Triptolide Induces Cell Damage in Lipopolysaccharide (LPS)-Treated Macrophages in an LPS-Signaling Cascade-Dependent Manner
Received: 16-Aug-2017 / Accepted Date: 24-Aug-2017 / Published Date: 31-Aug-2017 DOI: 10.4172/2576-3881.1000117
Abstract
Triptolide, a Chinese plant medicine from Tripterygium wilfordii Hook F, has been shown to have inhibitory effects on macrophage activities, and we recently found that it induces sudden macrophage cell death in the presence of bacterial lipopolysaccharide (LPS). In this present study, we examined precise mechanisms underlying induction of the cytotoxicity of triptolide toward macrophages in relation to the action of LPS, and thereby showed that the cytotoxic effects depended on the concentrations of both triptolide and LPS. More than 10 ng/mL LPS was necessary in combination with 300 ng/mL triptolide. However, pre-treatment with 1 ng/mL LPS for 60 min abolished the cytotoxicity induced by 100 ng/mL LPS and 300 ng/mL triptolide, showing that the cytotoxicity was regulated by LPS-tolerance. Besides, in primary macrophages obtained from mouse peritoneum, those from C3H/HeN mice, an LPS-responder, showed similar susceptibility to triptolide and LPS-induced cell damage; whereas those from C3H/HeJ mice, an LPS hypo-responder, did not, suggesting that the cytotoxic effect of triptolide was linked to the LPS/Toll-like receptor 4 (TLR4)-signaling cascade. These results suggest that the cytotoxicity of triptolide toward macrophages was regulated by the LPS-signaling cascade through both down-regulation known as LPS-tolerance and the TLR4 receptor.
Keywords: Macrophage cell death; Triptolide; Lipopolysaccharide (LPS); Apoptosis; C3H/HeN mouse; C3H/HeJ mouse
6888Introduction
Macrophages are known to play important roles in host-defense mechanisms, including anti-bacterial, anti-viral, and other antimicrobial actions as well as participate in the regulation of immune reactions and inflammation [1]. Many of these macrophage functions, such as the production of inflammatory cytokines, reactive oxygen species (ROS), nitric oxide (NO), and prostaglandins (PGx), are characteristics of the so-called activated-macrophage phenotypes, which are readily induced by bacterial lipopolysaccharide (LPS), one of the major components of Gram (-) bacteria [2]. These effector molecules from LPS-activated macrophages show cytotoxic effects not only toward pathogens, degenerated cells or tumor cells but also toward the macrophages themselves [3]. However, if LPS is added to macrophages in the presence of cycloheximide (CHX), a protein synthesis inhibitor, the cells rapidly become damaged through apoptosis without macrophage activation [4]. Extensive studies on the LPS and CHX-induced rapid cell death have shown that inhibited synthesis of certain proteins that should be immediately induced by LPS-treatment seems to be involved in the induction of apoptosis [5,6]. Besides, our recent studies have revealed that shikonin, a Chinese plant medicine from Lithospermum erythrorhizon [7], and triptolide, a Chinese plant medicine from Tripterygium wilfordii Hook F [8], both induce apoptotic cell death in a mouse macrophage-like cell line, J774.1/JA-4 cells, in the presence of LPS but without inhibited protein synthesis [9]. Our preceding paper suggested the involvement of Mapkinase phosphatase-1 (MKP-1) in the regulation of LPS+triptolideinduced macrophage cell death in J774.1/JA-4 cells, which study showed that the induction/synthesis of MKP-1 by LPS was strongly inhibited by triptolide or CHX and that sustained phosphorylation of p38 MAPK accompanied the inhibition of MKP-1 induction/synthesis by these reagents [9,10].
However, the mechanism underlying the induction of the cytotoxicity of triptolide toward macrophages in relation to the action of LPS has been remained to be elucidated. In this present study using J774.1/JA-4 macrophage-like cells and peritoneal macrophages from C3H/HeN and C3H/HeJ mice, we examined precisely this mechanism.
Materials and Methods
Reagents
Escherichia coli (055: B5) LPS was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.), and Ham’s F-12 and fetal bovine sera were purchased from Life Technologies (Carlsbad, CA, U.S.A.). Penicillin– streptomycin mixed solution came from Nacalai Tesque (Kyoto, Japan); and triptolide, from Tripterygium wilfordii.
