, we investigate the ability of tannic acid to selectively chelate with titanium ions from a multi-element solution containing iron, vanadium, silicon, aluminium and other related ions, prepared by dissolving a complex ore such as ilmenite. It has been reported that hydroxyl-aromatic compounds can selectively complex with titanium compounds at pH 4-5 [9-11]. Tannic acid, being a compound of such category and possess carboxylic acids expected to show a strong affinity towards metal ions having high oxidation states [9,12]. Also, tannic acid in water is hydrolysed to digallic acid and glucose [13]. Figure 1 shows the molecular structure of tannic and digallic acids. By adjusting a pH of the solution between 4 and 5 during precipitation, titanium-tannic acid complex can be precipitated selectively from a multi-element solution, containing impurities such as iron, magnesium, vanadium, and manganese as ions. The titanium-tannic acid complex so obtained has a strong potential to be used for titanium production following a scheme very similar to that developed by Fang et al. [14]. Metallic titanium can be produced by dehydrogenation of titanium hydride (TiH2), obtained by the reduction of titanium-tannic acid complex in the presence of hydrogen after the purification process of remaining impurities [15]. Moreover, tannic acid is commonly found in beverages and food stuffs and plant materials [16]. Using such an eco-friendly material to separate titanium can minimize the environmental and health concerns associated with current industrial titanium production process. To get a deeper scientific insight of the complication and selectivity for titanium chelation, density functional theory (DFT) simulations have been performed on the complex besides the exciting experimental result.
Materials and Methods
Preparation of titanium solution from ilmenite
16 M sulphuric acid was chosen as a lixiviant to prepare a titanium containing multi-element solution from ilmenite. 10 g of ilmenite was added into 50 ml of sulphuric acid in a 500 ml round-bottom boiling flask. 20 g of ammonium sulphate ((NH4)2SO4) was also added to elevate the boiling point of the sulphuric acid solution during leaching. A 200 ml Graham condenser which has an inner coil for additional surface area was applied on the neck of the flask to maintain the volume. The solution was refluxed and the leaching was performed for 8 hours. A magnetic stirring bar at 1,000 RPM was used for homogenizing the solution. After the digestion process, the solution is allowed to settle. A dark brownish multi-element sulphate solution was obtained as supernatant liquid was collected for chelation.
Synthesis of titanium-tannic acid complex
50 g of tannic acid in 200 ml water was taken in a 500 ml three neck flask, constantly stirred by a magnetic stirring bar at a rotational speed of 500 RPM. A 200 ml separator funnel fitted with a stopcock, containing titanium bearing solution, was installed on one of necks of the flask. A pH probe was also inserted through another neck and the third neck was sealed. A formate buffer solution was added from the separatory funnel so that the pH was maintained at 4.5 during the precipitation of the dark orange-colored precipitate. The experiment was carried out at room temperature. The precipitate was collected by a vacuum filtration, washed with 2% hydrochloric acid solution, followed by cold deionized water and isopropyl alcohol, dried at room temperature, and ground into powder. The filtrate was investigated by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICPOES). The solid samples were characterized using Scanning Electron Microscopy (SEM), Energy-dispersive X-ray Spectroscopy (EDS) and Fourier Transform Infrared Spectroscopy (FTIR).
Methodology of DFT simulation
DFT simulation using general ab initio quantum chemistry package, Gaussian 09, was carried out to determine optimized geometry of titanium-tannic acid complex and the stability based on highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). B3LYP [17,18] for the exchange-correlation energy functional calculation was employed with a basis of 6-31G, which has been known as a most effective method for the simulation of organic molecules [19]. Also, the optimization was conducted without symmetry constraints.
