Research Article Open Access
The Role of Diffusion Weighted MR Imaging in the Diagnosis of Acute Pancreatitis
Hasan Aydin*, Idil Gunes Tatar, Baki Hekimoglu
Diskapi Yildirim Beyazit Education and Research Hospital, Radiology Department, Turkey
Visit for more related articles at International Journal of Emergency Mental Health and Human Resilience
Abstract
Objective: To understand the utility of Diffusion-weighted MR imaging(DWI) in the diagnosis of acute pancreatitis. Conclusion: DWI can analyse the major manifestations of acute pancreatitis without any contrast agent use and may replace Abdominal CT and routine Pancreas MRI as a primary investigation tool for acute pancreatitis.
Keywords
Pancreas, DWI, acute, MRI, pancreatitis
Introduction
Acutepancreatitis (AP) is a potentially fatal disease which has to be accurately diagnosed with a mortality rate of over 10% (Thomas et al., 2012; Shinya et al., 2008; Shinya et al., 2009; Lankisch et al., 1999). AP is mostly caused due to the obstruction extra-hepatic bile ducts and ampulla vateri by gall bladder stones and choledocholithiasis, by the tumors of bile ducts especially Klatskin tumor, by the acute and chronic inflammation of bile ducts, other etiologic conditions are: Chronic alcoholism, primary hyperparathyroidism and hypercalcemia, hypertriglyceridemia, some drugs and toxins, pregnancy, pancreatic divisum, idiopathic and etc. (Sarles, 1963; Wang et al., 2009; Carroll et al., 2007; Wada et al., 2010). AP is a reversible inflammatory process of the pancreas, may occur as an isolated attack or may be recurrent, range in severity from mild to severe and life threatening, mild form has a low mortality rate, but patients with severe AP are more likely to develop complications and have a much higher deathrate(Thomas et al., 2012; Shinya et al., 2008; Lankisch et al., 1999; Wang et al., 2009; Carroll et al., 2007; Wada et al., 2010; Triester et al., 2002; Mitchell et al., 2003; Malangoni et al., 2005). Serum amylase and lipase levels remain the most widely used diagnostic assays for acute pancreatitis, other biomarkers and inflammatory mediators such as trypsinogens are being investigated for clinical use(Smotkin et al., 2002; Frossard et al., 2001; Neoptolemos et al., 2000). Although the disease process may be limited to pancreatic tissue, it also can involve peripancreatic tissues causing peri-pancreatic hemorrhage and inflammation or more distant organ sites which can assess the differentiation of acute and chronic inflammation of pancreatic parenchyma(Thomas et al., 2012; Carroll et al., 2007; Triester et al., 2002). Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation(APACHE II) scale, and CT Severity Index are systems for classifying severity of this disease; Atlanta classification is widely used to compare these systems and standardize clinical trials, new developments in imaging modalities such as endoscopic ultrasonography and MR cholangiopancreatography (MRCP) increase the options available to physicians for determining the cause of pancreatitis and assessing for complications(Ranson, 1982; Chatzicostas et al., 2003; Leung et al., 2005; Chatzicostas et al., 2002; Prasad et al., 2001; Matos et al., 2002; Makary et al., 2005). DWI has been recently applied in the evaluation of AP due to the latest soft and hardware advances in MR technology, DWI assesses the Brownian motion of free water within intra and extracellular spaces, is initially used in the detection of acute cerebral stroke then has become available for the detection of abdominal and pelvic tumors especially the renal and prostate tumors, focal hepatic lesions, thyroid nodules, meniscal tears, soft tissue pathologies, bone marrow edema etc. (Thomas et al., 2012; Shinya et al., 2008; Balci et al., 2009; Aydin et al., 2012a; Aydin et al., 2012b; Koh et al., 2007; Aydin, 2012c; Aydin et al., 2012d; Zhang et al., 2012; Daggulli et al., 2011; Onur et al., 2012; Aydin et al., 2011). In this review, we will discuss the utility and contribution of DWI in the accurate diagnosis of AP.