Cell culture
Culturing of the JA-4 cell line, an LPS-sensitive subline of the murine macrophage-like cell line J774.1 was performed as described previously [11]. In brief, the cells were cultured in Ham’s F-12 medium containing 10% (v/v) heat-inactivated fetal bovine serum (FBS), 50 U of penicillin, and 50 μg of streptomycin per mL, and were maintained in culture dishes (Falcon #1001, Corning, NY, U.S.A.)) under a humidified atmosphere with 5% CO2 at 37°C in a CO2 incubator. The cells were maintained as described above, and were used for various assays after several passages and before the 25th passage from the time of defrosting of the stored cells in order to maintain stable phenotypes of the cells [11].
Cell toxicity assay
The cell toxicity assay used was performed as described previously [4,9,12]. In brief, cells were harvested and seeded at 1 × 105 cells/0.25 mL per well onto 48-well plates (Corning #3548, Corning, NY, U.S.A.) and incubated at 37°C for about 4 h. Then the medium was exchanged for 0.25 mL of fresh medium, after which triptolide or CHX was added to the cultures, followed by incubation with or without 100 ng/mL LPS at 37°C, usually for 240 min. Next, the cells were chilled on ice to terminate the cultures. The culture supernatants were collected into microfuge tubes and centrifuged at 10,000 rpm for 1 min at 4°C. The resultant supernatants were used to assess lactate dehydrogenase (LDH) activity as a cytosolic enzyme marker whose release into the culture supernatant corresponds to cell damage [4,9,12]. The assay was performed by using an LDH-Cytotoxic Test Wako (Wako Pure Chemicals, Osaka, Japan) according to the manufacturer’s protocol. Absorbance was measured at 450 nm with subtraction at 620 nm by using a MultiSkan FC (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.). Culture supernatants collected at 0-time incubation were used to determine the background release of LDH. To determine the total LDH activity, we added Triton X-100 at a final concentration of 0.1% to the cultures of non-treated cells at the end of the incubation at 37°C for 240 min, and then incubated them for an additional 30 min at 37°C for complete lysis of the cells. Cytotoxicity was expressed as % of the total activity according to the following formula:
% of total={(experimental release)–(background release)/total activity} × 100
Preparation of mouse peritoneal macrophages
Preparation of mouse peritoneal macrophages was performed as described before [13]. In brief, female C3H/HeN and C3H/HeJ mice, 6 weeks of age, were obtained from Japan SLC, Inc., (Hamamatsu, Shizuoka, Japan) as SPF mice. The mice were sacrificed by dislocating the cervical vertebra, and peritoneal resident macrophages were immediately collected by injecting sterile ice-cold saline (Otsuka Pharmaceutical Co., Tokushima, Japan) into the peritoneal cavity, followed by massaging and re-collection of the peritoneal washing fluid. The peritoneal cells were washed with ice-cold saline by centrifugation at 2,000 rpm for 3 min, and then the cell pellets were suspended in ice-cold 5 mL of 1/3 concentration of saline/H2O on ice for 1 min, followed by the addition of ice-cold 5 mL of saline to rupture contaminating red blood cells. After centrifugation at 2,000 rpm for 3 min, the cell pellets were suspended in F-12 culture medium containing 10% FBS and 50 U of penicillin and 50 μg of streptomycin/ mL. The number of cells was counted with a hemocytometer, and more that 98% of the cells were macrophages as revealed by staining with Giemsa stain (Merck). The cells were seeded onto 48-well plates as described in section 2.3, but were incubated at 37°C for 1 h to obtain the adherent cells (i.e., macrophages); and the non-adherent cells, including lymphocytes, were removed by thorough washing with the culture medium. Cytotoxicity assays were performed as described in section 2.3. All animal experiments complied with the approved animal care protocols of the Osaka University of Pharmaceutical Sciences.
Induction of LPS-tolerance in J774.1/JA-4 macrophages
LPS-tolerance assay was performed as described previously [12]. In brief, cells were seeded and incubated at 37°C for about 4 h, as described in section 2.3. The medium was exchanged for 0.25 mL of fresh medium containing 0, 1, or 10 ng/mL LPS, followed by incubation at 37°C for 60 min. Then the cells were rinsed twice with the culture medium, and incubated in the fresh medium containing nothing, 100 ng/mL LPS and/or 300 ng/mL triptolide at 37°C for 240 min. Cell damage was estimated by determining LDH release as described above.
Morphologic observation
Cells were observed under an Olympus CKX41 phase-contrast microscope, and photographs were taken in random fields of each sample with original magnification × 100.
