Review Article Open Access
Tolerabilities of Artemisinin-Based Combination Drugs among Patients with Uncomplicated Malaria in a Tertiary Institution Benin City, Nigeria
| Aghahowa SE1*, Obianwu HO1 and Isah AO2 | |
| 1Department of Pharmacology and Toxicology, University of Benin, Benin City, Nigeria | |
| 2Department of Medicine, School of Medicine, University of Benin, Benin City, Nigeria | |
| Corresponding Author : | Aghahowa SE Department of Pharmacology and Toxicology Faculty of Pharmacy, PMB1154 University of Benin, Benin City, Nigeria Tel: +2348055219550 E-mail: pharma_sea@yahoo.com |
| Received September 08, 2014; Accepted September 29, 2014; Published Octorber 01, 2014 | |
| Citation: Aghahowa SE, Obianwu HO, Isah AO (2014) Tolerabilities of Artemisinin-Based Combination Drugs among Patients with Uncomplicated Malaria in a Tertiary Institution Benin City, Nigeria. Clin Pharmacol Biopharm 3:123. doi:10.4172/2167-065X.1000123 | |
| Copyright:2014 Aghahowa SE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: The policy governing the treatment of malaria in Nigeria was changed in 2005 instituting the Artemisinin-Based Combination Therapies [ACTs] as first-line Drugs instead of Chloroquine due to rapidly developing resistance of Plasmodium falciparum.
Methodology: The tolerability to [ACTs] was assessed in patients using a structured questionnaire and Visual analogue scale. These instruments were distributed systematically among the patients that presented with uncomplicated malaria after obtaining consent from the University of Benin Teaching Hospital, Benin City, Nigeria.
Results: Among the five hundred and twenty patients that participated in the tolerability assessment of four different types of ACTs, male and female ratio was 1.3:1, Mean age 32.06 ± 2.3 years [Mean ± SD]. Artemether-Lumefantrine was the most frequently utilized and most tolerable. Two hundred and five [52.69%] respondents reported with forty-one different types of adverse effects of nine major systemic classes. Body weakness was the most frequent adverse effects reported in 112[54.63%] respondents. Adverse effects were significantly dependent on the type of ACTs used p<0.05; these were found to be most common with Artesunate-Mefloquine. Eleven patients were hospitalized due to very severe Body weakness and Dizziness. These severities were found common with Artesunate-Amodiaquine and Artesunate- Mefloqunie respectively. Despite the adverse effects, four hundred and seventy-six respondents (91.53%) were willing to repeat ACTs another time they have malaria. There were significant differences in symptomatic response on Days 0, 1, 2, 3, 7, 14 and 28 (p<0.05). 93% respondents felt extremely sick on Day 0, 68% had persistent fever on the third day of treatment and 71% had improved response after day 7.
Conclusion: Anecdotal reports showed that ACTs have a modest tolerability, they are therefore recommended for patients that have uncomplicated malaria.