General Knowledge About Current Dwi Technique
DWI is a new promising, non-invasive imaging technique that is being used increasingly in body applications, based on the random translational motion of water protons, indirectly proportional to the diffusion barriers, generating apparent diffusion coefficient (ADC), a net motion of water molecules (Thomas et al., 2012; Shinya et al., 2008; Shinya et al., 2009; Balci et al., 2009; Aydin et al., 2012a; Aydin et al., 2012b; Koh et al., 2007; Wang et al., 2011; Takeuchi et al., 2008; Ichikawa et al., 2007). Structural changes in benign or malignant tissues, may result in different signals on DWI which may be quantified by calculating the ADC values which is an objective parameter that reflects the quantitative expressions of the tissuespecific diffusions, that are related to the proportion of extracellular and intracellular components (Thomas et al., 2012; Shinya et al., 2008; Shinya et al., 2009; Balci et al., 2009; Aydin et al., 2012a; Aydin et al., 2012b; Koh et al., 2007; Wang et al., 2011; Takeuchi et al., 2008).
In general, Rapidly growing tumors are characterized by increased tissue cellularity and cellular density with the increased amount of diffusion barriers, the motion of diffusion capacity is restricted and water diffuses from extracellular components to the intracellular space, resulting to low ADC values and high signal intensity on DWI, however high ADC values are attributed to the free motion of water molecules in fluid-rich biologic environments (Thomas et al., 2012; Shinya et al., 2009; Aydin et al., 2012a; Aydin et al., 2012b; Wang et al., 2011; Takeuchi et al., 2008; Ichikawa et al., 2007). DWI is influenced by both water diffusion and T2 relaxation time(T2-shinethrough effect) which is the non-pure ADC maps of DW images, containing mixed contributions from T2 effects and spin-density phenomenon, leading to high signal appearing simple cysts on DWI with b value 0 sec/mm2, low signal intensity on high-b value images and high signal intensity on ADC maps, but however hyper intensity of abscesses, hydatid cysts and neoplastic cysts on 1000 sec/mm2 b factor images can’t be totally attributed to the T2-shine-through effect (Wang et al., 2011; Takeuchi et al., 2008; Wiggermann et al., 2012; Inan et al., 2008). T2-shine-effect exhibit high signal both in DWI and ADC maps, may be mistaken for restricted diffusion and as DWI has significant T2-weighting because of long TE, this effect can be overcome by use of short TE and high b-value, but can’t be avoided easily in DWI (Balci et al., 2009; Le Bihan et al., 1988; Koh et al., 2006). Diffusion can be quantitatively evaluated by ADC, which is free of T2-shine-through effect, only influenced by the magnitude of diffusion gradients (Inan et al., 2008; Koh et al., 2006). Diffusion measurement is eventually sum of vectoral quantities, 3 orthogonal diffusion vector gradients; GX, Gy, Gz are used for calculation of ADC, sum of the square root of these vector magnitudes is the real ADC value and mostly favorable in isotropic diffusion(Balci et al., 2009; Yoshikawa et al., 2006).
Free or isotropic diffusion occurs when movement of water molecules is completely random and restricted, anisotropic diffusion occurs when the motion of water is restricted due to the barriers within cell membranes and macromolecules, signal measured from MRI can be sensitized to water diffusion by addition of balanced diffusion-sensitive, magnetic field gradients and this diffusion-sensitizing gradients which acquire MR signals, are the real good contrast DW images because of the diffusion of water protons (Balci et al., 2009; Aydin et al., 2012a; Koh et al., 2007; Le Bihan et al., 1988). The MR signal obtained by diffusion-sensitizing gradients are equated by: S (b) = So-(bxADC), S (b) is the measured diffusion signal, So is the signal intensity without diffusion, b is the b-value (sec/mm2) for determining the magnitude, duration and time between the pair of gradients and ADC of water molecules (Balci et al., 2009; Aydin et al., 2012a; Koh et al., 2007). Increasing b-value in the above equation, increases the MR signal loss with a constant ADC value: A b-value of 0 sec/mm2 regards to the equal S(b) and So, a b-value of 50 sec/mm2 is sensitive to large net diffusion of water protons whereas b-value of 500-1000 sec/mm2 is sensitive to small motion of water molecules, nonetheless free diffusing molecules with high ADC values and large motion can produce a greater MR signal loss than that for water protons, under sampling restricted diffusion with lower ADC values for a constant b-value (intracellular space) (Balci et al., 2009; Koh et al., 2007; Koh et al., 2006). B-values between 50-500 sec/mm2 are recommended for DWI of abdominal organs as b-values lower than 50 sec/mm2 may cause signal loss due to tissue perfusion and higher b-values may acquire decrease in signal-to-noise (SNR) ratio (Balci et al., 2009; Yoshikawa et al., 2006). As DWI and ADC mapping have to be performed for each b-value, breath-hold Echo Planar Imaging (EPI) is preferred due to its short duration time and adequate SNR, but however motion artifacts, susceptibility artifacts at tissue-air interfaces and blurring of long interval acquisition are its disadvantages, nevertheless free-breathing or respiratory–gated and parallel imaging techniques are usually performed on DWI, cardiac motion like some artifacts can’t be minimized by breath-hold approaches (Balci et al., 2009; Koh et al., 2007; Koyama et al., 2006).
