The Use of Vestibular Rehabilitation for Individuals with Migraines: A Systematic Review
Received: 17-Nov-2017 / Accepted Date: 28-Nov-2017 / Published Date: 05-Dec-2017 DOI: 10.4172/2161-119X.1000334
Abstract
Introduction: Up to 50% of all individuals with migraines experience vertigo. The purpose of this systematic review was to evaluate the effectiveness of vestibular rehabilitation at managing individuals who experience vertigo associated with their migraine headaches.
Methods: The CINAHL Complete, ProQuest Medical Library and PubMed databases were accessed using the following search terms: “migraine” AND “vestibular rehabilitation” OR “vestibular therapy” AND “vertigo” OR “dizziness”. A tool developed by the Oxford Centre for Evidence-Based Medicine was used to examine the evidence level of each included research study and a tool developed by Medlicott and Harris was used to examine the methodological rigor of each included research study.
Results: Vestibular rehabilitation was generally more effective for individuals with a non-migrainous vestibular disorder than it was for individuals with vestibular migraines. However, in all 5 studies, every group of participants benefitted to some degree from a customized vestibular rehabilitation program. Discussion: 2 of the studies proposed that behavioral therapies may benefit those individuals who have been diagnosed with vestibular migraines. 2 other studies suggested that the use of migraine medications may decrease an individual’s sensitivity to head movements and may allow the individual to more fully participate in a vestibular rehabilitation program.
Conclusion: Although it is difficult to draw any definitive conclusions from this systematic review, vestibular rehabilitation should be seriously considered when treating individuals who experience vertigo associated with their migraine headaches.
Keywords: Dizziness; Migraines; Systematic review; Vertigo; Vestibular rehabilitation
Introduction
According to the Migraine Research Foundation [1], migraine is the third-ranked disease in terms of prevalence. Approximately 1 billion individuals worldwide, including 38 million Americans, have been diagnosed with this medical condition. These statistics include almost 10% of all children in the United States. Although a majority of these children are males, migraines become more common in females by adolescence. By adulthood, migraines affect approximately 18% of all American females and 6% of all American males. In addition to its high prevalence, migraine is the sixth-ranked disease in terms of disability. In the United States al1, over 35 billion dollars are annually consumed by the healthcare costs and the productivity losses associated with migraines. Most individuals report significant difficulties performing their activities of daily living while they are experiencing a migraine.
Up to 50% of all individuals with migraines experience vertigo [2]. This medical condition, known as vestibular migraine, appears to be caused by the physiologic link between the trigeminal afferent neurons in the brainstem (which play a role in the headache) and the vestibular nuclei in the brainstem (which play a role in the vertigo) [3]. Although vestibular migraine was not described in the first or second editions of the International Classification of Headache Disorders, it has been recognized in the appendix of the third edition [4]. According to this most recent international classification, the headache part of a vestibular migraine resembles either a migraine without an aura or a migraine with an aura. The vestibular part of the medical condition includes moderate to severe vertigo that lasts 5 min to 72 h. A recent research study [5] reported that individuals who have vestibular migraines without an aura tend to experience non-spinning vertigo that is provoked by head movements or visual stimuli, whereas individuals who have vestibular migraines with an aura tend to experience spinning vertigo that is more spontaneous in nature.
The 2 primary types of migraine treatments are preventive (or prophylactic) approaches and acute (or abortive) approaches [6]. Most of the treatment options associated with each of these approaches are pharmacologic in nature. In a 2012 journal article [7], the author reported that divalproex sodium, propranolol, timolol and topiramate are effective as preventive treatment options for migraines. In a 2015 journal article [8], the authors reported that ergotamine derivatives, nonsteroidal anti-inflammatory drugs, opioids, triptans and combination medications are effective as acute treatment options for migraines. The purpose of a recent systematic review [9] was to ascertain which preventive approaches and which acute approaches are optimal for the treatment of vestibular migraines. The authors of this systematic review concluded that “patients with [vestibular migraine] need to be managed with similar prophylactic and abortive strategies as those used for migraine in adults” [9] (p. 711). Although the authors of another recent systematic review arrived at a similar conclusion, they also proposed that “vestibular rehabilitation therapy may be effective in vestibular migraine regardless of the used medical therapy” [10] (p. 260).
