Toxicological Studies of Aqueous Extract of Adenia cissampeloides in Clarias batrachus Fish
Received: 27-Jan-2018 / Accepted Date: 13-Mar-2018 / Published Date: 19-Mar-2018 DOI: 10.4172/2476-2024.1000137
Abstract
The study was aimed at investigating effects of aqueous stem extract of Adenia cissampeloides on selected liver function biomarkers of Clarias batrachus fish. We determined lethal concentration (LC 50) of the extract to the fish; assayed aspartate aminotran sferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP); and determined concentration of unconjugated bilirubin (UB). A total of one hundred and sixty (160) fish of 122 g each on average were used and grouped into five (A, B, C, D and E) in triplicate. For a total of eighths, blood sample was collected from one fish from each group at one hour interval and assayed. The analysis was done using one factor completely randomized ANOVA design. The LC50was 5.00 g/L, 2.50 g/L and 2.50 g/L for 24 h, 48 h and 72 h respectively. The results showed significant (p<0.05) increases in AST activities and concentrations of UB whereas increases in the activities of ALT and ALP were not significant (p>0.05). Large effect size (ω2) of 0.42 and 0.52 for UB and AST, respectively, were obtained. All the means in AST activity and two in UB concentration with the exception of the control were significantly different from each other in the post hoc (Turkey’ HSD) test. AST/ALT ratio of 1.5 indicated cytotoxic damages to liver cells in the fish. The same effects may occur in man; hence, such fish should be properly heat treated or avoided for human consumption.
Keywords: Adenia cissampeloides; Cytotoxic; Biomarkers; Clarias batrachus
Introduction
Adenia cissampeloides is a fish poisoning semi-woody climber plant which contains the toxin modeccin [1]. It belongs to the family PASSIFLORACEAE. Its fresh leaves are used as vegetables in Ngbo, Ebonyi State; in Ivory Coast, the leaf extract is rubbed on the breast after child birth to promote lactation [2]. Leaves and roots are used in the treatment of itching and ringworm, haemorrhoids, sores and wounds [3]. The stem of this plant is used as fish poison (Figure 1). Many plants contain some active principles which can make the fish dizzy or kill the fish out rightly thereby making them easy to catch. Notable among them are saponins and rotenones [4]. Some plants which liberate cyanide in water are also used as fish poison, as well as plants which contain high level of ichthyothereol, triterpene and other ichthyotoxins.
Leaves, stems and bark used as fish poison with varying levels of toxicity include Acacia ditricha , soapy wattle, Alphitomia sp , Eucalyptus Confertiflora , Barringtonia acutagula , Monkshood, Persea americana , Phytolaccaceae americana , Quercus, Asclepiadacene, Zigadenus , Agave, Chlorogalum pomeridianum [5], OlaX and Adenia cissampeloides [6]. Saponins and rotenones as well as cyanide in plants account for nearly all varieties of fish poisons. Sufficient levels of ichthyothereol, triterpene and other ichthyotoxins are also used. The most common use of fishing poisons documented is plant containing saponin, a glycoside poison. Saponin is one of many secondary metabolites abundant in plant species. It is one of the non-ionic surfactants or tensoactive compounds that have a wide range of industrial applications. It is used as wetting agents, emulsifiers, foaming agents and dispersing agents [4].
Saponins are amphipathic glycosides grouped in terms of phenomenology, by the soap-like foaming they produce when shaken in aqueous solutions, and in terms of structure by their composition of one or hydrophilic glycoside moieties combined with a lipophilic triterpene derivative [7]. It undergoes complexation with cholesterol to form pores in cell membrane bilayers leading to red cell lysis [8]. Saponin normally breaks down in the digestive system and must enter the blood stream to be toxic, but fish assimilate saponin directly into their blood stream via their gills. Fish poisoned by saponin become stupefied and float to the surface of water where they can easily be collected [7].