Results and Discussion
Experiment result
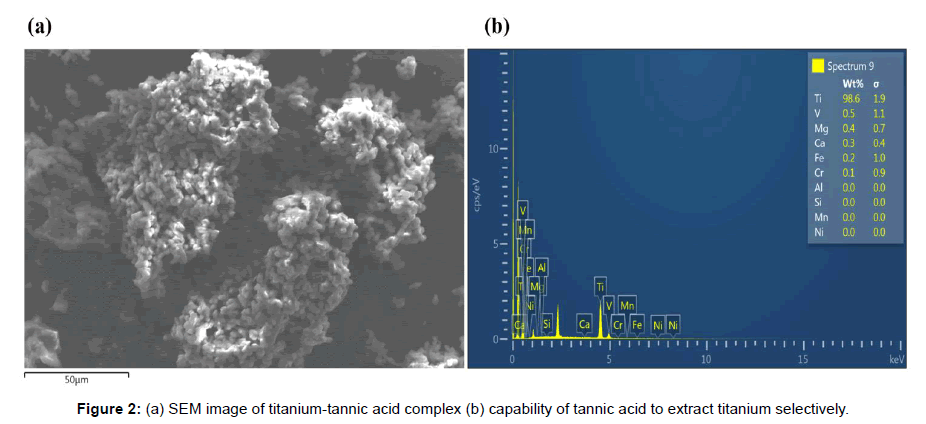
The multi-element solution prepared by dissolving ilmenite in concentrated sulphuric acid was composed of 22.64% (w/w) of titanium, 71.28% (w/w) of iron, and 6.08% (w/w) of other impurities determined by ICP-OES. After precipitating out titanium-tannic acid complex with tannic acid containing ammonium hydroxide at a pH of 4.5, the yield of titanium was found to be 95.58% (w/w). Figure 2 shows the surface morphology and Table 1 exhibits the chemical composition of titanium-tannic acid as analysed by SEM and EDS, respectively. The particle shape looks irregular and it forms agglomerate. The sizes of individual particle are only few micron meters. The powder has 98.6% (w/w) of titanium, 0.2% (w/w) of iron, and 1.2% (w/w) of other impurities. When it is considered that iron is a difficult impurity to be removed in titanium production, Figure 2b shows a remarkable capability of tannic acid to extract titanium selectively.
| Elements | Ti | V | Mg | Ca | Fe | Cr |
|---|---|---|---|---|---|---|
| Wt (%) | 98.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 |
Table 1: The composition of titanium-tannic acid complex analyzed by EDS.
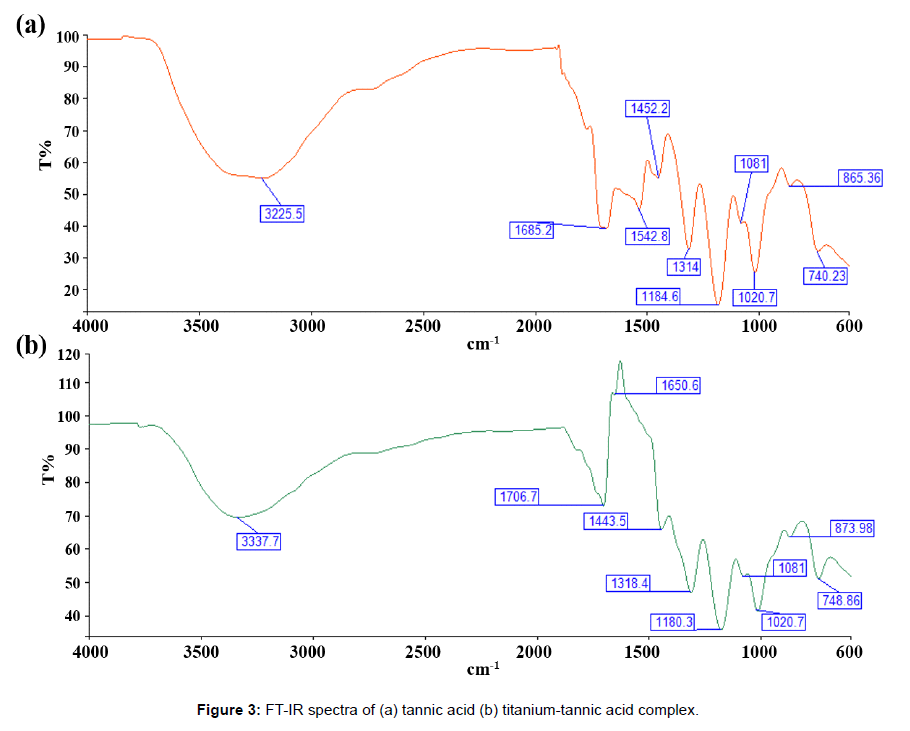
Figure 3 shows IR spectra of titanium and titanium-tannic acid complex. The IR spectra present peaks at a broad range of 3500~3200 cm-1 due to O-H stretching vibration in both tannic acid and titaniumtannic acid complex [20]. However, the positive shift and decreased intensity