The main goals of vestibular rehabilitation are to decrease an individual’s vertigo level, to stabilize the individual’s eyes during head movements, to stabilize the individual’s body during head movements and to improve the individual’s functional status [11]. Except in the case of benign paroxysmal positional vertigo, these goals are primarily achieved through the use of habituation exercises, adaptation exercises and substitution exercises [12]. The purpose of habituation exercises is to decrease vertigo through repetitive exposure to provocative stimuli. Adaptation exercises result in physiologic compensation by enhancing the vestibulo-ocular and vestibulo-spinal reflexes. Substitution exercises result in functional compensation by enhancing the visual and somatosensory systems. In order to assess the impact of a vestibular rehabilitation program, the primary participation outcome measures used clinically are the Activities of Daily Living Questionnaire, the Activities-Specific Balance Confidence Scale, the Dizziness Handicap Inventory, the Prototype Questionnaire, the UCLA-Dizziness Questionnaire, the Vertigo Handicap Questionnaire, the Vestibular Disorders Activities of Daily Living Scale and the Vestibular Rehabilitation Benefit Questionnaire [13]. In addition, several different visual analog scales have been used for this purpose. Although vestibular rehabilitation was originally designed to treat individuals diagnosed with specific disorders of the vestibular system, the purpose of this systematic review was to evaluate the effectiveness of vestibular rehabilitation at managing individuals who experience vertigo associated with their migraine headaches.
Methods
The CINAHL Complete, ProQuest Medical Library and PubMed databases were accessed using the following search terms: “migraine” AND “vestibular rehabilitation” OR “vestibular therapy” AND “vertigo” OR “dizziness”. The Cochrane Library did not contain any similar published systematic reviews. A language bias may have been created by limiting the database search to research studies written in English.
This systematic review used the following inclusion criteria: (1) individuals who experience vertigo associated with their migraine headaches, (2) the use of vestibular rehabilitation techniques as the intervention, (3) the use of a non-vestibular intervention or a sham intervention as the comparison if the research study is a randomized controlled trial and (4) the use of a participation outcome measure described by Whitney and Sparto13 and/or the use of a visual analog scale as the outcome measure. The following exclusion criteria were used in this systematic review: (1) individuals who have not been diagnosed with migraines, (2) individuals who do not experience vertigo associated with their migraine headaches, (3) studies that do not use vestibular rehabilitation techniques as a comp1nt of the treatment plan and (4) research studies that employ inferential connections between the intervention and the outcome.
A tool developed by the Oxford Centre for Evidence-Based Medicine [14] was used to examine the evidence level of each included research study (Table 1). This tool rates evidence level from 1 to 5. Research studies with a rating of 1 are considered to possess the strongest evidence level and research studies with a rating of 5 are considered to possess the weakest evidence level. To decrease the risk of bias while using this tool, each included research study was independently examined by the 3 authors. Following this independent screening process, the authors discussed any discrepancies that may have occurred and ultimately reached a consensus on the evidence level of each research study.
| Hierarchy | EL Criteria |
|---|---|
| 1 | Systematic review of randomized trials or n-of-1 trials |
| 2 | Randomized trial or observational study with dramatic effect |
| 3 | Non-randomized controlled cohort/follow-up study |
| 4 | Case-series, case-control studies, or historically controlled studies |
| 5 | Mechanism-based reasoning |
Table 1: Evidence level (EL) overview [14].