Rotenione is the second major fish poison in plants. Rotenone is otherwise known as flavonoid and is found almost exclusively among legumes especially in the family of FABACEAE [9]. When rotenone is introduced to water by crushing or mashing of the appropriate plant parts (usually roots), fish respiration is damaged and they are forced to gulp the air at the water surface where they are vulnerable it inhibits cellular processes, depriving fish of oxygen in their tissue cells. Complex 1 of the respiratory chain is inhibited leading to neurotoxicity induced by an energetic stress [10]. Tannins are naturally occurring plant polyphenols. Their main characteristic is that they bind and precipitate proteins, although not only tannins bind and precipitate proteins. There are three large classes of secondary metabolites in plants: nitrogen containing compounds, terpenoids and phenolics. Tannins belong to the phenolics class and are formed via the shikimic acid pathway (phenylpropanoid pathway).
Tannins are responsible for astringent taste we experience when we partake of wine or unripe fruits and for enhancing colors of flowers. In microorganisms, tannins have toxic action on membranes, enzyme inhibition, substrate and metal ion deprivation. Hydrolysable tannins poisoning to animals include being hemorrhagic, gastroenteritis, necrosis of the liver, kidney damage, decreased methionine availability, increase toxicity of cyanogenic glycosides, damage to mucosal lining of the digestive tract and low protein utilization [11]. Ichthyothereol is a polyacetylene compound that is toxic to fish. The leaves of Ichthyothere terminalis are inicorporated into baits to be swallowed by fish. This is followed by extreme agitation and death after few minutes to ichthyothereol [12]. Cyanogenic compounds are defense-related secondary metabolites in plants. Cyanogenic glycosides are found in over 2000 species and their structures allow the release of cyanide, a poison produced by certain bacteria, fungi and algae that is found in numerous plants. Cyanide is a chemical compound that contains the cyano group -CON, which consists of a carbon atom triple-bonded to a nitrogen atom. Most cyanide is highly toxic. The cyanide anion is an inhibitor of the enzyme cytochrome c oxidase in the fourth complex of the electron transport chain. It attaches to the iron within this protein.
The binding of cyanide to the cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. The electron transport chain gets disrupted and can no longer aerobically produce ATP or energy. Tissues that depend highly on aerobic respiration such as the CNS and the heart are particularly affected. The most hazardous compound is hydrogen cyanide [13]. Fish poisons affect a number of cellular processes in the fish. They inhibit Na+_K+_ATPase, interfere with oxidative capacity and glutathione metabolism of major organs and nerves; and cause other toxic effects [12,14]. The ancient practice of poisoning fish was an important method of securing food and has continued to flourish in many cultures today. People consume fish produced by this method in almost every part of the world. In Ebonyi State, and possibly in many other parts of Nigeria, the stem extract of Adenia cissampeloides is used as fish poison and such fish are widely consumed in the state. No research evidence is available on the biochemical effect of using this plant as fish poison. Hence, the study aimed at determining the effects of the stem extract on selected biomarkers of liver function such as Aspartate aminotansferase (AST), Alanine aminotransferase (ALT), Alkaline phosphatase (ALP) and Unconjugated bilirubin (UB).
Material And Methods
Extraction
The extraction process was done by soaking 400 g of shredded fresh stem of the plant in 400 ml of distilled water for 24 h. To circumvent the effect of temperature on the active principle during drying, as was the case in the pilot study where both aqueous and ethanol extracts after evaporation could not kill the fish, fresh aqueous extract yield was estimated by soaking 400 g of the shredded fresh stem in 400 ml of distilled water and taking the weight (792 g). The chaff weighed 391.94 g after filtration with Whatman filter paper No 42 (125 mm). By subtracting the weight of water and chaff (783.94 g) from the weight of stem and water (792 g) which gave 8.06 g and dividing by 400 g of the stem, 1 g of the stem gave 0.02 g of the extract. This guided the amount/ quantity of the shredded fresh stem added to the aquaria directly without drying process.