Use Of Dwi For Acute Pancreatitis In The Routine Practice
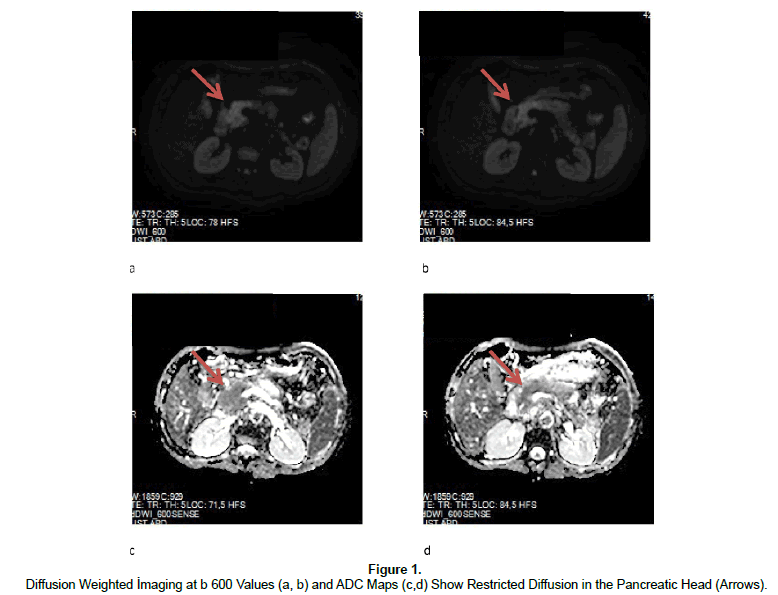
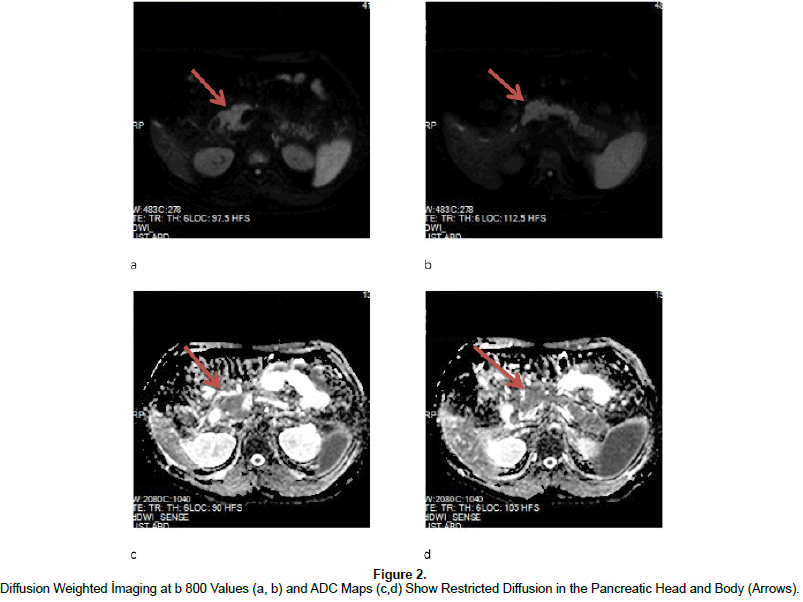
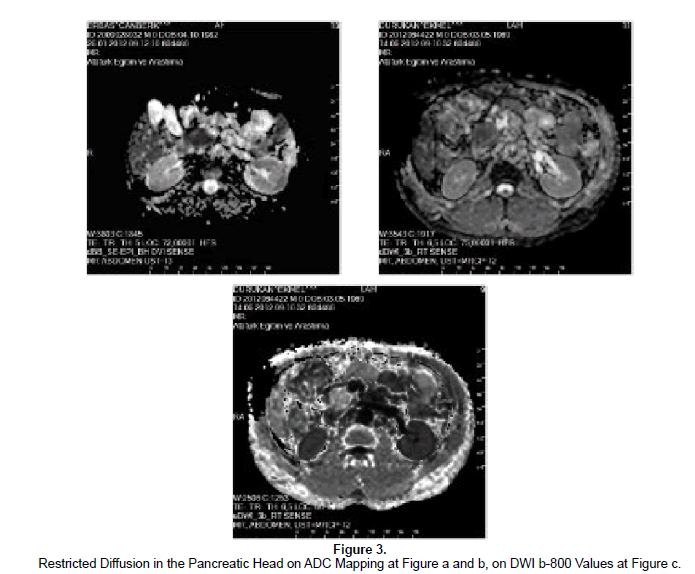
DWI can be performed via single-shot EPI with fat-saturation pulse sequence, high b-value use, integrated paralel imaging and higher gradient amplitudes with multiple excitations allow improved SNR and fast breath-hold imaging (Thomas et al., 2012; Koh et al., 2007; Aydin, 2012c; Aydin et al., 2012d, Wang et al., 2011; Ichikawa et al., 2007). Standard application of DWI is based on quantitative approach and limited scanning time acquired by breath-holding, motion artefacts does not permit thin-slice DW 94images with adequate SNR so multiple excitations with applied three dimensional gradients allow accurate images with good SNR for multiplanar reconstruction (Balci et al., 2009; Koh et al., 2007, Aydin, 2012c; Ichikawa et al., 2007; Le Bihan et al., 1988; Takahara et al., 2004). Conventional pancreatic MR imaging is more widely used in the diagnosis of cystic and solid pancreatic neoplasms due to its high soft-tissue resolution, T1 weighted (T1W) gradient echo-T2 weighted (T2W) single-shot turbo spin echo fat suppressed images and dynamic contrast-enhanced T1W gradient-echo with fat saturation are involved on conventional MR imaging (Wang et al., 2011; Takahara et al., 2004; Pamuklar et al., 2005). In addition, heavily T2W coronal and oblique MRCP sequence can be used