A tool developed by Medlicott and Harris [15] was used to examine the methodological rigor of each included research study (Table 2). This tool rates methodological rigor from 0 to 10. Research studies with a rating of 8 to 10 are considered to possess strong methodological rigor, research studies with a rating of 6 or 7 are considered to possess moderate methodological rigor and research studies with a rating of 5 or less are considered to possess weak methodological rigor. To decrease the risk of bias while using this tool, each included research study was independently examined by the 3 authors. Following this independent screening process, the authors discussed any discrepancies that may have occurred and ultimately reached a consensus on the methodological rigor of each research study.
| Item | MR Criteria |
|---|---|
| 1 | Randomization |
| 2 | Inclusion and exclusion criteria were listed for the subjects |
| 3 | Similarity of groups at baseline |
| 4 | The treatment protocol was sufficiently described to be replicable |
| 5 | Reliability of data obtained with the outcome measures was investigated |
| 6 | Validity data obtained with the outcome measures was addressed |
| 7 | Blinding of patient, treatment provider, and assessor |
| 8 | Dropouts were reported |
| 9 | Long-term results were assessed via follow-up |
| 10 | Adherence to home programs was investigated |
Table 2: Methodological rigor (MR) overview [15].
Results
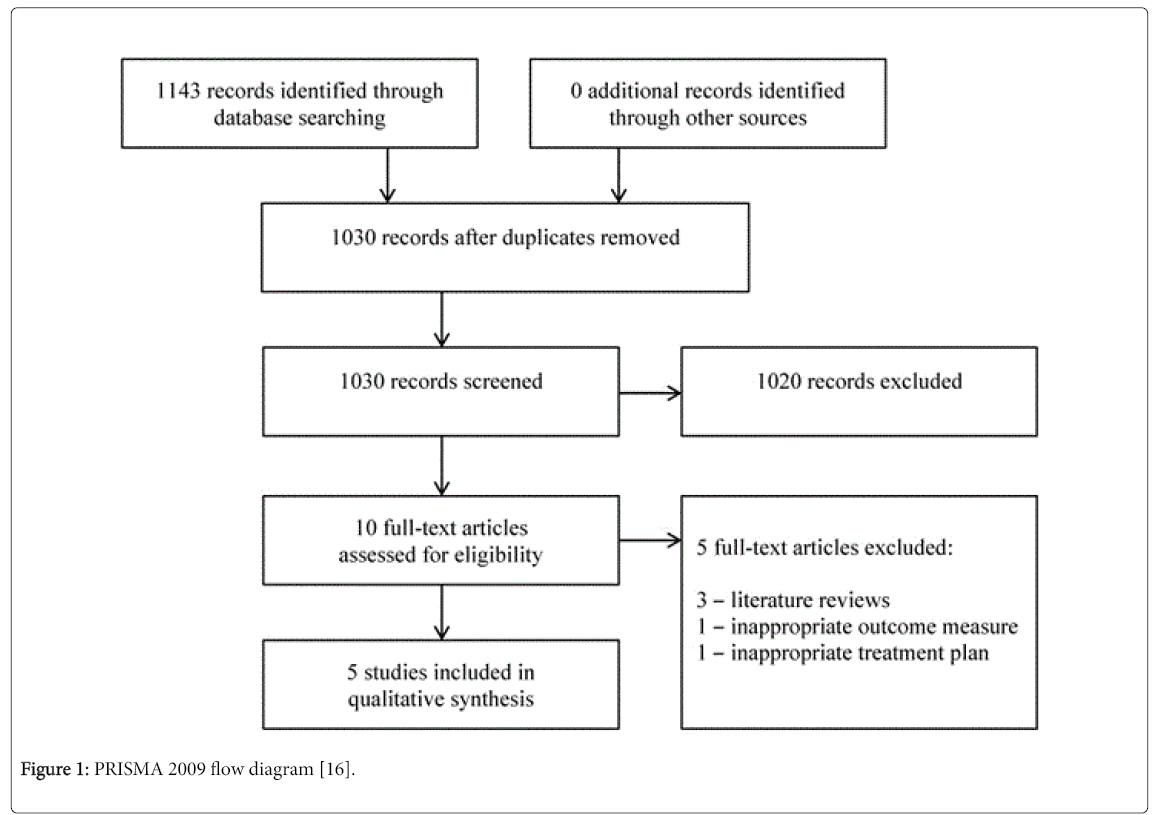
As displayed in the PRISMA 2009 flow diagram (Figure 1) [16], 1143 records were located through a search of the CINAHL Complete, ProQuest Medical Library and PubMed databases. No related records were located through other means. After duplicates were eliminated, 1030 records were screened by title and abstract. Ten articles were then assessed by a full-text review and the systematic review was eventually comprised of 5 articles [17-21] that met the inclusion criteria.