of the peak for O-H bond in titanium tannic acid complex were observed due primarily to coordination of phenolic –OH group with titanium ion by means of deprotonation [10,21]. This may suggest that tannic acid tends to form complexes with titanium using hydroxyl groups in benzene rings. Several peaks below 1800 cm-1 are due to tannic acid ligands including benzene rings and carboxylic acids. C=O stretching vibration at 1685 and 1706 cm-1 for tannic acid and titanium-tannic acid complex was observed [20]. The peaks 1500~1400 cm-1 account for C-C stretching vibration in aromatic rings. Significant changes in spectra profile of titanium-tannic acid complex at a range of 1700~1450 cm-1 are due to the chelation process for the formation of metal complex. The peaks due to C-O stretching vibration and O-H bending vibration in benzene rings and carboxylic acids appear at 1300~1000 cm-1 in both Figure 3a and 3b [20]. The peaks below 900 cm-1 can be explained by C-H bonding in benzene rings. It is worthwhile to be noted that peaks below 1452 cm-1 in tannic acid match with peaks in titanium-tannic acid complex, which may mean that there exists no significant participation of carboxylic group in coordination with titanium ion.
Simulation
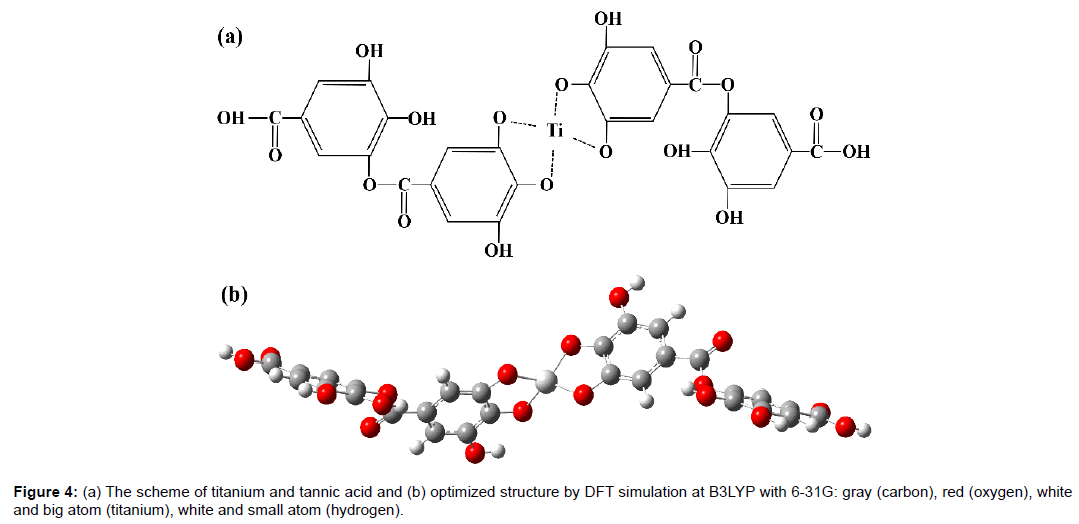
Based on the fact that titanium ion forms an ionic bonding with two –OH groups in a gallic acid [11], Figure 4 shows a probable schematic titanium-tannic acid complex and optimized geometry determined by DFT simulation. The geometry was fully stabilized.