| Keywords |
| Tolerability; Adverse effects; ACTs; Malaria; Policy |
| Introduction |
| In recent years new drug therapies were introduced in the treatment of major tropical diseases. Since large populations are often involved on repetitive exposure as in case of malaria, there is need for close monitoring of possible adverse reactions as suggested [1]. |
| The antimalarial programs in the sub-Saharan African countries are recent examples of public health initiatives where safety has been a priority. Due to the frequent report of resistance to many monotherapeutic antimalarials, up to 106 countries have adopted Artemisinin– Based Combination Therapies as first-line agents for the treatment of uncomplicated malaria [2]. This treatment policy was adopted by the Federal ministry of health Abuja- Nigeria in the year 2005. Following this, many clinicians have imbibed the prescription of ACTs in the treatment of uncomplicated malaria within the country. The major drawback of these new therapies in our environment is the cost. This has been estimated to be ten times more expensive than monotherapies [3]. |
| Although combination therapies are accepted as the rational approach in malaria management in Africa, current evidence of their effectiveness within the African region is limited. There is little or no information on the safety and efficacy of combination treatment in a large populace most especially the high-risk group such as pregnant woman and children [2]. The use of drugs in therapy irrespective of the drugs choice and disease condition can result in tolerable side-effects, and intolerable adverse effects among patients [4]. |
| The scope of this work is to assess the tolerability among individuals that reported with uncomplicated malaria and to validate whether malaria symptoms were potentiated or resolved by Artemisinin-Based Combination Therapies in the University of Benin Teaching Hospital, Benin City Nigeria. |
| Materials and Methods |
| Before the study was carried out the approval was sought and obtained from the Ethical committee in the University of Benin City Teaching Hospital Benin City, Nigeria. Sample size calculated using an adapted formula [5]. Five hundred and twenty patients that met the inclusion criteria were randomly selected and administered a three page structured questionnaire adapted from past authors [4]. Methods of administration were by self-administration and face-face interview for literates and non-literates respectively. The procedures for filling were explained to the participants prior to the exercise. ACTs assessed for tolerability were Artesunate-Lumefantrine, Artesunate-Amodiaquine, Artesunate-mefloquine and Artesunate- Sulfamethoxypyrazine- Pyrimethemine. Duration of study was between January and December 2008. Patients recruited for the study were those that presented with features of uncomplicated malaria such as fever, headache, chills or rigors, general weakness, vomiting, loss of appetite and profuse sweating. They were prescribed ACTs. Patients that withdrew their consent, unable to report back, presenting with intolerable side/adverse effects necessitating hospitalization, emergence of concomitant disease, degeneration to severe malaria, utilization of antimalarials or antibiotics with antimalarial outside the ACTs prescribed and inappropriately filled questionnaire were not included in the study. |
| Visual analogue scale measures developed by previous authors [6] were modified and used as instruments to assess the responses to Artemisinin-Based Combination Drugs. This evaluated whether malaria symptoms were potentiated or resolved during utilization. Meanwhile, two hundred patients that met the inclusion criteria were administered the Visual Analogue Scale measures. All measures were administered by face-to-face interviews to ensure adequate reporting. Respondents were expected to indicate their responses to therapy by marking a perpendicular line the between 0mm and 100mm scale. The scale between 0mm and 100mm scale with extreme adjectives represents a measure between “extremely well and extremely sick”; “extremely calm and extremely restless”; “extremely strong and extremely weak”. These Visual analogue scale measures were administered on Days 0, 1, 2, 3, 7, 14, and 28 of the study. |
| Statistical methods |
| Data generated were grouped as respondents characteristics, types of ACTs used, antimalarials used before policy and pattern of adverse effect reported. Data were entered into Microsoft excel SPSS version 11.0 (SPSS, Inc. Chicago, IL) Where necessary, they were computed as percentages, mean ± SD. Non-parametric data were assessed for significance using Chi-square, parametric data were assessed using student t-test and analysis of variance. p-values less than 0.05 were regarded as significant. |