Figure 1: PRISMA 2009 flow diagram [16].
Based upon the tool developed by the Oxford Centre for Evidence- Based Medicine [14], all 5 of the included studies [17-21] possessed an evidence level rating of 3 because each of them was a non-randomized controlled study (Table 3).
| Author and Date | Hierarchy | EL |
|---|---|---|
| Whitney et al. [17] | non-randomized controlled study | 3 |
| Wrisley et al. [18] | non-randomized controlled study | 3 |
| Gottshall et al. [19] | non-randomized controlled study | 3 |
| Vitkovic et al. [20] | non-randomized controlled study | 3 |
| Sugaya et al. [21] | non-randomized controlled study | 3 |
Table 3: Evidence level (EL) results [14].
Based upon the tool developed by Medlicott and Harris [15], 1 of the 5 included studies [20] possessed a rating of 8 which is considered strong methodological rigor. 1 included study [21] possessed a rating of 4, another included study [19] possessed a rating of 3 and 2 included studies [17,18] possessed a rating of 1. Therefore, each of these other 4 studies [17-19,21] had weak methodological rigor (Table 4).
| Author and Date | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | MR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Whitney et al. [17] | N | Y | N | N | N | N | N | N | N | N | 1 |
| Wrisley et al. [18] | N | N | Y | N | N | N | N | N | N | N | 1 |
| Gottshall et al. [19] | N | N | N | Y | N | N | N | N | Y | Y | 3 |
| Vitkovic et al. [20] | N | Y | N | Y | Y | Y | Y | Y | Y | Y | 8 |
| Sugaya et al. [21] | N | Y | N | Y | N | N | N | Y | N | Y | 4 |
Table 4: Methodological rigor (MR) results [15], Item 1=randomization; Item 2=inclusion and exclusion criteria were listed for the subjects; Item 3=similarity of groups at baseline; Item 4=the treatment protocol was sufficiently described to be replicable; Item 5=reliability of data obtained with the outcome measures was investigated; Item 6=validity data obtained with the outcome measures was addressed; Item 7=blinding of patient, treatment provider, and assessor; Item 8=dropouts were reported; Item 9=long-term results were assessed via follow-up; Item 10=adherence to home programs was investigated.
Whitney et al. [17] completed a study that had an evidence level of 3 and a methodological rigor of 1 (Table 5). In this study, 39 individuals were instructed in a customized vestibular rehabilitation program that included habituation, balance, gait, strengthening and stretching exercises. Of the 39 participants, 14 had vestibular migraines and 25 had a vestibular disorder and a migraine history. At the completion of the 4 month program, the impact of the vestibular rehabilitation exercises was assessed with the Activities-Specific Balance Confidence Scale, the Dizziness Hand icap Inventory and the Perception of Dizziness Symptoms tool. The Activities-Specific Balance Confidence Scale [22] examines an individual’s balance-related confidence while performing 16 different gait activities. The individual’s score on this outcome measure ranges from 0 (no confidence) to 100 (maximum confidence). The Dizziness Handicap Inventory [23] examines an individual’s dizziness-related handicap while considering 25 different life situations. The individual’s score on this outcome measure ranges from 0 (no handicap) to 100 (maximum handicap). The Perception of Dizziness Symptoms tool [17] is a visual analog scale that examines an individual’s vertigo from 0 (no vertigo) to 100 (maximum vertigo). The outcomes of this study may be found in Table 5.