Statistical analysis
Results of the experiments using J774.1/JA-4 cells were expressed as the means ± S.E.M. of at least 3 experiments performed. Data for 2 groups were analyzed by using Student’s t-test, and data for more than 2 groups were compared by using one-way ANOVA with Bonferroni multiple comparisons as a post hoc test (Pharmaco basic software Ver. 15; Three S, Tokyo, Japan). Statistical significance was set at p
Results
Induction of cell damage in J774.1/JA-4 macrophages treated with triptolide and LPS
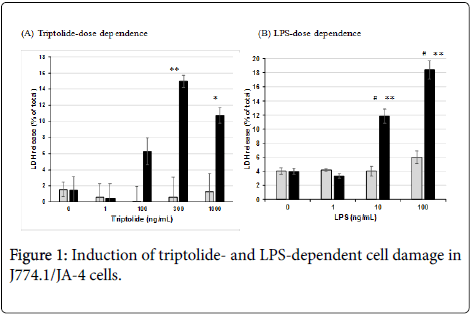
When J774.1/JA-4 macrophages were treated with 0, 1, 100, 300 or 1000 ng/mL triptolide at 37°C for 30 min, and then incubated without or with 100 ng/mL LPS followed by further incubation at 37°C for 240 min, the cells became damaged dependent on triptolide doses; and about 15% and 11% of the total LDH was significantly released in the presence of LPS (Figure 1A). However, no such damage was observed without LPS addition. In addition, the cells pre-incubated with 300 ng/mL triptolide showed cell damage dependent on the LPS dose; and more than 10 ng/mL LPS, but not 1 ng/mL LPS, induced significant cell damage (Figure 1B). These results showed that the induction of cell damage to J774.1/JA-4 macrophages was dependent on the concentration of the both triptolide and LPS and that the presence of the both was necessary for the induction.
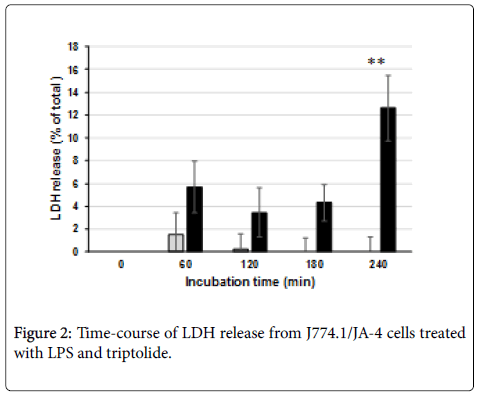
As for the time-course of the induction of the cell damage, J774.1/ JA-4 macrophages treated with 300 ng/mL triptolide alone showed little cell damage throughout the incubation period; but those treated with both 300 ng/mL triptolide and 100 ng/mL LPS showed slight, but not significant, cell damage by 180 min after LPS addition. However, the damage was significantly increased by 240 min (Figure 2). Similar results were obtained in the preceding study not only in terms of LDH release but also in terms of the population of TUNEL (+) cells [9].
Induction of LPS-tolerance to the cell death of the macrophages treated with LPS and triptolide
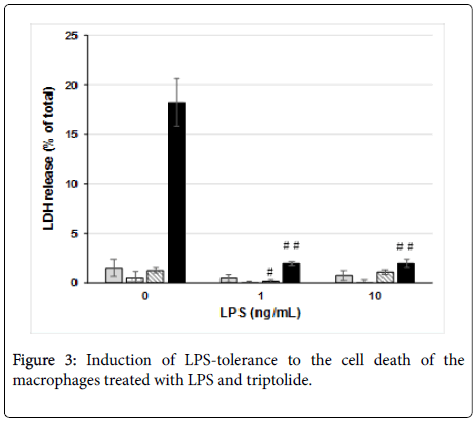
In order to know whether the cell damage induced by triptolide and LPS was regulated by the LPS-signaling cascade, we examined LPStolerance by using J774.1/JA-4 macrophages. Pre-incubation of the cells with 1 or 10 ng/mL LPS at 37°C for 60 min abolished the cell damage induced by 300 ng/mL triptolide and 100 ng/mL LPS (Figure 3). However, pre-incubation of the cells without LPS did not, showing that this phenomenon was LPS dependent. These results suggest that the cell damage induced by triptolide and amounts of LPS higher than 10 ng/mL (Figure 1B) were regulated by the LPS-signaling cascade through down-regulation, referred to as “LPS-tolerance” [14].