to detect pancreatic ductal system and relation of pancreatic masses to the pancreatic major and minor ducts (Prasad et al., 2001; Makary et al., 2005; Wang et al., 2011). Conventional Pancreatic MR imaging has limitations in differentiating acute from chronic pancreatitis and signal changes in some mild forms of AP (Thomas et al., 2012; Sica et al., 2002). Besides depicting cystic and solid neoplasms of pancreas, DWI has become recently applied in imaging of acute and chronic inflammation of the pancreas, regarding increased signal at DWI and decreased ADC values (Thomas et al., 2012; Shinya et al., 2009; Balci et al., 2009; Takeuchi et al., 2008; Wiggermann et al., 2012) (Figures 1 and 2). Acute inflammation causes increased vascular permeability and edema, fluid accumulation in the affected area which lead to increased diffusion and ADC values with some variations and exceptions, if fibrosis exists with acute inflammation, restricted diffusion and lower ADC values are quantified, Diffusion-weighted images-with different b-values, ADC map and the calculated ADC value is really important for the diagnosis of acute and/or chronic inflammatorydiseases, the perfusion value, however which can also be calculated DWI and ADC maps, is also different in the acute and chronic form of pancreatitis that can aid in the diagnosis and depiction of inflammatory pancreatic disorders (Figure 3a and b) (Shinya et al., 2008; Balci et al., 2009; Inan et al., 2008).
