Research Article Open Access
The Development, Evaluation and In Vitro Release Study of the Terbinafine Transdermal Patch
Vikas G Rajurkar1*, Sambhaji Zarekar1, Vilas B Ghawate1 and Inayat B Pathan2
1Department of Quality Assurance, MES’s College of Pharmacy, Affiliated to Savitribai Phule Pune University, Sonai, Ahmednagar District, Maharashtra, India
2Department of Quality Assurance, Government College of Pharmacy, Aurangabad, Maharashtra, India
- *Corresponding Author:
- Rajurkar VG, Professor
Department of Quality Assurance
MES’s College of Pharmacy, Sonai-414 105
Taluka - Newasa, District - Ahmednagar
Maharashtra, India
Tel: +919860482926
E-mail: vikas_rajurkar_1973@yahoo.co.in
Received date: October 01, 2015; Accepted date: October 23, 2015; Published date: October 30, 2015
Citation: Rajurkar VG, Zarekar S, Ghawate VB, Pathan IB (2015) The Development Evaluation and In Vitro Release Study of the Terbinafine Transdermal Patch. Ind Chem Open Access 3:107. doi: 10.4172/2469-9764.1000107
Copyright: © 2015 Rajurkar VG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
Transdermal drug delivery is an alternative route for systemic drug delivery, which minimizes the absorption and increase the bioavailability. Orally Terbinafine undergoes extensive metabolism and frequent high doses are required to maintain the therapeutic level as a result, dose development toxic effect. The purpose of this research work was to formulation and evaluation of transdermal drug delivery system of Terbinafine using various polymers such as HPMC E25, Eudragit-RS100 and PVP K25 with different proportions by solvent evaporation technique. The Fourier transform infrared study revealed no physical or chemical interactions between Terbinafine and excipients. The prepared formulations were evaluated for different physicochemical characteristics such as thickness, folding endurance, drug content, percentage moisture absorption, and percentage moisture loss. The diffusion studies were performed by using modified Franz diffusion cells. The result of dissolution studies shows that formulation, SA12 showed maximum release of 92.56% in 06 h, whereas SA22 showed minimum release of 45.89% in 06 h. Based on the drug release and physicochemical values obtained the formulation SA 12 is considered as an optimized formulation, which shows higher percentage of drug release.
Keywords
Terbinafine; Eudragit-RS 100; PVP K25; Transdermal patch; Solvent evaporation technique
Introduction
The potential of intact skin as the route of drug administration has been known for years; the inspiration for using skin for the delivery of drug is obtained from ancient times [1]. Utilization of skin as a route for delivering drugs as has been an alternative to conventional methods including injections and tablets. Advantages of transdermal drug delivery include the avoidance of pain, hepatic first-pass metabolism, sustain drug release, easy use and withdrawal in case of side effects. However, the major limitation for transdermal drug delivery system (TDDS) is that the skin has the outmost layer of the epidermis; the stratum corneum (SC) provides an outstanding barrier towards the absorption of substances [2]. Oral delivery of complex molecules such as peptides and proteins has been hampered by the degradation in the gastrointestinal tract. As a result, various types of particulate systems such as biodegradable microspheres and liposomes have been proposed as potential delivery vehicles to protect these drugs in the gastrointestinal tract. Unfortunately, these particulates generally display low oral absorption efficiencies [3]. Millions of unsafe injections are delivered in developing countries, and the transmission of certain blood borne pathogens via this route is thought to be a major public health problem [4]. Several technological advances developed to overcome this challenge. These advances can be broadly divided into two categories; physical and chemical methods. Physical methods employed for increasing transport of molecules across the skin uses mechanical, electrical, magnetic or thermal energy source to promote transport of macromolecules by disrupting the skin membrane. Examples of physical approaches include the use of micro needle array, ballistic liquid jet, high velocity particles, ultrasound, electric current, abrasion, ablation, lasers, pressure waves, radiofrequency thermal ablation, magnetophoresis of diamagnetic solutes and thermophoresis [5].
To overcome the barrier properties of the skin for drugs is an incorporation of suitable vehicles. Substances that promote the penetration of topically applied drugs through stratum corneum and epidermis are commonly referred to as skin permeation enhancers, accelerants, adjuvant, or sorption promoters [6]. Dimethylsulfoxide (DMSO) is widely studied penetration enhancers in many areas of pharmaceutical sciences as a “universal solvent”. DMSO denatures the intercellular structural protein of the stratum corneum [7]. Over the last 2-3 decades, the skin has become an important route for the delivery of drugs for topical, regional, or systemic action. The skin has evolved as a physical and biochemical protective barrier, prevents the loss of water from the body, and guards against entry into the body of external toxic chemicals and infectious agents. The stratum corneum, which is the outermost layer of the skin and comprised of keratin-rich cells embedded in multiple lipid bilayers, has been considered the ratelimiting structure governing percutaneous absorption of many kinds of permeants [8].