Fish exposure and serum preparation
A total of one hundred and sixty (160) Clarias batrachus fish of average weight of 122 g each were used in the study. Clarias batrachus is the primary fish harvested in the area. Eight fish were exposed to 0.0 g/L 0.625 g/L, 1.25 g/L, 2.5 g/L, 5.0 g/L, 10 g/L and 20 g/L of the stem extract in five aquaria (A, B, C, D, E) for LC50 determination Eight fish were exposed to 0.0 g/L, 0.625 g/L, 1.25 g/L, 2.5 g/L and 5.0 g/L in five different aquaria each in triplicate for determinations of effects on biomarkers. Blood was collected from one fish taken from each aquarium at one hour interval during the eight hour exposure for serum preparation.
Determination of aspartate aminotransferase (AST) in serum:
Principle:
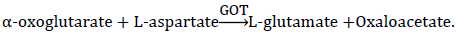
AST was measured by monitoring the concentration of oxaloacetate hydrazone formed with 2,4-dinitrophenylhydrazine. A red color is produced on the addition of sodium hydroxide (0.4 Mol/L). Intensity of color is related to enzyme activity [15,16].
Procedure: AST kits manual (source: Randox Laboratories Ltd, UK).
AST activity (u/L) was obtained from AST Standard graph of activity vs. absorbance.
Determination of alanine aminotransferase (ALT) in serum
Principle:
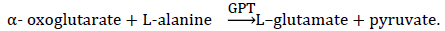
Glutamic–pyruvic transaminase was measured by monitoring the concentration of pyruvate hydrazone formed with 2, 4- dinitrophenylhydrazine [15,16].
Procedure: ALT kits manual (source: Randox Laboratories Ltd, UK). ALT activity (u/L) was obtained from ALT Standard graph of activity vs. absorbance
Determination of alkaline phosphatase (ALP) in serum
Principle:
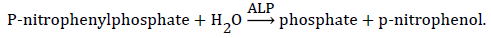
Procedure: ALP kits manual (source: Randox Laboratories Ltd, UK). ALP activity (u/L)=2760 × change in absorbance at 405 nm/min.
Determination of total and direct bilirubin in serum
Principle: Direct (conjugated) bilirubin reacts with diazotized sulphanilic acid in alkaline medium to form a blue colored complex. Total bilirubin is determined in the presence of caffeine, which releases albumin bound bilirubin by the reaction with diazotised sulphanilic acid [17].
Procedure: Bilirubin kits manual (source: Randox Laboratories Ltd, UK). Total bilirubin concentration (mg/l)=10.8 × Absorbance TB (578 nm). Direct bilirubin concentration (mg/dl)=14.4 × Absorbance DB. Unconjugated bilirubin (UB) concentraion (mg/dl)=Total bilirubin concentration–Direct bilirubin concentration.
Data Analysis
Analysis of variance (ANOVA): one factor completely randomized design was used. The causative relationship between the independent variable (extract) and dependent variable (activity/concentration of the biomarkers over time) was determined. This is because there is only single independent variable (extract of Adenia cissampeloides ) with five levels or conditions (groups). The focus was upon different types of variance in the groups. Hence, a null hypothesis was tested which stated that there was no difference among the five group means. The null hypothesis was rejected when the derived F-value exceeded the tabled critical value of F or otherwise accepted. When the null hypothesis was rejected the effect size, omega–squared (ω2), which is the measure of the magnitude of the effect of independent variable upon the dependent variable, was determined and multiple comparison test (Turkey’s Honestly Significant Difference, HSD) was used to tell which of the means were significantly different from each other [18].
Results And Discussion
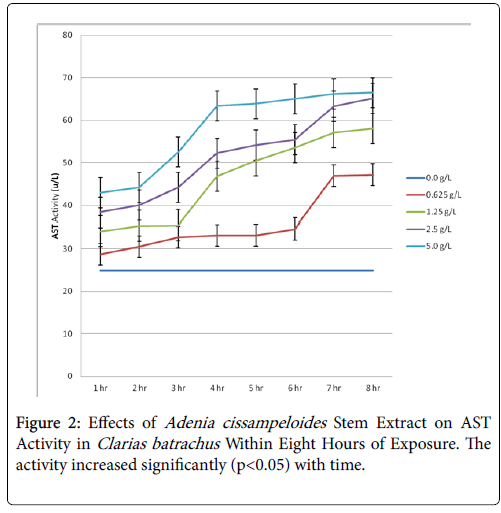
The lethal concentration (LC50) of the stem extract to the fish was 5.00 g/L, 2.50 g/L and 2.50 g/L for 24 h, 48 h and 72 h respectively. This guided the amount of extract that the fish were exposed to (Table 1). The results showed significant increases in AST activities which were both time and concentration dependent (Figure 2).