At histologic sections, AP is characterized by polymorphonuclear leukocytes, deposition of fibrin in intercellular spaces and microthrombi in blood vessels, decreased ADC values observed in AP may be due to any of these factors (Thomas et al., 2012; Bockman et al., 1986). Akisik et al. (Akisik et al., 2009) regarded 100% sensitivity and 73% specificity in the differentiation of chronic pancreatitis from normal pancreas parenchyma via DWI with secretin stimulation, also revealed lower baseline ADC values with the increasing severity of pancreatitis. Taniguchi et al. (Taniguchi et al., 2009) reported lower ADC values in patients with autoimmune pancreatitis, compared to normal pancreas and patients with chronic pancreatitis. Shinya et al (Shinya et al., 2009) showed qualitative evaluation of DWI signal changes, lacking quantifiable ADC measurements in the setting of AP in a series of 11 patients. Thomas et al (Thomas et al., 2012) presented 93% sensitivity and 87% specificity in the detection of AP with b values 0 and 800 sec/mm2, predicted lower ADC values in the AP group than the normal controls. Wiggermann et al. (Wiggermann et al., 2012) revealed significant differences for ADC values between pancreatic cancerand normal pancreas, between focal pancreatitis and normal parenchyma which can clearly differentiate the normal pancreatic tissue and abnormal pancreas parenchyma, without any statistical differences between pancreatic cancer and pancreatitis in a series of 64 patients. The consequent obstacle of a significant overlap of ADC values for pancreatic cancer and chronic pancreatitis can be due to fibrotic and focal inflammatory reactions in chronic pancreatitis, causing difficulty in the differentiation of both of these pancreatic lesions (Takeuchi et al., 2008; Wiggermann et al., 2012; Taniguchi et al., 2009). Head and body of pancreas reveal slightly higher ADC values when compared with the tail, ADC values of pancreatic adenocarcinoma tend to be lower than normal pancreas parenchyma due to dense fibrosis and increased cellular elements, whereas higher or lower ADC values than the normal appearing pancreas exist in AP, due to the nature of inflammation in the gland (Balci et al., 2009; Wiggermann et al., 2012; Fattahi et al., 2009; Momtahen et al., 2008). Acute relapsing pancreatitis in the setting form of chronic pancreatitis show increased ADC values, predominant loose fibrosis, replacement of normal parenchyma with edematous fibrosis and or reduced exocrine function which reduces the diffusion of water in tissues, result in decreased ADC values (Balci et al., 2009; Wiggermann et al., 2012; Bockman et al., 1986) (Figure 3c).
As the limitations; DWI may not be capable of helping definitively characterize solid lesions as inflammatory or neoplastic because of an overlap in the ADC values between them, poorly differentiated adenocarcinomas have a low ADC value similar to that of mass-forming acute or chronic pancreatitis due to extensive fibrosis (Balci et al., 2009; Wang et al., 2011; Wiggermann et al., 2012; Fattahi et al., 2009). At low b-values (0 or 50 sec/mm2); Small benign pancreatic endocrine neoplasms, focal pancreatitis and normal parenchyma may show overlapping high signal intensity and relatively confusing high ADC values in DWI: In comparison, malignant islet cell tumors may show hyperintensity on DWI and low ADC values with high b-values (>500 sec/mm2), similar to AP and mass-forming chronic pancreatitis which is consistent with the restricted Brownian motion of water molecules due to dense cellularity (Balci et al., 2009; Wang et al., 2011; Herwick et al., 2006). Endocrine tumors of pancreas generally have varying ADC values due to differentiation, hemorrhage, necrosis etc. like variable underlying histologic patterns; DWI can be helpful in the differentiation of such neoplasms without hemorrhage or cystic degeneration from AP or benign inflammatory process by ADC values and signal changes on DWI (Wang et al., 2011 Herwick et al., 2006). DWI may also show limited help in the differentiation of Acute Peripancreatic Fluid Collections (APFC) from Acute Necrotic Collections (ANC) due to acute hemorrhagic pancreatitis, predict variable ADC values on those circumstances due to nature of collections (Hemorrhage or necrosis), acute or chronic inflammatory edema. For distinguishing APFC from ANC, Contrast-enhanced CT is the standard gold reference (Wang et al., 2009; Carroll et al., 2007; Wada et al., 2010; Triester et al., 2002; Chatzicostas et al., 2003; Leung et al., 2005; Chatzicostas et al., 2002; Wiggermann et al., 2012; Pamuklar et al., 2005; Sica et al., 2002). In the differentiation of Pancreatic Pseudocyst (PP) and Walled of Necrosis (WON) due to pancreatitis, DWI may supply important information as ADC values are extremely higher in PP than in WON, restricted diffusion and low ADC values are quite common in WON than in PP so DWI can be easily be performed in the routine practice instead of CT, nevertheless both PP and WON may reveal confusing enhancements which may acquire mis and/or over diagnosis (Thomas et al., 2012; Shinya et al., 2008; Shinya et al., 2009; Wang et al., 2009; Wada et al., 2009; Matos et al., 2002; Inan et al., 2008; Akisik et al., 2009; Fattahi et al., 2009).