Effects of triptolide on LPS-treated macrophages from C3H/HeN and C3H/HeJ mice
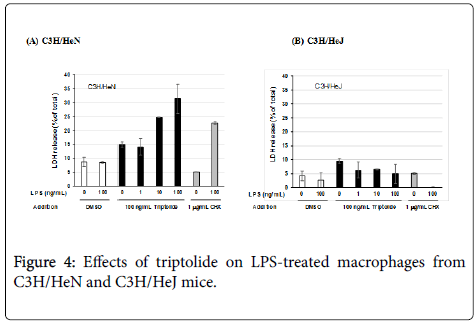
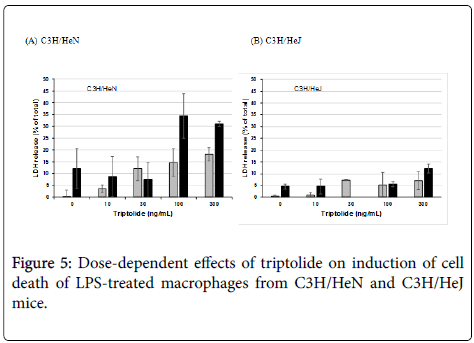
Induction of the cell damage caused by triptolide and LPS was also examined by using resident peritoneal macrophages from C3H/HeN and C3H/HeJ mice in order to know whether the cell damage was not limited to J774.1/JA-4 macrophage-like cells but could also be reproduced in primary mouse macrophages as well. Although the optimal dose of triptolide was different for these primary macrophages (100 ng/mL; Figure 5A) from that for J774.1/JA-4 cells (300 ng/mL; Figure 1A), the macrophages from the C3H/HeN mice showed cell damage after treatment with triptolide and LPS at more than 10 ng/mL (Figure 4A), whereas those from C3H/HeJ mice did not (Figure 4B). The different susceptibility of these macrophages to LPS and CHXinduced cell damage was also observed in these experiments, as reported previously [13].
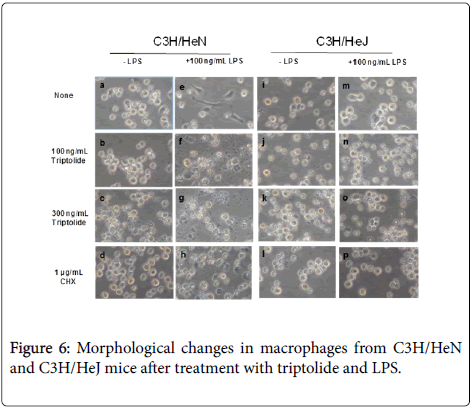
To examine the effective concentrations of triptolide, the primary macrophages were treated with 0, 10, 30, 100 and 300 ng/mL triptolide in the presence or absence of 100 ng/mL LPS. Although a little LDH release, around 12-18%, was seen in the macrophages from C3H/HeN mice after treatment with more than 100 ng/mL triptolide alone, much more release, about 30-35%, was observed in combination with 100 ng/mL LPS, showing that the optimal concentration of triptolide for the cytotoxicity was 100 ng/mL (Figure 5A). To the contrary, the macrophages from C3H/HeJ mice did not show cell damage after treatment with both more than 100 ng/mL triptolide and 100 ng/mL LPS ((Figure 5B). Besides, morphological changes in the primary macrophages also supported the results shown in Figures 4 and 5: As seen in Figure 6, the macrophages from C3H/HeN mice without any additive (a) showed round and adherent shapes, which became elongated by the addition of LPS alone (e). Although the addition of 100 ng/mL triptolide elevated the number of shrunken cells (b), that of both 100 ng/mL triptolide and 100 ng/mL LPS induced apparent cell damage, as indicated by the swollen and ruptured morphology (f). More severe cell damage was observed in the cells after treatment with 300 ng/mL triptolide alone (c) and in those with both 300 ng/mL triptolide and 100 ng/mL LPS (g). Instead, although the macrophages from C3H/HeJ mice without any additive (i) showed round and adherent shapes, they did not become elongated after incubation with LPS (m). These macrophages showed few changes after treatment with 100 or 300 ng/mL triptolide alone (j, k) or with both triptolide and LPS at these concentrations (n, o). As the controls, the addition of 1 μg/mL CHX alone induced round but non-damaged cell shapes in these macrophages from either strain of mice (d, l). The addition of both 1 μg/mL CHX and 100 ng/mL LPS induced severe cell damages with round and swollen shapes in the macrophages from C3H/HeN mice (h) but not in those from C3H/HeJ mice (p).
These results in Figures 4-6 showed that the cell damage induced by triptolide and LPS were seen in the macrophages from an LPSresponsive strain, C3H/HeN mice, but not in those from an LPS-hyporesponsive strain, C3H/HeJ mice, suggesting the regulation of the cytotoxicity by the LPS-signaling cascade acting through the LPS/ TLR4 pathway.