Terbinafine is a well-established antifungal agent, widely used in fungal infections, its chemically [(2E)-6, 6-dimethylhept-2-en-4-yn-1- yl] (methyl) (naphthalen-1-ylmethyl) amine. Upon oral administration of Terbinafine tablets as a result of first-pass metabolism is approximately 40%. A single oral dose of 250 mg Terbinafine results in peak plasma concentration (Cmax) of 0.83 μg/ml within 2 h of administration. The absorption half-life is 0.8 h and the distribution half-life is 4.6 h. This originates the need of an alternative route of administration, which can bypass the hepatic first-pass metabolism. Transdermal route is an alternative choice of route of administration for such drugs. Efforts offer added advantages, such as maintenance of constant and prolonged drug level, reduced frequency of dosing, minimization of inter and intra patient variability, self administration and easy termination of medication, leading to patient compliance by using different polymers ratio and penetration enhancers in optimized quantity [9].
Materials and Methods
Terbinafine was received as a gift sample from Shreya Life Sciences (Aurangabad, India). Poly Vinyl Pyrrolidone K25 (PVP K25) and Eudragit RS-100 were generous gift from Evonik India Pvt. Ltd. (Mumbai, India). Dimethyl sulfoxide (DMSO) and di-nbutyl- phthalate (DBP) were procured from Loba Chemie, Mumbai. Other materials used in the study ethanol, chloroform, methanol, dichloromethane were of analytical grade. Double-distilled water was used throughout the study.
Partition coefficient determination
The partition coefficient studies were performed by using n-octanol as non-aqueous phase and water as an aqueous phase. The two phases were mixed in equal quantities and kept for saturation with each other in separating the funnel. After mixing the system remain undisturbed for half an hour. About 10 mg of drug added to this solution and was occasionally shaken in separating the funnel. After shaken the resulting solution was kept a site for 24 hr. After 24 hr, two phases were separated in a separating funnel. The aqueous phase was filtered. Suitably diluted and amount of Terbinafine in an aqueous phase was determined by measuring absorbance at 223.5 nm using ultraviolet (UV) spectrophotometer. The partition coefficient of Terbinafine was calculated from the ratio between the concentration of Terbinafine in organic and aqueous phases from the below mentioned formula [10].

Fourier transforms infrared (FT-IR)
FT-IR technique was used to study the physical and chemical interaction between drug and excipients. The FT-IR study revealed no physical or chemical interactions between drug and polymer [11,12].
Differential scanning calorimetry (DSC)
DSC analysis is a thermo analytical tequnique used to identify the difference in the amount of heat required to increase the temperature of a sample and reference as a function of temperature. DSC, thermo analysis gives characteristic and comparable results for the pure drug and the prepared formulation as patch [13].
Preparation of terbinafine transdermal patches
The transdermal patches containing Terbinafine were prepared by solvent evaporation technique in glass ring; contain mixture of polymer I (HPMC E5) and polymer II (PVPK 25 OR Eudragit RS-100) were dissolved in 3 ml solvent Methanol: Chloroform, (1:1) by using magnetic stirrer; then the 72 mg plasticizer polyethylene glycol 400, 24 mg penetration enhancer dimethyl sulfoxide and finally added 6 mg Terbinafine in the above polymeric solution and stirred for 15 minutes. Final volume of above solutions was placed on the aluminium foil wrapped and placed in Petri dish to facilitate the evaporation of solvent at a controlled rate over the drying period of 12 hr by using inverted funnel. The dried films were removed; cut into specified area and kept in desiccators until used (Table 1) [14].
| Batch | Polymer I (mg) | Polymer II*(mg) | Batch | Polymer I(mg) | Polymer II** (mg) | Polymer II* (mg) |
|---|---|---|---|---|---|---|
| SA1 | 200 | 10 | SA25 | 160 | 10 | - |
| SA2 | 200 | 20 | SA26 | 160 | 20 | - |
| SA3 | 200 | 30 | SA27 | 160 | 30 | - |
| SA4 | 200 | 40 | SA28 | 160 | 40 | - |
| SA5 | 180 | 10 | SA29 | 140 | 10 | - |
| SA6 | 180 | 20 | SA30 | 140 | 20 | - |
| SA7 | 180 | 30 | SA31 | 140 | 30 | - |
| SA8 | 180 | 40 | SA32 | 140 | 40 | - |
| SA9 | 160 | 10 | SA33 | 200 | 10 | 10 |
| SA10 | 160 | 20 | SA34 | 200 | 20 | 20 |
| SA11 | 160 | 30 | SA35 | 200 | 30 | 30 |
| SA12 | 160 | 40 | SA36 | 200 | 40 | 40 |
| SA13 | 140 | 10 | SA37 | 180 | 10 | 10 |
| SA14 | 140 | 20 | SA38 | 180 | 20 | 20 |
| SA15 | 140 | 30 | SA39 | 180 | 30 | 30 |
| SA16 | 140 | 40 | SA40 | 180 | 40 | 40 |
| SA17 | 200 | 10** | SA41 | 160 | 10 | 10 |
| SA18 | 200 | 20** | SA42 | 160 | 20 | 20 |
| SA19 | 200 | 30** | SA43 | 160 | 30 | 30 |
| SA20 | 200 | 40** | SA44 | 160 | 40 | 40 |
| SA21 | 180 | 10** | SA45 | 140 | 10 | 10 |
| SA22 | 180 | 20** | SA46 | 140 | 20 | 20 |
| SA23 | 180 | 30** | SA47 | 140 | 30 | 30 |
| SA24 | 180 | 40** | SA48 | 140 | 40 | 40 |
*PVP K25; **EudragitRS-100
Table 1: Formulation of Terbinafine transdermal patches SA1-SA48.