| Author and Date | EL and MR | Population | Interventions | Outcomes |
|---|---|---|---|---|
| Whitney et al. [17] | EL=3 | Group 1=14 participants with vestibular migraines | Vestibular rehabilitation for both groups | Activities-Specific Balance Confidence Scale: There was no significant difference between the pre-test scores and the post-test scores for group 1. Group 2 reported significantly improved scores from pre-test to post-test (p<0.05). |
| MR=1 | Group 2=25 participants with a vestibular disorder and a migraine history | Dizziness Handicap Inventory: Both groups reported significantly improved scores from pre-test to post-test (p<0.05). | ||
| Perception of Dizziness Symptoms tool: There was no significant difference between the pre-test scores and the post-test scores for group 1. Group 2 reported significantly improved scores from pre-test to post-test (p<0.05). | ||||
| Wrisley et al. [18] | EL=3 | Group 1=31 participants with a vestibular disorder and a migraine history | Vestibular rehabilitation for both groups | Activities-Specific Balance Confidence Scale: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2. |
| MR=1 | Group 2=31 participants with a non-migrainous vestibular disorder | Dizziness Handicap Inventory: The participants in group 2 reported a significantly greater improvement in scores than did the participants in group 1 (p<0.05). | ||
| Perception of Dizziness Symptoms tool: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2. | ||||
| Gottshall et al. [19] | EL=3 | Group 1=6 participants with idiopathic vestibular migraines | Vestibular rehabilitation for all groups | Activities-Specific Balance Confidence Scale: There was no significant difference between the pre-test scores and the post-test scores for group 1 or group 2. Group 3 and group 4 reported significantly improved scores from pre-test to post-test (p<0.05). |
| MR=3 | Group 2=4 participants with idiopathic vestibular migraines and BPPV | Dizziness Handicap Inventory: All 4 groups reported significantly improved scores from pre-test to post-test (p<0.05). | ||
| Group 3=17 participants with traumatic vestibular migraines | ||||
| Group 4=7 participants with traumatic vestibular migraines and BPPV | ||||
| Vitkovic et al. [20] | EL=3 | Group 4=7 participants with traumatic vestibular migraines and BPPV | Vestibular rehabilitation for both groups | Activities-Specific Balance Confidence Scale: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2 at either the short-term or long-term follow-up. |
| MR=8 | Group 1=20 participants with vestibular migraines | Dizziness Handicap Inventory: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2 at either the short-term or long-term follow-up. | ||
| Group 2=16 participants with a non-migrainous vestibular disorder | Vestibular Rehabilitation Benefit Questionnaire: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2 at either the short-term or long-term follow-up. | |||
| Sugaya et al. [21] | EL=3 | Group 1=28 participants with vestibular migraines | Vestibular rehabilitation for all groups | Vestibular Symptom Index: There was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2 at either the short-term or long-term follow-up. |
| MR=4 | Group 2=79 participants with vertigo and non-migrainous headaches | Dizziness Handicap Inventory: All 3 groups demonstrated improvements at both the short-term and long-term follow-up. | ||
| Group 3=144 participants with vertigo and no headaches | Frequency of Dizziness tool: All 3 groups demonstrated improvements at both the short-term and long-term follow-up. |
Table 5: Summary of studies, BPPV: Benign Paroxysmal Positional Vertigo; EL: Evidence Level; MR: Methodological Rigor.
Wrisley et al. [18] completed a study that had an evidence level of 3 and a methodological rigor of 1 (Table 5). In this study, 62 individuals were instructed in a customized vestibular rehabilitation program that included habituation, balance, gait, strengthening and stretching exercises as well as maneuvers for benign paroxysmal positional vertigo if necessary. Of the 62 participants, 31 had a vestibular disorder and a migraine history and 31 had a non-migrainous vestibular disorder. At the completion of the program, the impact of the vestibular rehabilitation exercises was assessed with the Activities- Specific Balance Confidence Scale, the Dizziness Hand icap Inventory and the Perception of Dizziness Symptoms tool. All 3 of these outcome measures have been previously described. The outcomes of this study may be found in Table 5.