| Results |
| Among the five hundred and twenty patients that participated in the tolerability assessment of four different types of ACTs, male and female ratio was 1.3:1, Mean age 32.06 ± 2.3years [Mean ± SD]. Artemether- Lumefantrine was the most frequently utilized and most tolerable. Two hundred and five [52.69%] respondents reported with forty-one different types of adverse effects of nine major systemic classes as in Table 1. Body weakness was the most frequent adverse effects reported in 112[54.63%] respondents. Adverse effects were significantly dependent on the type of ACTs used p<0.05; these were found to be most common with Artesunate-Mefloquine. Eleven patients were hospitalized due to very severe Body weakness and Dizziness. These severities were found common with Artesunate-Amodiaquine and Artesunate-Mefloqunie respectively. Despite the adverse effects, four hundred and seventysix respondents (91.53%) were willing to repeat ACTs another time they have malaria. There were significant differences in symptomatic response on Days 0, 1, 2, 3, 7, 14 and 28 (p<0.05). 93% respondents felt extremely sick on Day 0, 68% had persistent fever on the third day of treatment and 71% had improved response after day 7. |
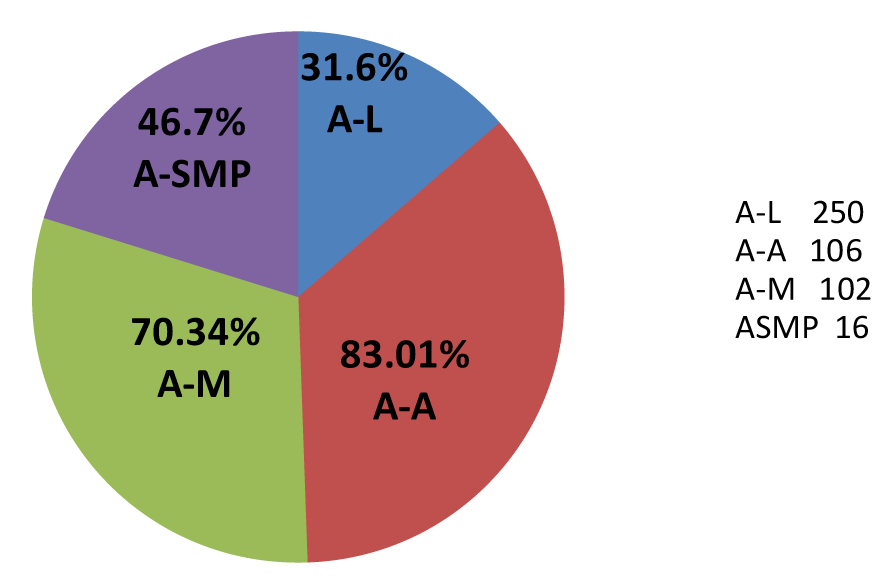
| The number of respondents that used individual ACTs was Artemether-Lumfantrine 250 (48.08%), Artesunate-Amodiaquine 106(21.00%), Artesunate-Mefloquine 145(28.0%), Artesunate- Sulphamethoxypyrazine-Pyrimethamine 19(3.65%). They reported with adverse-effects as Artemether-Lumfantrine 79(31.60%), Artesunate- Amodiaquine 88(83.01%), Artesunate-Mefloquine 102(70.34%) and Artesunate-Sulphamethoxypyrazine-Pyrimethamine 16(84.21%) as in Figure 1. Malaria symptoms were resolved significantly resolved rather than being potentiated during the period of investigation as in Figure 2. |
| Discussion |
| The study showed that Artemether-Lumefantrine was the most tolerable being that least proportion of respondents presented with adverse effects after its use. This may be related to suiTable doses that will not cause harm. This is similar to findings previous studies [7-12]. |
| Other combinations utilized that have shown intolerabilities could be due to high doses in the combinations. Artemisinin derivatives component may have acted in synergy with the other drugs, thus potentiated the reported adverse effects. These adverse effects seem similar to those reported in animal and human subjects when used singly [13]. It is interesting to note that when new agents are introduced into therapy, it does not necessarily mean they are safe even when such have been certified at preliminary phases. There may be need for withdrawal most especially when there are reports of toxicities at postmarketing stage. |
| As seen in this study, few cases of intolerabilities that resulted in hospitalization of affected patients suggest retaining of ACTs for malarial therapy in the environment. The intolerabilities were found to be more common with Artesunate-Mefloquine and Artesunate- Amodiaquine presenting as body weakness and dizziness respectively. All these serious adverse effects were managed by the physicians symptomatically and patients were discharged home within one day. This aspect further suggests that the doses in these combinations should be adjusted in order to improve their tolerance. |
| Serious adverse effects leading to prolongation of hospital stay or deaths not reported in this study could be attributed to the remarkable level of tolerance to ACTs. This can be attributed to the artemisinin derivative components that are known to have wide therapeutic index [13,14]. Although in animals such as rats, dogs, and monkeys, artemether, the methyl ether derivative of artemisinin, has been reported to be associated with an unusual toxicity pattern in specific brain nuclei involving the auditory and vestibular pathways [15,16]. This has not been observed in humans and studies using auditory brainstem responses in patients exposed to artemisinin derivatives [17,18]. |
| Previous studies on tolerability of ACTs have been reports of a meta-analyses pooled from twenty-six different randomized studies of sample size n=10,266 in different regions [19]. In the series, two or three ACTs were assessed and few cases were reported in Nigeria. Among the few cases as reported [20], tolerability studies of Artemetherlumefantrine were reported among few children below ten years in the use of in Benin City. All these had some limitations. Interestingly, this study therefore reports some adverse effects after assessing four ACTs among different age groups. |