Evaluation of patches
Physical appearance: All the prepared patches were visually inspected for color, clarity, flexibility and smoothness [15].
Thickness of the patch: The thickness of the drug loaded patch was measured in different points by using a digital micrometer and determines the average thickness and standard deviation for the same to ensure the thickness of the prepared patch, (Table 2) [16].
| Batch | T | WU | FE | Batch | T | WU | FE |
|---|---|---|---|---|---|---|---|
| SA1 | 0.161 ± 0.0103 | 0.305 | 98 | SA25 | 0.152 ± 0.0028 | 0.210 | 105 |
| SA2 | 0.160 ± 0.0241 | 0.295 | 96 | SA26 | 0.151 ± 0.0025 | 0.230 | 70 |
| SA3 | 0.158 ± 0.0165 | 0.310 | 97 | SA27 | 0.151 ± 0.0025 | 0.265 | 98 |
| SA4 | 0.152 ± 0.0132 | 0.275 | 95 | SA28 | 0.156 ± 0.0110 | 0.235 | 97 |
| SA5 | 0.156 ± 0.0110 | 0.265 | 55 | SA29 | 0.153 ± 0.0143 | 0.260 | 70 |
| SA6 | 0.157 ± 0.0095 | 0.250 | 67 | SA30 | 0.152 ± 0.0086 | 0.281 | 89 |
| SA7 | 0.161 ± 0.0047 | 0.283 | 70 | SA31 | 0.150 ± 0.0080 | 0.295 | 92 |
| SA8 | 0.155 ± 0.0075 | 0.273 | 78 | SA32 | 0.156 ± 0.0110 | 0.278 | 78 |
| SA9 | 0.156 ± 0.0062 | 0.230 | 96 | SA33 | 0.151 ± 0.0131 | 0.305 | 98 |
| SA10 | 0.158 ± 0.0085 | 0.240 | 95 | SA34 | 0.152 ± 0.0050 | 0.320 | 99 |
| SA11 | 0.161 ± 0.0050 | 0.280 | 97 | SA35 | 0.155 ± 0.0057 | 0.310 | 97 |
| SA12 | 0.160 ± 0.0057 | 0.250 | 99 | SA36 | 0.151 ± 0.0062 | 0.311 | 98 |
| SA13* | - | - | - | SA37 | 0.152 ± 0.0057 | 0.330 | 95 |
| SA14* | - | - | - | SA38 | 0.152 ± 0.0050 | 0.340 | 97 |
| SA15* | - | - | - | SA39 | 0.152 ± 0.0050 | 0.310 | 98 |
| SA16* | - | - | - | SA40 | 0.151 ± 0.0062 | 0.320 | 95 |
| SA17 | 0.156 ± 0.0086 | 0.265 | 98 | SA41 | 0.156 ± 0.0075 | 0.270 | 98 |
| SA18 | 0.157 ± 0.0050 | 0.280 | 99 | SA42 | 0.155 ± 0.0057 | 0.266 | 97 |
| SA19 | 0.152 ± 0.0050 | 0.290 | 98 | SA43 | 0.152 ± 0.0086 | 0.360 | 95 |
| SA20 | 0.152 ± 0.0050 | 0.278 | 99 | SA44 | 0.153 ± 0.0075 | 0.298 | 97 |
| SA21 | 0.150 ± 0.0080 | 0.252 | 99 | SA45 | 0.155 ± 0.0057 | 0.278 | 87 |
| SA22 | 0.150 ± 0.0147 | 0.315 | 99 | SA46 | 0.157 ± 0.0121 | 0.260 | 79 |
| SA23 | 0.152 ± 0.0095 | 0.283 | 98 | SA47 | 0.151 ± 0.0050 | 0.256 | 92 |
| SA24 | 0.152 ± 0.0050 | 0.283 | 84 | SA48 | 0.152 ± 0.0050 | 0.265 | 96 |
T: Thickness** (mm); WU: Wt. Uniformity (mg); FE: Folding Endurance; *Patch is not formed; **SD mean (n=6)
Table 2: Thickness, Weight Uniformity and Folding Endurance of Terbinafine transdermal patches SA1-SA24.