Gottshall et al. [19] completed a study that had an evidence level of 3 and a methodological rigor of 3 (Table 5). In this study, 34 individuals were instructed in a customized vestibular rehabilitation program that included habituation, balance and aerobic exercises. Of the 34 participants, 6 had idiopathic vestibular migraines, 4 had idiopathic migraines and benign paroxysmal positional vertigo, 17 had traumatic vestibular migraines and 7 had traumatic vestibular migraines and benign paroxysmal positional vertigo. At the completion of the 6 to 8 week program, the impact of the vestibular rehabilitation exercises was assessed with the Activities-Specific Balance Confidence Scale and the Dizziness Hand icap Inventory. Both of these outcome measures have been previously described. The outcomes of this study may be found in Table 5.
Vitkovic et al. [20] completed a study that had an evidence level of 3 and a methodological rigor of 8 (Table 5). In this study, 36 individuals were instructed in a customized vestibular rehabilitation program that included habituation, adaptation, substitution, balance and gait exercises. Of the 36 participants, 20 had vestibular migraines and 16 had a non-migrainous vestibular disorder. At the completion of the 9- week program, the short-term impact of the vestibular rehabilitation exercises was assessed with the Activities-Specific Balance Confidence Scale, the Dizziness Handicap Inventory, the Vestibular Rehabilitation Benefit Questionnaire and the Vestibular Symptom Index. The longterm impact of the exercises was assessed after 6 months using the same outcome measures. The first 2 outcome measures have been previously described. The Vestibular Rehabilitation Benefit Questionnaire [24] examines the benefits an individual receives from having participated in a vestibular rehabilitation program. The individual’s score on this outcome measure ranges from 0% (maximum benefit because no vertigo remains) to 100% (no benefit because maximum vertigo remains). The Vestibular Symptom Index [20] is a visual analog scale that examines an individual’s vertigo from 0 (no vertigo) to 60 (maximum vertigo). The outcomes of this study may be found in Table 5.
Sugaya et al. [21] completed a study that had an evidence level of 3 and a methodological rigor of 4 (Table 5). In this study, 251 individuals were instructed in a customized vestibular rehabilitation program that included adaptation, substitution, balance and gait exercises. Of the 251 participants, 28 had vestibular migraines, 79 had vertigo and nonmigrainous headaches and 144 had vertigo and no headaches. At the completion of the 1-month program, the short-term impact of the vestibular rehabilitation exercises was assessed with the Dizziness Handicap Inventory and the Frequency of Dizziness tool. The longterm impact of the exercises was assessed after 4 months using the same outcome measures. The first outcome measure has been previously described. The Frequency of Dizziness tool [21] is a visual analog scale that examines an individual’s vertigo from 0 (vertigo is never present) to 8 (vertigo is always present). The outcomes of this study may be found in Table 5.
Discussion
The purpose of this systematic review was to evaluate the effectiveness of vestibular rehabilitation at managing individuals who experience vertigo associated with their migraine headaches. 5 articles [17-21] met the inclusion criteria and were included in the qualitative synthesis.
The Vitkovic et al. [20] study included a group of participants with vestibular migraines (group 1) and a group of participants with a nonmigrainous vestibular disorder (group 2). Although there was no significant difference between the improvement in scores of group 1 and the improvement in scores of group 2 at either the 9 week or 6 month follow-up as measured by 3 participation outcome measures and a visual analog scale, the participants in group 1 both began and ended the study with worse scores than did the participants in group 2. Because of this finding, the authors concluded that “vestibular migraineurs subjectively perceive their symptoms more severely than other patients despite having similar peripheral vestibular function, physical performance and symptom chronicity” [20] (p. 3046). The author’s conclusion is supported by Yavuz et al. [25] who found a positive correlation between the presence of migraine symptoms and the amplification of somatic discomfort. Individuals with migraines also tend to experience high levels of anxiety and depression [25,26]. In addition, they tend to perceive that their quality of life is greatly reduced by their medical condition [25]. This perception is especially true for those individuals who have migraines that are more chronic in nature [27]. Therefore, Vitkovic et al. [20] proposed that “vestibular migraineurs may benefit from the implementation of additional behavioral therapies and a continuance of rehabilitation in order to improve their subjective symptoms to a level equivalent to their nonmigrainous counterparts” (p. 3047).