| Gastrointestinal upsets that were found to be common with Artesunate-Mefloquine followed by Artesunate-Amodiaquine; this may be related to the direct irritant effect of the drug on mucosal wall, indirect influence on the cytoprotective mechanism of prostaglandin E2 or alteration of the cholinergic function as observed [21]. The study therefore suggests ACTs should be taken after meals to reduce the gastrointestinal effects. Anxiety/confusion that was commonly noted with Artesunate-mefloquine may be due to mefloquine in the combination. This could be related to the neuropsychiatric effect that is common with mefloquine as reported previously [22]. Cardiac adverseeffect that was more common with Artemether-Lumefantrine may be related to Lumefantrine component which is similar to Halofantrine in the prolongation of QTc interval as previously reported [22]. Similar cardiac adverse-effects have been reported with other antimalarials, such as quinine, quinidine, in the prolongation of the QTc interval at therapeutic doses [11,23]. They attributed this effect to the aryl alcohol analogue common in their structures. Patients reporting with body weakness, blurred vision and dizziness as found to be most frequently linked with Artesunate-Mefloquine. Sweating as seen common with Artemether-Lumefantrine may reflect symptomatic relief rather than adverse-effect of the drug. Adverse-effects reported may be related to the primary symptoms of uncomplicated malaria before the onset of therapy as observed by some previous authors [24]. It is worthy to note that malarial symptoms were not potentiated rather they were resolved gradually by ACTs despite the reported adverse-effects seen in early days of initiating therapy. |
| All the adverse-effects were found to be dependent on the type of ACTs utilized by the patient (p<0.05 Chi-square), their significant differences can be attributed to the varied adverse effects that may be peculiar to the individual drugs in the combination. Patients claiming that they would never take ACTs another time they had malaria may be related to the severity of adverse-effects experienced. Higher proportion willing to repeat the drugs may be related to subjective response to efficacy. As a follow up to this study, it is desirable to carry out efficacy study to ascertain the claim by past workers [25]. Patients reported that they had been using chloroquine and sulphadoxine-pyrimethamine as most common monotherapeutic antimalarials before the adoption of the new policy, may be related to the cost and availability of the agents [3]. Report of pruritus, though rare with Artesunate – mefloquine and Artesunate-amodiaquine as in this study, this may be related to the quinoline analogue that is in chloroquine [26,27]. In Nigeria, reports of chloroquine, pyrimethamine-sulfadoxine failures have been documented [28]. Higher proportion of patients withdrawing from their use may be related to their side-effects and poor efficacy associated with the drugs. |
| Reports of adverse-effects as seen in this study showed a pattern of acute toxicities during the period of therapy. There may be other possible adverse-effects that are inherent among these agents during repeated/long term use. The study therefore suggests more exploratory studies involving sampling of biological fluid to know the effect on the major organs. |
| Conclusion |
| ACTs were found to be tolerable among most patients. Therefore ACTs are recommended for the treatment of uncomplicated malaria. |
| Acknowledgements |
| We wish to appreciate the staff of Pharmacy department of the University of Benin Teaching Hospital, Benin City Nigeria, for their contribution in retrieving the questionnaire distributed to respondents. |
| References |
References
- Olsson S (2005) Drug Safety Surveillance a concern for everyone. World Health Organization Essential Drug Monitor 24-25.
- World Health Organization (2010) World Malaria Report 2010. WHO, Geneva.
- Salako L (2006) Chemotherapy of Malaria: A Historical Overview. Keynote Address at the 18th Annual General and Scientific Meeting of the West African Postgraduate College of Pharmacist, Abuja 1-16.
- Whitebread S, Hamon J, Bojanic D, Urban L (2005) Keynote review: in vitro safety pharmacology profiling: an essential tool for successful drug development. Drug Discov Today 10: 1421-1433.
- Bartlett JE, Kotrlik JW and Higgins CC (2001) Organizational Research: Determining Appropriate Sample Size in Survey Research. Information Technology, Learning, and Performance Journal 19.