Weight uniformity: The prepared patches were dried at 60°C for 4 hr before testing. A specified area 3 × 3 cm of the patch was cut in different parts of the patch and weigh in digital balance. The average weight and standard deviation values are to be calculated from the individual weight, (Table 2) [16].
Folding endurance: A strip of a specific 2 × 2 cm area was cut evenly and repeatedly folded at the same place till it broke. The number of times the film could be folded at the same place without breaking gave the value of the folding endurance, (Table 2) [17].
Percentage moisture absorption
The weighed films were kept in desiccators at room temperature for 24 hr containing a saturated solution of potassium chloride in order to maintain 84% RH. After 24 hr the films are to be reweighed and determine the percentage moisture uptake from the below mentioned formula, (Table 3) [17].
| Batch | MA % | ML % | WVTR | Batch | MA % | ML % | WVTR |
|---|---|---|---|---|---|---|---|
| SA1 | 04.73 | 03.51 | 0.0388 | SA25 | 12.63 | 07.31 | 0.0277 |
| SA2 | 06.72 | 04.45 | 0.0277 | SA26 | 10.73 | 10.43 | 0.0290 |
| SA3 | 02.20 | 01.85 | 0.0390 | SA27 | 09.61 | 06.98 | 0.0277 |
| SA4 | 07.12 | 02.12 | 0.0277 | SA28 | 12.75 | 10.44 | 0.0277 |
| SA5 | 04.49 | 03.40 | 0.0297 | SA29 | 12.30 | 11.53 | 0.0280 |
| SA6 | 05.68 | 03.75 | 0.0240 | SA30 | 10.32 | 05.69 | 0.0518 |
| SA7 | 08.03 | 06.46 | 0.0277 | SA31 | 10.16 | 05.08 | 0.0555 |
| SA8 | 09.90 | 06.10 | 0.0277 | SA32 | 10.34 | 03.44 | 0.0462 |
| SA9 | 05.24 | 03.88 | 0.0925 | SA33 | 10.00 | 08.19 | 0.0462 |
| SA10 | 06.25 | 04.50 | 0.0211 | SA34 | 08.75 | 05.06 | 0.0314 |
| SA11 | 07.67 | 05.96 | 0.0365 | SA35 | 09.20 | 04.61 | 0.0462 |
| SA12 | 05.60 | 03.24 | 0.0370 | SA36 | 10.95 | 08.57 | 0.0462 |
| SA13* | - | - | - | SA37 | 10.57 | 03.66 | 0.0460 |
| SA14* | - | - | - | SA38 | 08.82 | 07.30 | 0.0450 |
| SA15* | - | - | - | SA39 | 11.47 | 01.63 | 0.0668 |
| SA16* | - | - | - | SA40 | 12.11 | 02.95 | 0.0555 |
| SA17 | 09.43 | 02.64 | 0.0277 | SA41 | 08.00 | 07.40 | 0.0462 |
| SA18 | 08.07 | 05.35 | 0.0240 | SA42 | 10.90 | 01.50 | 0.0450 |
| SA19 | 12.06 | 03.44 | 0.0370 | SA43 | 10.55 | 04.16 | 0.0370 |
| SA20 | 10.93 | 05.08 | 0.0278 | SA44 | 11.11 | 02.81 | 0.0277 |
| SA21 | 11.11 | 03.17 | 0.0324 | SA45 | 10.32 | 08.16 | 0.0462 |
| SA22 | 06.82 | 04.76 | 0.0185 | SA46 | 09.37 | 06.12 | 0.0648 |
| SA23 | 09.18 | 01.06 | 0.0370 | SA47 | 10.78 | 07.24 | 0.0370 |
| SA24 | 08.88 | 04.61 | 0.0370 | SA48 | 12.45 | 09.45 | 0.0462 |
MA: Moisture Absorption; ML: Moisture Loss; WVTR: Water Vapor Transmission Rate; *Patch is not formed
Table 3: Percent Moisture Absorption, Percent Moisture Loss and Water Vapour. Transmission Rate of Terbinafine transdermal patch SA1-SA48.
Percentage Moisture Uptake=Final Weight-Initial Weight/Initial Weight × 100
Percentage moisture content/loss
The prepared films were weighed individually and kept in desiccators containing fused calcium chloride at room temperature for 24 hr. After 24 hr the films were reweighed and determined the percentage moisture content from the below mentioned formula, (Table 3) [18].