Conclusion
Acute inflamed pancreatitis demonstrate restricted diffusion, increased DWI signals and decreased ADC values so DWI can be a powerful imaging technique without any need of contrast agent use, for evaluating AP and may have a serious potential to replace CT as a primary diagnostic approach for the diagnosis of AP and a complementary screening method to the standard Pancreatic MR imaging protocol. Conventional MR imaging combined with functional DWI provides important information in the detection and characterization of a variety of pancreatic abnormalities, including AP, chronic pancreatitis, solid or cystic pancreatic tumors. In conjunction to standard MR imaging technique, DWI may also challenge in the differentiation of mass-forming AP or chronic pancreatitis from pancreatic adenocarcinomas. Nevertheless, further prospective studies with large number of cases and clinical settings, are necessary to investigate whether DWI can improve the diagnostic accuracy of MR imaging for AP. To our knowledge, it is also important for radiologists to be aware of the advantages and limitations of DWI in the routine pancreatic imaging.
References
- Aydin, H., Kizilgoz, V., Tatar, I., Damar, C., Guzel, H., Hekimoglu, B., Delibasi, T.(2012a). The role of proton-MR-spectroscopy and apparent diffusion coefficient values in the diagnosis of malignant thyroid nodules, preliminary results. Clinical Imaging, 36, 323-333
- Aydin, H., Kizilgoz, V., Tatar, I., Damar, C., Ugan, A.R., Paker, I., et al. (2012b). Detection of Prostate Cancer With Magnetic Resonance Imaging: Optimization of T1-Weighted, T2-Weighted, Dynamic-Enhanced T1-Weighted, Diffusion-Weighted Imaging Apparent Diffusion Coefficient Mapping Sequences and MR Spectroscopy, Correlated With Biopsy and Histopathological Findings. Journal of Computer Assisted Tomography, 36, 30-45
- Aydin, H. (2012c). A new approach for prostate cancer diagnosis: Perfusion and Diffusionmeasurements.American Journal of Roentgenology, doi: 10.2214/AJR.12.10110, in press
- Aydin, H., Hekimoglu, B., & Kizilgoz, V. (2012d). A brief review for the combined use of T2-Weighted MR imaging and Diffusion Weighted Imaging for prostate cancer diagnosis. American Journal of Roentgenology, doi: 10.2214/AJR.12.9629,in press
- Aydin, H., Kizilgoz, V., &Hekimoglu, B. (2011). Is the quantitative Diffusion-Weighted MR Imaging and ADC mapping with b-values of 50, 400, and 800 sec/mm2 a reliable method for evaluation of meniscal tears in the knee? Polish Journal of Radiology, 76, 30-40
- Akisik, M.F., Aisen, A.M., Sandrasegaran, K., Jennings, S.G., Lin, C., Sherman S, et al. (2009).Assessment of chronic pancreatitis: Utility of diffusion-weighted MR imaging with secretin enhancement. Radiology, 250, 103-109
- Balci, N.M., Perman, W.H., Saglam, S., Akisik, F., Fattahi, R.,&Bilgin, M.I. (2009).Diffusion-weighted Magnetic Resonance imaging of the pancreas.Topics in Magnetic Resonance Imaging, 20, 43-47
- Bockman, D.E., Buchler,&M., Beger, H.G. (1986).Ultrastructure of human acute pancreatitis.International Journal of Pancreatology, 2, 141-153
- Chatzicostas, C., Roussomoustakaki, M., Vlachonikolis, I.G., Notas, G., Mouzas, I., Samonakis, D., et al. (2002). Comparison of Ranson, APACHE II and APACHE III scoring systems in acute pancreatitis. Pancreas, 25, 331–335
- Chatzicostas, C., Roussomoustakaki, M., Vardas, E., Romanos, J., &Kouroumalis, E.A. (2003).Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome.Journal of Clinical Gastroenterology, 36, 253–260
- Carroll, J.K., Herrick, B., Gipson, T., &Lee, S.P. (2007). Acute pancreatitis: diagnosis, prognosis and treatment. American Family Physician, 75, 1513-1520