| Number of dead fish | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time interval | Total fish | 0.00 g/L | 1.25 g/L | 2.50 g/L | 5.00 g/L | 10.00 g/L | 20.00 g/L | LC 50 |
| 24 h | 48 | 0 | 0 | 2 | 4* | 8 | 8 | 5.00 g/L |
| 48 h | 48 | 0 | 1 | 4* | 6 | 8 | 8 | 2.50 g/L |
| 72 h | 48 | 0 | 2 | 4* | 6 | 8 | 8 | 2.50 g/L |
*where half population of the fish died in each group indicating LC50
Table 1: Lethal Concentration (LC50)
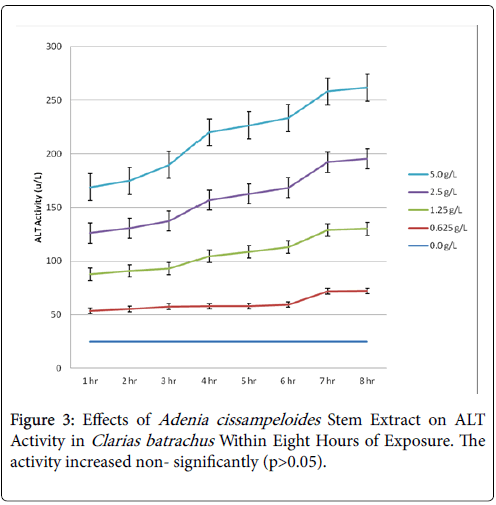
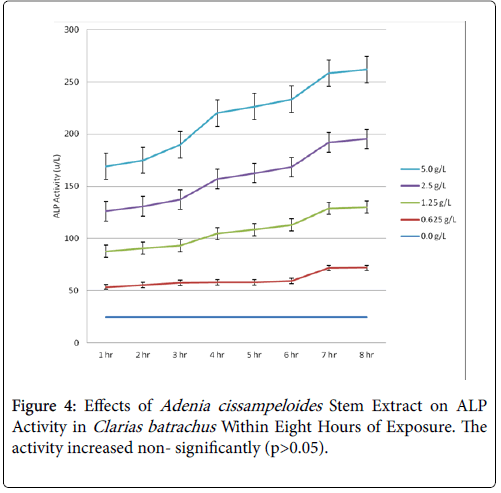
Within the first one hour of exposure, the activity increased from 17.52 u/L of the control to 46.25 u/L of the highest concentration of extract (5.0 g/L) and increased to 76.0 u/L in eighths. The post hoc test showed that all the means of the activities of AST with the exception of the control were statistically significant (P<0.05) in the test since the Turkey’ HSD critical value (19.98) was exceeded by the absolute values of those means. There was increase in ALT activity, though not statistically significant (p>0.05) (Table 2 and Figure 3). However, AST/ALT ratio of 1.5 obtained was indicative of damage to the liver cells causing release of membrane bound AST, reaction which is normally irreversible. This could be attributed to the toxic effects of the extract on the liver of the exposed fish (Figure 4).
| Parameter | Source | Some of squares | D f | Mean squares | F-cal | F-tab | Ho | Ώ2 |
|---|---|---|---|---|---|---|---|---|
| AST | Treatment | 8984.681 (SSB) | 4 | 2246.170 (MSB) | 11.64* | 2.64 | Rejected | 0.52 |
| Error | 6753.250 (SSW) | 35 | 192.950 (MSW) | - | - | - | ||
| Total | 15737.931 (SST) | 39 | - | - | - | - | ||
| ALT | Treatment | 515.925 | 4 | 128.981 | 0.99 | 2.64 | Accepted | - |
| Error | 4544.05 | 35 | 129.83 | - | - | - | ||
| Total | 5059.98 | 39 | - | - | - | - | ||
| ALP | Treatment | 747.506 | 4 | 186.877 | 0.06 | 2.64 | Accepted | - |
| Error | 102370 | 35 | 2924.85 | - | - | - | ||
| Total | 103117 | 39 | - | - | - | - | ||
| UB | Treatment | 0.438 | 4 | 0.11 | 8.46* | 2.64 | Rejected | 0.43 |
| Error | 0.471 | - | - | - | - | - | ||
| Total | 0.909 | 4 | - | - | - | - |
Table 2: Analysis of variance on the parameters.