Percentage Moisture Content=Initial Weight-Final Weight/Final Weight × 100
Water vapor permeability/Transmission rate
Glass vials of 5 ml capacity were washed thoroughly and dried to a constant weight in an oven. About 1 g of fused calcium chloride was taken in the vials and the polymer films were fixed over the brim with the help of an adhesive tape. Then the vials were weighed and stored in a humidity chamber at 85% RH condition for a period of 24 hrs. The vials were removed and weighed at various time intervals like 3, 6, 12, 18 and 24 hrs to note down the weight gain, (Table 3) [19].
Drug content
A specified area of size 2 × 2 cm transdermal patch SA1-SA48 was dissolved in a phosphate buffer 7.4 up to 10 ml. Then the solution was filtered through a whatman filter paper and the drug content were analyzed with the spectroscopic method (Table 4) [20].
| Batch | DC (%) | Batch | DC (%) | Batch | DC (%) |
|---|---|---|---|---|---|
| SA1 | 80.22 | SA17 | 84.00 | SA33 | 76.27 |
| SA2 | 80.22 | SA18 | 70.00 | SA34 | 85.00 |
| SA3 | 85.62 | SA19 | 76.21 | SA35 | 91.77 |
| SA4 | 87.16 | SA20 | 75.36 | SA36 | 85.77 |
| SA5 | 81.63 | SA21 | 86.20 | SA37 | 76.44 |
| SA6 | 89.93 | SA22 | 88.81 | SA38 | 79.92 |
| SA7 | 86.53 | SA23 | 75.21 | SA39 | 74.22 |
| SA8 | 82.14 | SA24 | 84.36 | SA40 | 89.95 |
| SA9 | 89.55 | SA25 | 70.66 | SA41 | 90.15 |
| SA10 | 88.77 | SA26 | 79.76 | SA42 | 88.66 |
| SA11 | 80.51 | SA27 | 85.96 | SA43 | 87.50 |
| SA12 | 92.89 | SA28 | 72.58 | SA44 | 84.74 |
| SA13* | - | SA29 | 84.24 | SA45 | 71.50 |
| SA14* | - | SA30 | 87.54 | SA46 | 76.34 |
| SA15* | - | SA31 | 86.95 | SA47 | 78.67 |
| SA16* | - | SA32 | 86.95 | SA48 | 82.56 |
DC: Drug Content; *Patch is not formed
Table 4: Drug content (%) of Terbinafine transdermal patches SA1-SA48.
In vitro drug diffusion study
In vitro skin permeation studies were performed by using a Franz diffusion cell with a receptor compartment capacity of 20 ml. The rat skin was mounted between the donor and receptor compartment of the diffusion cell, the formulated patches were cut into size of 2 cm2 and placed over the drug release membrane and the receptor compartment of the diffusion cell was filled with phosphate buffer pH 7.4. The whole assembly was fixed on a magnetic stirrer, and the solution in the receptor compartment was constantly and continuously stirred using magnetic beads at 100 rpm, the temperature was maintained at 37 ± 0.50C. The samples of 1 ml were withdrawn at time interval of 1, 2, 3, 4, 5 and 6 hr analyzed for drug content UV-Visible spectrophotometrically at 223.5 nm. The receptor phase was replenished with an equal volume of phosphate buffer at each time of sample withdrawal. The cumulative amounts of drug permeated per square centimeter of patches were plotted against time (Table 5 and Figure 1) [21].
| Time (h)Time (h) | SA1 | SA2 | SA3 | SA4 | SA5 | SA6 | SA7 | SA8 | SA9 | SA10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.30 | 11.84 | 14.29 | 25.72 | 26.27 | 20.12 | 19.53 | 15.23 | 16.56 | 16.45 |
| 2 | 38.40 | 19.62 | 27.85 | 35.23 | 36.27 | 39.45 | 30.13 | 24.15 | 27.67 | 32.27 |
| 3 | 55.15 | 33.57 | 32.19 | 54.49 | 45.49 | 56.04 | 36.15 | 36.64 | 39.87 | 36.46 |
| 4 | 59.17 | 48.15 | 49.42 | 61.94 | 52.86 | 67.51 | 39.51 | 45.90 | 48.56 | 42.46 |
| 5 | 61.50 | 60.13 | 64.73 | 68.11 | 59.69 | 71.81 | 49.50 | 56.77 | 54.64 | 48.24 |
| 6 | 65.70 | 64.70 | 68.34 | 87.45 | 67.04 | 80.75 | 61.14 | 62.34 | 64.15 | 55.92 |