The Sugaya et al. [21] study included a group of participants with vestibular migraines (group 1), a group of participants with vertigo and non-migrainous headaches (group 2) and a group of participants with vertigo and no headaches (group 3). All 3 groups demonstrated improvements at both the 1 month and 4 month follow up as measured by 1 participation outcome measure and a visual analog scale. However, similar to the results in Vitkovic et al. [20], the participants in group 1 both began and ended the study with worse scores than did the other participants. Therefore, because of the link previously described between the presence of migraine symptoms and the decline in an individual’s quality of life due to increased somatic discomfort, anxiety and depression [25,26], the authors proposed that individuals with vestibular migraines may be more effectively treated through a “combination of vestibular rehabilitation and intervention for emotional distress” [21] (p. 128).
Whitney et al. [17] and Wrisley et al. [18] both included a group of participants with a vestibular disorder and a migraine history. Whitney et al. [17] compared this group to a group of participants with vestibular migraines and Wrisley et al. [18] compared this group to a group of participants with a non-migrainous vestibular disorder. The participants with vestibular migraines tended to demonstrate a worse outcome than did the participants with a vestibular disorder and a migraine history [17]. Whitney et al. [17] stated that “although the patients in the [vestibular migraine] group improved their physical performance, they still felt emotionally handicapped” (p. 1533). In addition, the participants with a vestibular disorder and a migraine history tended to demonstrate a worse outcome than did the participants with a non-migrainous vestibular disorder [18]. Wrisley et al. [18] stated that “even though their physical function was similar to physical function in those without a history of migraine (the participants with a migraine history) perceived that they were more disabled” (p. 486). Despite these findings, both Whitney et al. [17] and Wrisley et al. [18] recommended that vestibular rehabilitation be considered as a viable intervention option for those individuals who experience vertigo in conjunction with their migraine headaches.
The fifth study included in this systematic review [19] found that, in general, the 2 groups of participants with traumatic vestibular migraines demonstrated greater improvements by the conclusion of the investigation than did the 2 groups of participants with idiopathic vestibular migraines. However, the authors of the study admitted that this finding may have been due to the demographic composition of each group. Most of the participants with traumatic vestibular migraines were individuals who had sustained a head injury while participating in a military activity or in an athletic event. Therefore, their motivation, physical conditioning and younger age level may have been the reason why they responded better to the vestibular rehabilitation program than did the individuals whose vestibular migraines were idiopathic in nature.
The most notable strengths of the systematic review were: (1) the Cochrane Library did not contain any similar published systematic reviews; and (2) to decrease the risk of bias while using the evidence level and methodological rigor tools, each included research study was independently examined by the 3 authors. The most notable weaknesses were: (1) only 5 studies [17-21] met the inclusion criteria and were included in the qualitative synthesis; (2) all of the included studies [17-21] possessed an evidence level rating of 3 because n1 of them was a rand omized controlled trial; (3) 4 of the 5 included studies [17-19,21] had weak methodological rigor; and (4) a language bias may have been created by limiting the database search to research studies written in English.
Additional research needs to be completed in this area, especially in terms of rand omized controlled trials that compare the effectiveness of vestibular rehabilitation to the impact of using either a non-vestibular intervention or a sham intervention. Because Vitkovic et al. [20] and Sugaya et al. [21] proposed that behavioral therapies may benefit those individuals who have been diagnosed with vestibular migraines, a randomized controlled trial that compares the effectiveness of a combined vestibular rehabilitation-behavioral therapy approach to the impact of using vestibular rehabilitation al1 should be conducted. Vikovic et al. [20] also implied that future studies should investigate the long-term effects of vestibular rehabilitation. In addition, Whitney et al. [17] and Wrisley et al. [18] suggested that the use of migraine medications may decrease an individual’s sensitivity to head movements and may allow the individual to more fully participate in a vestibular rehabilitation program.