- Isah AO, Rawlins MD, Bateman DN (1992) The pharmacokinetics and effects of prochlorperazine in elderly female volunteers. Age Ageing 21: 27-31.
- Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, et al. (2005) Efficacy and safety of artemether-lumefantrine (Coartem) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg 99: 459-467.
- Abdulla S, Sagara I, Borrmann S, D'Alessandro U, González R, et al. (2008) Efficacy and safety of artemether/lumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trial. Lancet 372: 1819-1827.
- Hatz C, Soto J, Nothdurft HD, Zoller T, Weitzel T, et al. (2008) Treatment of acute uncomplicated falciparum malaria with artemether/lumefantrine in non-immune populations: a safety, efficacy and pharmacokinetic study. Am J Trop Med Hyg 78: 241-247.
- Van Vugt M, Looareesuwan S, Wilairatana P, McGready R, Villegas L, et al. (2000) Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg 94: 545-548.
- Vugt MV, Wilairatana P, Gemperli B, Gathmann I, Phaipun L, et al. (1999) Efficacy of six doses of artemether-lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg 60: 936-942.
- Lefèvre G, Looareesuwan S, Treeprasertsuk S, Krudsood S, Silachamroon U, et al. (2001) A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg 64: 247-256.
- Hien TT (1994) An overview of the clinical use of artemisinin and its derivatives in the treatment of falciparum malaria in Viet Nam. Trans R Soc Trop Med Hyg 88: S7-S8.
- McGready R, Cho T, Keo NK, Thwai KL, Villegas L, et al. (2001) Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis 33: 2009-2016.
- Brewer TG, Peggins JO, Grate SJ, Petras JM, Levine BS, et al. (1994) Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg 88: S33-S36.
- Petras JM, Kyle DE, Gettayacamin M, Young GD, Bauman RA, et al. (1997) Arteether: risks of two-week administration in Macaca mulatta. Am J Trop Med Hyg 56: 390-396.
- Hien TT, Turner GD, Mai NT, Phu NH, Bethell D, et al. (2003) Neuropathological assessment of artemether-treated severe malaria. Lancet 362: 295-296.
- Kissinger E, Hien TT, Hung NT, Nam ND, Tuyen NL, et al. (2000) Clinical and neurophysiological study of the effects of multiple doses of artemisinin on brain-stem function in Vietnamese patients. Am J Trop Med Hyg 63: 48-55.
- Falade C, Manyando C (2009) Safety profile of Coartem: the evidence base. Malar J 8: S6.
- Aghahowa SE (2010) Safety profile of Artemether-Lumefantrine combination therapy among children below ten years in the University of Benin Teaching Hospital, Benin City, Nigeria. Archives of Applied Science Research 4: 216-223.
- Hoogerwerf WA, Pasricha PJ (2006) Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. Chapter 36, In: Goodman and Gilman's The pharmacological basis of therapeutics, 11th edition, McGraw-Hill 967-981.
- White NJ (1999) Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41: 301-308.
- Nosten F, Ter Kuile FO, Luxemburger C, Woodrow C, Kyle DE, et al. (1993) Cardiac effects of antimalarial treatment with halofantrine. Lancet 341: 1054-1056.
- Bakshi R, Hermeling-Fritz I, Gathmann I (2000) An integrated assessment of the clinical safety of artemether-lumefantrine: a new oral fixed-dose combination antimalarial drug. Trans R Soc Trop Med Hyg 94: 419-424.
- Mårtensson A, Strömberg J, Sisowath C, Msellem MI, Gil JP, et al. (2005) Efficacy of artesunate plus amodiaquine versus that of artemether/lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 41: 1079-1086.
- Sowunmi A, Walker O, Salako LA (1989) Pruritus and antimalarial drugs in Africans. Lancet 2: 213.
- George AO (1996) Chloroquine pruritus: a possible fixed cutaneous reaction. Int J Dermatol 35: 323-324.
- Federal Ministry of Health Nigeria (2005) National Drug Policy on Malaria Treatment. 1-31.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 13876
- [From(publication date):
November-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 9252
- PDF downloads : 4624