| Time (h) | SA11 | SA12 | SA13 | SA14 | SA15 | SA16 | SA17 | SA18 | SA19 | SA20 |
| 1 | 22.16 | 19.45 | 23.12 | 22.64 | 18.34 | 15.79 | 23.18 | 16.09 | 24.17 | 17.87 |
| 2 | 45.96 | 38.35 | 42.45 | 35.12 | 34.16 | 38.74 | 42.51 | 25.28 | 42.08 | 36.48 |
| 3 | 55.56 | 56.67 | 52.55 | 51.20 | 55.67 | 43.03 | 46.92 | 37.45 | 56.76 | 48.81 |
| 4 | 61.25 | 67.78 | 65.70 | 66.19 | 66.17 | 46.62 | 50.49 | 46.74 | 68.43 | 57.97 |
| 5 | 69.78 | 81.94 | 78.61 | 77.23 | 78.52 | 54.62 | 55.04 | 51.33 | 77.03 | 64.97 |
| 6 | 87.35 | 92.56 | 85.99 | 89.00 | 88.89 | 62.61 | 64.87 | 59.17 | 88.30 | 70.43 |
| Time (h) | SA21 | SA22 | SA23 | SA24 | SA25 | SA26 | SA27 | SA28 | SA29 | SA30 |
| 1 | 21.06 | 7.91 | 19.16 | 24.45 | 22.56 | 18.14 | 19.16 | 20.58 | 16.65 | 13.34 |
| 2 | 34.12 | 10.55 | 35.55 | 49.93 | 41.96 | 20.27 | 29.87 | 31.87 | 29.69 | 23.75 |
| 3 | 40.98 | 14.59 | 40.26 | 54.90 | 55.25 | 26.03 | 36.71 | 36.66 | 37.73 | 36.41 |
| 4 | 55.69 | 19.48 | 44.58 | 60.24 | 60.25 | 32.30 | 42.86 | 44.34 | 44.85 | 48.86 |
| 5 | 65.39 | 25.88 | 48.12 | 64.93 | 70.73 | 38.78 | 47.15 | 47.12 | 50.65 | 54.97 |
| 6 | 72.46 | 45.89 | 65.57 | 71.27 | 76.29 | 46.65 | 54.65 | 53.93 | 61.54 | 65.33 |
| Time (h) | SA31 | SA32 | SA33 | SA34 | SA35 | SA36 | SA37 | SA38 | SA39 | SA40 |
| 1 | 23.54 | 13.06 | 20.16 | 14.65 | 17.51 | 16.65 | 18.25 | 21.31 | 19.67 | 17.65 |
| 2 | 46.29 | 18.56 | 34.83 | 21.47 | 27.48 | 25.25 | 34.79 | 41.10 | 35.21 | 34.24 |
| 3 | 65.10 | 36.65 | 49.60 | 31.58 | 32.02 | 38.18 | 44.36 | 52.36 | 45.61 | 43.16 |
| 4 | 77.51 | 42.86 | 57.60 | 36.56 | 39.89 | 48.54 | 50.09 | 58.13 | 52.49 | 47.60 |
| 5 | 82.73 | 47.32 | 63.75 | 40.34 | 43.83 | 54.23 | 53.97 | 64.75 | 60.70 | 56.29 |
| 6 | 88.52 | 58.99 | 69.64 | 49.57 | 57.57 | 63.81 | 64.09 | 72.46 | 74.19 | 68.15 |
| Time (h) | SA41 | SA42 | SA43 | SA44 | --- | --- | --- | --- | --- | --- |
| 1 | 17.14 | 19.15 | 15.17 | 19.89 | --- | --- | --- | --- | --- | --- |
| 2 | 25.19 | 28.46 | 22.87 | 29.71 | --- | --- | --- | --- | --- | --- |
| 3 | 38.39 | 35.47 | 38.70 | 34.12 | --- | --- | --- | --- | --- | --- |
| 4 | 41.25 | 41.34 | 46.87 | 42.60 | --- | --- | --- | --- | --- | --- |
| 5 | 49.56 | 51.78 | 52.67 | 54.71 | --- | --- | --- | --- | --- | --- |
| 6 | 67.78 | 64.89 | 65.79 | 66.49 | --- | --- | --- | --- | --- | --- |
The percentage drug release in 6 h was found to be highest 92.56% for formulation SA12 carrying HPMC E5 and PVP K25 and minimum 45.89% for formulation SA22 carrying HPMC E5 and Eudragit RS-100.
Table 5: Percentage cumulative drug release of formulation SA1-SA10.
Results and Discussion
Physical appearance
The transdermal patches were transparent, smooth, uniform and flexible.
Thickness
The thicknesses of the prepared transdermal patches were observed in the range of 0.150 ± 0.0080 mm to 0.161 ± 0.0103 mm, (Table 2).
Weight uniformity
Weight uniformity of the prepared transdermal patches was observed in the range of 0.210 to 0.360 mg, (Table 2).
Folding endurance
Folding endurance of the prepared transdermal patches was observed in the range of 55 to 105, (Table 2).
Percent moisture absorption
The percent moisture absorption of the prepared transdermal patches was found to be between 2.20 to 12.75, (Table 3).
Percentage moisture content/loss
The percent moisture losses of the prepared transdermal patches were found to be between 1.06 to 11.53, (Table 3).