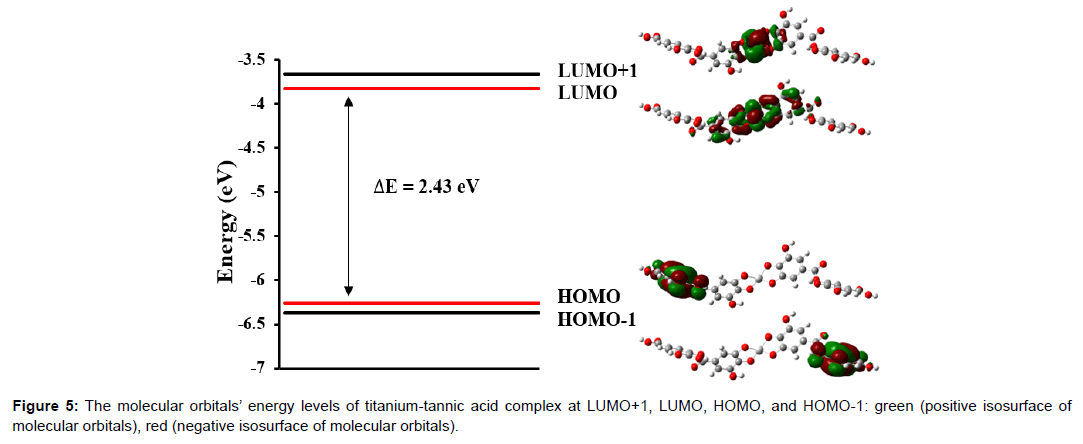
A LUMO-HOMO gap is known as the important parameter that can describe the degree of stability of the complexes [22]. A molecule having a larger LUMO-MOMO gap is more stable than a molecule having a smaller LUMO-HOMO gap. Also, according to a concept of hard and soft Lewis acids and bases (HSAB), the global hardness of a molecule is the representative stability index of complexes [23]. A high value of the global harness implies a highly stable complex. In the DFT simulation, The LUMO-HOMO gap was calculated to be 0.08934 a.u (2.43 eV) as shown in Figure 5 and the global hardness (h) was found to be 0.04467 a.u based on the eqn. (1).
h=(ELUMO-EHOMO)/2 (1)
Where h is the global hardness, the energy of the lowest unoccupied molecular orbitals and the energy of the highest occupied molecular orbitals. The LUMO-HOMO gap and the global hardness of titaniumtannic acid complex are large enough to indicate the energetic feasibility and stability for the formation of titanium-tannic acid complex.
Also, it is interesting to note that electron density mainly consists of carbon and oxygen from a conjugated gallic acid unit located at the end of the molecule in both HOMO and HOMO-1 levels in which there is no contribution from titanium atom. On the other hand, in LUMO level, molecular orbitals are uniformly distributed around titanium atom and adjacent gallic acid group, which has emptyorbitals of benzene rings. It is also observed in LUMO+1 level that the distribution of molecular orbitals is somewhat similar to that in LUMO level, but there are a more significant localization in titanium atom and less contribution from digallic acid group. This can be explained by the relative energetic alignment between molecular orbitals of titanium and gallic acid group. Titanium atom in LUMO is lower in energy than digallic acid groups. Thus, titanium in titanium-tannic acid complex has a possibility of forming titanium hydride in a hydrogen atmosphere as titanium atom can easily accept excited electrons.
Conclusion
The investigation of ability of tannic acid to extract titanium selectively from a multi-element bearing solution prepared by digesting ilmenite was performed through experiment and density functional theory simulation.
(1) Preparation of titanium-tannic acid complex coupled with careful control of pH of the solution was successfully performed and the complex showed a purity of 98.6%.
(2) A LUMO-HOMO gap of titanium-tannic acid complex was calculated to be 2.43 eV.
Acknowledgments
The authors hope to thank ARPA-E of the US Department of Energy for financial support of the Direct Reduction of Titanium Slag (DRTS) project (Grant # 55800707).