- Daggulli, M., Onur, M.R., Firdolas, F., Onur, R., Kocakoc, E., Orhan,I. (2011). Role of diffusion MRI and apparent diffusion coefficient measurement in the diagnosis, staging and pathological classification of bladder tumors. Urologia Internationalis, 87, 346-352
- Fattahi, R., Balci, N.C., Perman, W.H., Hsueh, E.C., Alkaade, S., Havlioglu, N., et al. (2009). Pancreatic diffusion-weighted imaging(DWI): Comparison between mass-forming focal pancreatitis(FP), pancreatic cancer(PC) and normal pancreas. Journal of Magnetic Resonance Imaging,29, 350-356
- Frossard, J.L., Hadengue, A., &Pastor, C.M. (2001). New serum markers for the detection of severe acute pancreatitis in humans. American Journal of Respiratory and Critical Care Medicine, 164, 162–170
- Herwick, S., Miller, F.H., &Keppke, A.L. (2006). MRI of islet cell tumors of the pancreas. American Journal of Roentgenology, 187, 472-480. Ichikawa, T., Erturk, S.M., Motosugi, U., Sou, H., Lino, H., Araki, T., et al. (2007).High-b-value Diffusion-weighted MRI for detecting Pancreatic Adenocarcinoma: Preliminary results. American Journal of Roentgenology, 188, 409-414
- Inan, N., Arslan, A., Akansel, G., Anik, Y., &Demirci, A. (2008).Diffusion-weighted Imaging in the differential diagnosis of cystic lesions of the pancreas.American Journal of Roentgenology,191, 1115-1121
- Koh, D.M., &Padhani, A.R. (2006). Diffusion-weighted MRI: a new functional clinical technique for Tumor imaging. The British Journal of Radiology, 79, 633-635
- Koh, D.M., &Collins, D.J. (2007). Diffusion weighted MRI in the body:applications and challenges in oncology. American Journal of Roentgenology, 188, 1622–1635
- Koyama, T., Tamai, K., &Togashi, K. (2006). Current status of body MR imaging: fast MR imaging and diffusion-weighted imaging. International Journal of Clinical Oncology, 11, 278-285
- Lankisch, P.G., Assmus, C., Pflichthofer, D., Struckmann, K., &Lehnick, D. (1999). Which etiology causes the most severe acute pancreatitis? International Journal of Pancreatology, 26, 55-57
- Le Bihan, D., Breton, E., Lallemand, D., Aubin, M.L., Vignaud, J., &Laval-Jeantet, M. (1988).Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging, Radiology, 168, 497-505
- Leung, T.K., Lee, C.M., Lin, S.Y., Chen, H.C., Wang, H.J., Shen, L.K., et al. (2005). Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World Journal of Gastroenterology, 11, 6049–6052
- Makary, M.A., Duncan, M.D., Harmon, J.W., Freeswick, P.D., Bender, J.S., Bohlman, M., et al. (2005).The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Annals of Surgery,241, 119–124
- Malangoni, M.A., &Martin, A.S. (2005). Outcome of severe acute pancreatitis. Annals of Surgery,189, 273–277
- Matos, C., Cappeliez, O., Winant, C., Coppens, E., Deviere, J., &Metens,T. (2002). MR imaging of the pancreas: a pictorial tour. Radiographics,22, e2
- Mitchell, R.M., Byrne, M.F., &Baillie, J. (2003). Pancreatitis.Lancet, 361, 1447–1455
- Momtahen, A.J., Balci, N.C., Alkaade, S., Akduman, E.I.,& Burton, F.R. (2008). Focal pancreatitis mimicking pancreatic mass: magnetic resonance imaging(MRI)/magnetic resonance cholangiopancreatography (MRCP) findings included diffusion-weighted MRI.Acta Radiologica, 49, 490-497
- Neoptolemos, J.P., Kemppainen, E.A., Mayer, J.M., Fitzpatrick, J.M., Raraty, M.G., Slavin, J., et al. (2000). Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet, 355, 1955–1960
- Onur,M.R., Cicekci, M., Kayali, A., Poyraz, A.K., & Kocakoc, E. (2012). The role of ADC measurement in differential diagnosis of focal hepatic lesions. European Journal of Radiology, 81, 171-176