Water vapor permeability/Transmission rate (WVTR)
The water vapour transmission rate of the prepared transdermal patches was found to be between 0.0185 to 0.0925 (Table 3).
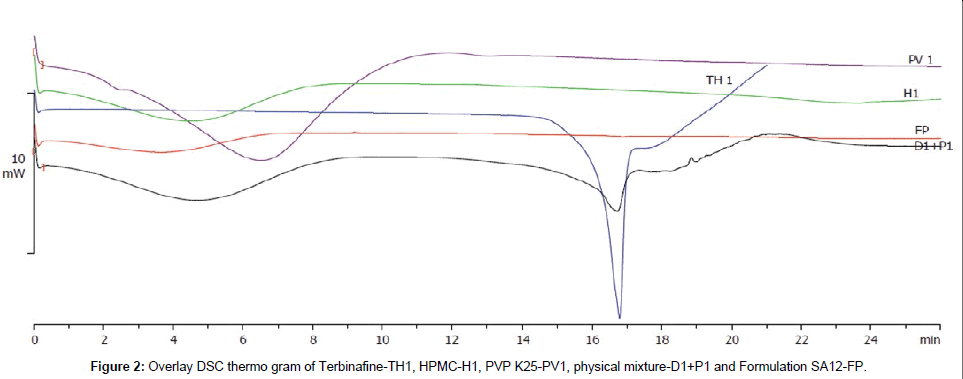
Differential scanning calorimetry
Differential scanning calorimetry enables the quantitative detection of all processes in which energy is required or produced (i.e., endothermic or exothermic phase transformations). The thermo grams of Terbinafine (TH1), HPMC E5 (H1), PVP K25 (PV1), physical mixture of Terbinafine, HPMC E5, PVP K25 (D1+P1), patch formulation (SA12-FP) are presented in Figure 2. The Terbinafine showed a melting peak at 206.99°C. Peak of Terbinafine at 206.77°C was present at the same position i.e., near to 206°C in the physical mixture of drug with both HPMC E5 and PVP K25 patch formulation excipients. This confirmed the physicochemical stability of drug with the formulation excipients used in the study.
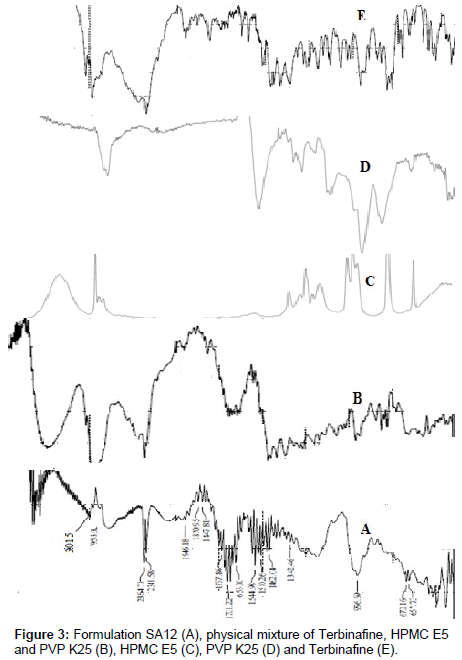
FT-IR
Drug - excipients interactions play a vital role with respect to release of drug from the formulation amongst others. FT-IR techniques have been used here to study the physical and chemical interaction between drug and excipients used. Infrared (IR) spectra of Terbinafine (E), physical mixture of Terbinafine, HPMC E5 AND PVP K25 (B), HPMC E5 (C), PVP K25 (D) and Terbinafine (E) are shown in Figure 3. Infrared absorption spectroscopy (IR) of Terbinafine showed sharp band at 2968, 1413 and 1361 cm-1 due to stretching vibration bands of C-H, C=C and C-N respectively. From the figure it was observed that there were no changes in these main peaks in FT-IR spectra of mixture of drug and polymers, which show there were no physical interactions because of some bond formation between drug and polymers.
Drug content
The drug content in all formulations SA1-SA48 was found to be ranging from 70.00% to 92.89%. That indicates that the drug was dispersed uniformly throughout the patches. The drug content of each formulation SA1-SA48 was evaluated (Table 4).
In vitro drug diffusion study
From the obtained data; concluded that the optimized batches SA4, SA12, SA13, SA19 and SA31 are selected on the basis of their drug content and percentage drug release. The optimized batches shows suitable physical and mechanical properties like thickness, weight uniformity, folding endurance, percentage moisture loss, percentage moisture absorption, water vapour transmission rate and drug content. The optimized batches SA4, SA12, SA13, SA19 and SA31 shows better percentage cumulative drug release as compared to other batches. The percentage drug release in 6 h was found to be highest 92.56% for formulation SA12 carrying HPMC E5 and PVP K25 and minimum 45.89% for formulation SA22 carrying HPMC and eudragit RS-100 (Table 5 and Figure 1).