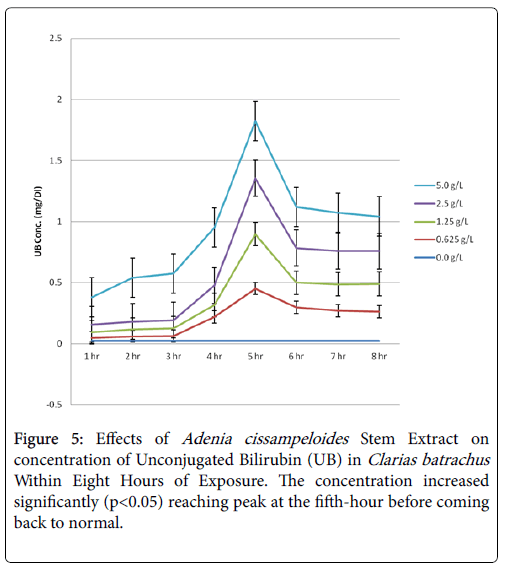
Equally, the concentration of unconjugated bilirubin (UB) increased significantly (p>0.05) with time of exposure reaching peak at the fifth before coming back to normal (Figure 5). The means of UB concentrations in the two highest extract concentrations exceeded HSD critical value (0.164) (Table 3). It suggests that hemoglobin was being destroyed or the liver was not actively treating the hemoglobin it received, however, it regained its functionality after the sixth. This agrees with the result in elevated AST activity. Increase in unconjugated bilirubin concentration up to 0.473 mg/dl coupled with AST/ALT ratio of 1.5 indicated liver damage [19,20].
| Parameters | 0.000 g/L | 0.625 g/L | 1.250 g/L | 2.500 g/L | 5.000 g/L | HSD Critical value |
|---|---|---|---|---|---|---|
| AST | 17.500 | 41.750* | 52.750* | 54.781* | 67.813* | 19.980 |
| ALT | 20.500 | 33.938 | 41.788 | 45.213 | 47.450 | - |
| ALP | 24.820 | 35.859 | 47.345 | 51.609 | 58.059 | - |
| UB | 0.024 | 0.185 | 0.170 | 0.205* | 0.355* | 0.164 |
Table 3: Tukey’s HSD.
These effects on the fish correlate the report that active principles in plants such as rotenone interfere with respiration by inhibiting the protective mechanisms which minimizes the toxic potential of oxygen intermediates; saponin has asphyxiation action while cardiac glycosides affect central nervous system and nerve mechanisms of heart [10]; alkaloids have neurologic effects, inhibit Na+-K+ ATPase, DNA polymerase, cytochrome P-450 system and cause damage to the liver cells; and tannins cause enzyme inhibition, substrate and metal ion deprivation and other slow acting effects [21].
Above all, the results revealed large effect size (ω2) of the extract on AST and UB as 0.52 and 0.43 respectively. There was no determination of effect size for ALT and ALP as they were not statistically significant (p>0.05).
Conclusion
The activities of AST increased significantly (p<0.05) with increasing concentration of the extract and with increasing time of exposure. Increases in the activities of ALT and ALP were not significant (p>0.05).
The concentration of unconjugated bilirubin increased but started reducing after 5 hours in all the extracts. Higher levels of unconjugated bilirubin (UB) indicated that too much hemoglobin was being destroyed or that the liver was not actively treating the hemoglobin it received; increased level of AST indicated damages to liver cells and tissues.