- Pamuklar, E., &Semelka, R.C. (2005).MR imaging of the pancreas.Magnetic Resonance Imaging Clinics of North America, 13, 313-330
- Prasad, S.R., Sahani, D., &Saini, S. (2001). Clinical applications of magnetic resonance cholangiopancreatography.Journal of Clinical Gastroenterology, 33, 362–366
- Ranson, J.H. Etiological and prognostic factors in human acute pancreatitis: a review. (1982). The American Journal of Gastroenterology. 77, 633–638
- Sarles, H. (1963).Pancreatitis symposium.Basel SK, Marseille
- Sica, G.T., Miller, F.H., Rodriguez, G., McTavish, J., &Banks, P.A. (2002).Magnetic resonance imaging in patients with pancreatitis: evaluation of signal intensity and enhancement changes. Journal of Magnetic Resonance Imaging, 15, 275-284
- Shinya, S., Sasaki, T., Nakagawa, Y., Guiquing, Z., Yamamoto, F., &Yamashita, Y. (2008). Acute Pancreatitis successfully diagnosed by diffusion-weighted imaging: A case report. World Journal of Gastroenterology, 14, 5478-5480. Shinya, S., Sasaki, T., Nakagawa, Y., Guiquing, Z., Yamamoto, F., &Yamashita, Y. (2009).The efficacy of Diffusion-weighted imaging for the detection and evaluation of acute pancreatitis.Hepatogastroenterology,56, 140-1410
- Smotkin, J., &Tenner, S. (2002). Laboratory diagnostic tests in acute pancreatitis. Journal of Clinical Gastroenterology, 34, 459–462
- Takahara, T., Imai, Y., Yamashita, T., Yasuda, S., Nasu, S., &Van Cauteren, M. (2004).Diffusion-weighted whole body imaging with background body signal suppression(DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiation Medicine, 22, 275-282
- Takeuchi, M., Matsuzaki, K., Kubo, H., &Nishitani, H. (2008).High b-value Diffusion-weighted Magnetic resonance imaging of pancreatic cancer and mass forming chronic pancreatitis: Preliminary results. ActaRadiologica, 4, 383-386
- Taniguchi, T., Kobayashi, H., Nishikawa, K., Iida, E., Michigami, Y., Morimoto, E., et al. (2009). Diffusion weighted magnetic resonance imaging in autoimmune pancreatitis. Japanese Journal of Radiology, 27, 138-142
- Thomas, S., Kayhan, A., Lakadamyali, H., &Oto, A. (2012).Diffusion MRI of acute pancreatitis and comparison with normal individuals using ADC values.Emergency Radiology, 19, 5-9
- Triester, S.L., &Kowdley, K.V. (2002). Prognostic factors in acute pancreatitis.Journal of Clinical Gastroenterology, 34, 167-176
- Wada, K., Takada, T., Hirata, K., Mayumi, T., Yoshida, M., Yokoe, M., et al. (2010).Treatment strategy for acute pancreatitis. Journal of Hepato-Biliary-Pancreatic Sciences, 17, 79-86
- Wang, G.J., Gao, C.F., Wei, D., Wang, C., &Ding, S.Q. (2009).Acute pancreatitis: Etiology and common pathogenesis.World Journal of Gastroenterology, 15, 1427–1430
- Wang, Y., Miller, F.H., Chen, Z.E., Merrick, L., Mortele, K.J., Hoff, F.L., et al. (2011). Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas.Radiographics, 31, 47-65. Wiggermann, P., Grutzmann, R., Weissenböck, A., Kamusella, P., Dittert, D.D., &Stroszczynski, C. (2012).Apparent diffusion coefficient measurements of the pancreas, pancreas carcinoma, and mass-forming focal pancreatitis.ActaRadiologica, 53, 135-139
- Yoshikawa, T., Kawamitsu, H., Mitchell, D.G., Ohno, Y., Ku, Y., Seo, Y., et al. (2006).ADC measurement of abdominal organs and Lesions using paralel imaging techniques.American Journal of Roentgenology, 187, 1521-1530
- Zhang, J.L., Sigmund, E.E., Rusinek, H., Chandarana, H., Storey, P., Chen, Q.,et al. (2012). Optimization of b-value sampling for diffusion-weighted imaging of the kidney.Magnetic Resonance in Medicine, 67, 89- 97.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15871
- [From(publication date):
June-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 11121
- PDF downloads : 4750