Conclusion
Transdermal patch showed good controlled release properties. The results of the present study demonstrated that Terbinafine can be considered for Transdermal patch containing HPMC E5 and Eudragit- RS100 as polymers, DMSO as permeation enhancer and plasticizer polyethylene glycol 400 for controlled release of the drug over a period of 06 h for the management of fungal infection. The Transdermal drug delivery system holds a promising future in effective Transdermal delivery of bioactive agents and opportunities for clinicians to experiment with various drugs to study their systemic and local effects.
Acknowledgements
The authors thank to Shri. Prashant Patil Gadakh, President, Mula Education Society’s, Sonai and Dr. V. K. Deshmukh, Principal, MES`s College of Pharmacy, Sonai for providing all laboratory facilities, UDCT Dr. BAMU, Aurangabad for recording FT-IR Spectra, Government College of Pharmacy, Aurangabad for recording DSC, Diya Labs, Mumbai for recording XRPD.
References
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, et al. (2010) Transdermal drug delivery: The inherent challenges and technological advancements. Asian J Pharm Sci 5:276-289.
- Hadgraft J (2004) Skin deep. Eur J Pharm Biopharm 58:291-299.
- Langer R, Chen H (1989) Oral particulate delivery: Status and future trends. Adv Drug Deliv Rev 34:339-350.
- Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M (1999) Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: Model-based regional estimates. Bull World Health Organ 77:801-807.
- Pankaj K, Samir M (2009) Enhancement of transdermal drug delivery via synergisticaction of chemicals. Biochimica et Biophysica Acta 1788: 2362-2373.
- Seeram NP, Lee R, Heber D (2004)Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta 348:63-68.
- Barry BW (1987) Mode of action of penetration enhancers on the kinetics. Journal of Controlled Release 6: 85-97.
- Sarunyoo S (2009) An overview of skin penetration enhancers: penetration enhancing activity, skin irritation potential and mechanism of action. Songklanakarin J Sci Technol 31:299-321.
- Sambhaji Z (2015) M Pharm Thesis, Savitribai Phule Pune University,Pune, Maharashtra, India. pp: 1-85.
- Dwarakanadha RP, Swarnalatha D, Sidda RB, Karthik Sai KP, Sardar UM (2015) Design, development and characterization of clopidogrel bisulfate transdermal drug delivery system. Asian J Pharm Clin Res 8:277-280.
- Manoj KM, Debajyoti R, Bhakti BB (2009) Microcapsules and transdermal patch: A comparative approach for improved delivery of antidiabetic drug. AAPS Pharm Sci Tech 10: 928-934.
- Yuxiu Z, Dongmei C, Xin K, Liang F (2014) Design and evaluation of a novel transdermal patch containing diclofenac and teriflunomide for rheumatoid arthritis therapy. Asian J Pharma Sci 9: 251-259.
- Vikas GR, Ajay LA, Sopan D (2015)Development of ezogabine co crystal formation: An efficient approach to enhance aqueous solubility. British Biomedical Bulletin 3: 350-363.
- Evrim AT, Özge I, Tamer B (2009) Studies on transdermal delivery enhancement of zidovudine. AAPS Pharm Sci Tech 10: 88-97.
- Amit KV, Om PM, Nimisha, Dipti S (2012) Formulation and evaluation of transdermal patch containing turmeric oil. International J Pharmacy and Pharma Sci 4: 358-361.
- Satheesh Madhavn NV, Abhay PY (2013) A novel translabial platform utilizing bio excipients from litchi chinesis for the delivery of rosiglitazone maleate. Acta Pharmaceutica Sinica B 3:408-415.
- Jirapornchai S, Laksana C, Fameera M, Chaowalit M, Apirak S, et al. (2015) Zingiber cassumunar blended patches for skin application: Formulation, physicochemical properties, and in vitro studies. Asian J Pharma Sci 10: 341-349.
- Janardhanan B, Ramachandra PV, Rajappan M, Thengungal KR, Probal Kumar M (2007) Formulation development and In Vitroand In Vivo evaluation of membrane-moderated transdermal systems of ampicillin sodium in ethanol: pH 4.7 buffer solvent system. AAPS Pharm Sci Tech 8: E50-55.
- Panner Selvam R (2010) Design and evaluation of transdermal drug delivery of an antihypertensive drug. M Pharm Thesis, Rajiv Gandhi University of Health Sciences, Bangalore, Karnataka, India.
- Prashant MS, Suniket VF, Avinash KD (2005) Evaluation of polymerized rosin for the formulation and development of transdermal drug delivery system: a technical note. AAPS Pharm Sci Tech 6: 1-6.
- Gupta JRD, Irchhiaya R, Garud N, Priyanka T, Prashant D, et al. (2009) Formulation and evaluation of matrix type transdermal patches of glibenclamide. International J Pharma Sci and Drug Res 1:46-50.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16719
- [From(publication date):
December-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11888
- PDF downloads : 4831