The destruction of hemoglobin and disruption of conjugation of bilirubin in the liver by phytochemicals from the extract might have elicited cytotoxic reactions and subsequent poisoning of the fish.
Recommendations
It is possible that the same effects may occur in man in a chronic manner if fish killed with Adenia cissampeloides is consumed; the extent of consumption and frequency of which are factors. Hence, proper cooking (heating) is required to denature the phenolics before eating such fish to avoid extended effects to humans.
References
- Hutchings A, Scott AH, Lewis G, Cuningham AB (1996) In: Hutchings A (eds.) Zulu Medicinal plants an inventory. Scottsville University of Natal Press, Scottsville, South Africa pp: 450.
- Neuwinger HD (2004) Plants used for poison fishing in tropical Africa. Toxicon 44: 417-430.
- Mabberley DJ (1997) In: G Natho (eds.) The Plant-Book. A portable dictionary of the vascularplants. (2nd ed) Cambridge University Press, Cambridge pp: 858.
- Bearez P (1998) Focus: First Archaeological Indication of Fishing by poisoning in a sea Environment by Engoroy population at Salango (Manabi Ecuador). J Arch Sci 25: 943-948.
- Rashtra V (2006) Floristic plants of the world-Amazon. Sarup & Sons, Delhi, India pp: 949.
- Brandt HD, Muller GJ (1995) Traditional Medicines and acute poisoning. J Med Edu 13: 1053-1060.
- Hostettmann K, Marston A (1995) In: GR Walle (eds.) Saponins: chemistry and pharmacology of natural products. Cambridge University Press, Cambridge, United Kingdom pp 8509-8511.
- Francis G, Zohar K, Harinder PS, Makkar HP, Klans B (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88: 587-605.
- Van-Andel T (2000) The diverse uses of fish-poison plants in North-west Guyana. Eco Botany 54: 500-502.
- Prance A, Balick J (1990) The occurrence of piscides and stupefactants in the plant kingdom. Adv Eco Botany 8: 256-273
- Hagerman AE, Klucher KM (1986) Tannin-protein interaction In: Cody V, Middleton Jr E, Harbone J-Alan, R. Liss (eds.) Plant flavoniods in biology and medicine: biochemical, pharmacological and structure-activity relationship. John Wiley & Sons, New York pp 67-76.
- Ray DE (1991) Pesticides derived from plants and other organism. In: Kamita GS, Casida SG (eds.) Handbook of Pestic, J.E. (2001).
- Biller J (2007) Interface of neurology and internal medicine (illustrated). Lippicott Williams and Wilkins, Philadelphia, pp 939.
- Ratra GS, Kamita SG, Casida JE (2002) In: Ratra GS (eds.) Role of human GABA Receptor beta 3 submitide Toxiciology. New york Academic press, New York, USA pp: 7-10.
- Ratra GS, Kamita SG, Casida JE (2002) Role of Human GABA Receptor β3 Subunit in Insecticide Toxicity. Toxicol Appl Pharmacol 172: 233-240.
- Recitman S, Franked S (1957) Assay of transaminase. Am J Clin Pathol 28: 56-63.
- Schmidt E, Schmidt FW (1963) Assay of transaminase. Enzymol Biol Clin J 3: 1.
- Jendvassik L, Grof P (1938) Bilirubin assay in liver disease. Biochemistry Z297: 81.
- Frederick LC (2002) Statistics: A gentle introduction (3rd edn) SAGE Publications Ltd, London. pp: 172-196.
- Price CP (1993) Multiple forms of human serum alkaline phosphatase: Detection and Quantitation. Ann clin Biochem 30: 355-372.
- Nyblom H, Bjornsson E, Simren M, Aldenborg F, Almer S et al. (2006) The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 26: 840-845.
Citation: Emeji EO, Ibiam UA (2018) Toxicological Studies of Aqueous Extract of Adenia cissampeloides in Clarias batrachus Fish. Diagn Pathol Open 3: 137. DOI: 10.4172/2476-2024.1000137
Copyright: © 2018 Emeji EO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4626
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 3724
- PDF downloads: 902