References
- Garabrant DH, Fine LJ, Oliver C, Bernstein L, Peters JM (1987) Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand J Work Environ Health 13: 47-51.
- Amarchand S, Rama Mohan TR, Ramakrishnan P (2000) A novel chemical solution technique for the preparation of Nano size titanium powders from titanium dioxide. Adv Powder Technol 11: 415-422.
- Boyer RR (1996) An overview on the use of titanium in the aerospace industry. Mater Sci Eng 213: 103-114.
- Niinomi M (2003) Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci Technol Adv Mater 4: 445-454.
- Hua F, Mon K, Pasupathi P, Gordon G, Shoesmith D (2005) A review of corrosion of titanium grade 7 and other titanium alloys in nuclear waste repository environments. Corrosion 61: 987-1003.
- Büchel KH, Moretto HH, Werner D, Woditsch P (2000) Industrial Inorganic Chemitry (2ndedn) Wiley-VCH, Mörlenbach.
- Froes FH (2015) Titanium: Physical Metallurgy, Processing, and Applications. Proceedings of ASM international.
- Middlemas S, Fang ZZ, Fan P (2013) A new method for production of titanium dioxide pigment. Hydrometallurgy 131: 107-113.
- Iffat AT, Maqsood ZT, Fatima N (2005) Study of complex formation of Fe(III) with tannic acid. J Chem Soc Pak 27: 174-178.
- Surleva A, Atanasova P, Kolusheva T, Costadinnova L (2014) Study of complex equilibrium between titanium (IV) and tannic acid. J Chem Technol Metall 49: 594-600.
- Araujo PZ, Morando PJ, Blesa MA (2005) Interaction of catechol and gallic acid with titanium dioxide in aqueous suspensions. Equilibrium Studies Langmuir 21: 3470-3474.
- Borgais BA, Cooper SR, Koh YB, Raymond KN (1984) Synthetic, structural and physical studies of titanium complexes of catechol and 3,5-di-tert-butylcatechol. Inorg Chem 23: 1009-1016.
- Hem JD (1962) Complexes of ferrous iron with tannic acid. In: Nolan TB (ed.) Chemistry of Iron in Natural Water. United States Government Printing office, Washington, pp: 75-94.
- Fang ZZ, Middlemas S, Fan P, Guo J (2013) A novel chemical pathway for producing low cost Ti by direct reduction of Ti-slag. J Am Chem Soc 135: 18248-18251.
- Cho J, Roy S, Sathyapalan A, Free M, Fang ZZ (2016) Purification of reduced upgraded titania slag by iron removal using mild acid. Hydrometallurgy 161: 7-13.
- Finar IL (1975) Organic Chemistry (4thedn), English Language Book Society & Longman Group Limited, London.
- Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98: 5648-5652.
- Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev 37: 785-789.
- Tirado-Rives J, Jorgensen WL (2008) Performance of B3LYP density functional methods for a large set of organic molecules. J Chem Theory Comput 4: 297-306.
- Larkin PJ (2011) IR and Raman Spectroscopy: Principles and Spectral Interpretation. Elsevier Amsterdam.
- Peres RS, Cassel E, Azambuja DS (2012) Blackwattle tannin as steel corrosion inhibitor, ISRN Corrosion 9-19.
- Zhou Z, Parr RG (1990) Activation hardness: new index for describing the orientation of electrophilic aromatic substitution. J Am Chem Soc 112: 5720-5724.
- Pearson RG (1963) Hard and soft acid and bases. J Am Chem Soc 85: 3533-3539.
Citation: Cho J, Roy S, Sathyapalan A, Free LM, Fang ZZ (2017) Tannic Acid Selectively Extracting Titanium from Ilmenite: Experimental and Theoretical Investigation. J Powder Metall Min 6: 183. DOI: 10.4172/2168-9806.1000183
Copyright: © 2017 Cho J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9245
- [From(publication date): 0-2017 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 8003
- PDF downloads: 1242