Conclusion
In summary, vestibular rehabilitation was generally more effective for individuals with a non-migrainous vestibular disorder than it was for individuals with vestibular migraines. In addition, it is difficult to draw any definitive conclusions from this systematic review because of the limitations previously menti1d as well as the heterogeneity of the outcome measures used in each of the included studies. However, in all 5 studies [17-21], every group of participants benefitted to some degree from a customized vestibular rehabilitation program. Therefore, vestibular rehabilitation should be seriously considered when treating individuals who experience vertigo associated with their migraine headaches. For an individual to receive full benefits, specific interventions for anxiety and/or depression may need to be incorporated into the customized vestibular rehabilitation program.
References
- Balaban CD (2011) Migraine, vertigo and migrainous vertigo: Links between vestibular and pain mechanisms. J Vestib Res 21: 315-321.
- Olesen J, Bendtsen L, Dodick D, Ducros A, Evers S, et al. (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33: 629-808.
- Pereira CB, Nader SN, Kanashiro AK, DeCarvalho WL (2016) Vestibular migraine: Vestibular symptom may identify different subgroups. Otol Neurotol 37: 281-283.
- Shapire RE (2012) Preventive treatment of migraine. Headache 52: 65-69.
- Marmura MJ, Silberstein SD, Schwedt TJ (2015) The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 55: 3-20.
- Fotuhi M, Glaun B, Quan SY, Sofare T (2009) Vestibular migraine: A critical review of treatment trials. J Neurol 256: 711-716.
- Obermann M, Strupp M (2014) Current treatment options in vestibular migraine. Front Neurol 5: 257.
- Han BI, Song HS, Kim JS (2011) Vestibular rehabilitation therapy: Review of indications, mechanisms and key exercises. J Clin Neurol 7: 184-196.
- Deveze A, Bernard-Demanze L, Xavier F, Lavieille JP, Elziere M (2014) Vestibular compensation and vestibular rehabilitation: Current concepts and new trends. Neurophysiol Clin 44: 49-57.
- Whitney SL, Sparto PJ (2011) Principles of vestibular physical therapy rehabilitation. NeuroRehabilitation 29: 157-166.
- Oxford centre for evidence-based medicine 2011 levels of evidence. Centre for Evidence-Based Medicine.
- Medlicott MS, Harris SR (2006) A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training and biofeedback in the management of temporom and ibular disorder. Phys Ther 86: 955-973.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 151: 264-269.
- Whitney SL, Wrisley DM, Brown KE, Furman JM (2000) Physical therapy for migraine-related vestibulopathy and vestibular dysfunction with history of migraine. Laryngoscope 110: 1528-1534.
- Wrisley DM, Whitney SL, Furman JM (2002) Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 23: 483-487.
- Gottshall KR, Moore RJ, Hoffer ME (2005) Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 11: 81-84.
- Vitkovic J, Winoto A, Rance G, Dowell R, Paine M (2013) Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 260: 3039-3048.
- Sugaya N, Arai M, Goto F (2017) Is the headache in patients with vestibular migraine attenuated by vestibular rehabilitation? Front Neurol 8: 124-130.
- Activities-Specific Balance Confidence Scale (2013) Rehabilitation Institute of Chicago.
- Dizziness Handicap Inventory (2013) Rehabilitation Institute of Chicago.
- Vestibular rehabilitation benefit questionnaire. Rehabilitation Institute of Chicago.
- Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C (2013) Association between somatic amplification, anxiety, depression, stress and migraine. J Headache Pain 14: 53-58.
- Lanteri-Minet M, Radat F, Chautard MH, Lucas C (2005) Anxiety and depression associated with migraine: influence on migraine subject’s disability and quality of life and acute migraine management. Pain 118: 319-326.
- Kim SY, Park SP (2014) The role of headache chronicity among predictors contributing to quality of life in patients with migraine: A hospital-based study. J Headache Pain 15: 68-75.
Citation: Kinne BL, Baker BJ, Chesser BT (2017) The Use of Vestibular Rehabilitation for Individuals with Migraines: A Systematic Review. Otolaryngol (Sunnyvale) 7: 334. DOI: 10.4172/2161-119X.1000334
Copyright: © 2017 Kinne BL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9005
- [From(publication date): 0-2017 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 7970
- PDF downloads: 1035