Discussion
Triptolide, a diterpene triepoxide, a major component of Tripterygium wilfordii Hook F [8], is known as a Chinese plant medicine with multiple pharmacological functions [15], including anti-inflammatory [16], anti-cancer [17], and anti-tumor metastatic [18] activities. We recently found that triptolide induces sudden macrophage cell death in the presence of bacterial lipopolysaccharide (LPS) [9]. In the preceding study using J774.1/JA-4 macrophage-like cells, we showed that triptolide induces apoptotic cell death and subsequent cell damage accompanied by LDH release, inhibits the induction of MKP-1 mRNA and protein, and exhibits sustained phosphorylation of p38 and JNK, suggesting a correlation among themselves and also implying that MKP-1 was the target of triptolide, which phosphatase is responsible for regulation of the LPS-signaling cascade that leads to sustained phosphorylation of p38 and subsequent apoptotic cell death of macrophages [9]. However, the mechanisms underlying the induction of the cytotoxicity by triptolide toward macrophages in relation to the action of LPS have been remained largely unknown.
In this study, we focused on the regulation by the action of LPS; Using J774.1/JA-4 macrophage-like cells, we examined dose-responses of triptolide (Figure 1A) and LPS (Figure 1B) and found that 300 ng/mL was the optimal concentration of triptolide and that more than 10 ng/mL LPS was necessary to induce the cell damage to the macrophages pre-incubated with 300 ng/mL triptolide. Because significant cell damage was observed in these macrophages only at 240 min after the addition of LPS (Figure 2), cell toxicity assessed in terms of LDH release was quantitatively estimated under these conditions in subsequent experiments.
LPS-tolerance is known to be one of the most remarkable phenotypes of macrophages and is closely linked to down-regulation of the LPS-signaling cascade [19]. As shown in the results in Figure 3, pre-culture of J774.1/JA-4 cells with 1 or 10 ng/mL LPS at 37°C for 60 min significantly and almost completely abolished the cell damage subsequently induced by 300 ng/mL triptolide and 100 ng/mL LPS. Because 1 ng/mL LPS failed to induce the cell damage in combination with 300 ng/mL triptolide (Figure 1B), the results shown in Figure 3 suggested that LPS-tolerance was efficiently induced in J774.1/JA-4 macrophage-like cells through the LPS-signaling cascade. It is not certain whether this LPS-tolerance was caused by induction of MKP-19 or not, and this is an important point to be addressed in the future.
In this study, we also used peritoneal macrophages from C3H/HeN and C3H/HeJ mice, because it had not been known whether the cell damage induced by triptolide and LPS is regulated by the LPSsignaling cascade acting through LPS/TLR4. C3H/HeJ mice are TLR4- deficient [20] and show hypo-responsiveness to LPS. In our previous study [13] and also in the present study (Figure 4), primary macrophages from C3H/HeJ mice showed much reduced responses to the cytotoxicity induced by 1 μg/mL CHX and 100 ng/mL LPS, compared with those from C3H/HeN mice, which are the wild-type mice of C3H/HeJ. Treatment with 100 ng/mL triptolide and 10 or 100 ng/mL LPS showed cell damage to peritoneal macrophages from C3H/HeN mice, whereas such treatment of C3H/HeJ mice did not (Figures 4-6). These results suggest that the cell damage to macrophages induced by triptolide and LPS was regulated by the LPSsignaling cascade acting through LPS/TLR4.
The effects of triptolide on the apoptotic cell death of macrophages were reported by Bao et al. [21]: In the absence of LPS, 15-25 ng/mL triptolide increased the generation of ROS and NO, and induced apoptosis of RAW264.7 cells during incubation at 37°C for 24 h. Wu et al. showed attenuation of oxidative stress and proinflammatory cytokine gene expression in LPS-treated mouse peritoneal macrophages incubated with 5-40 ng/mL triptolide at 37°C for 24 h, but they did not examine macrophage cell damage under their experimental conditions [22] Choi et al. reported apoptosis of human U937 monocyte cell line through activation of caspase-3 by 9-27 ng/mL triptolide during incubation at 37°C for 24 h in the absence of LPS [23]. Although these reports described the involvement of triptolide in macrophage cell damage and/or cell death, there are few reports concerning the induction of cell damage or apoptosis elicited by the combination of triptolide and LPS that we found in our previous study [9] and in the present one. It might be because the conditions of the experiments were different, including the length of incubation, coincubation with LPS, and the concentration of triptolide. For example, more than 300 ng/mL and 100 ng/mL triptolide was needed to induce cell damages in J774.1/JA-4 cells (Figure 1) and in peritoneal macrophages from C3H/HeN mice (Figure 5A), respectively, in combination with more than 10 ng/mL LPS (Figure 1B), and (Figure 4A). Besides, diversities of macrophage phenotypes among macrophage-like cell lines and those among primary macrophages seem to be an important factor for assessment of LPS-responses of these macrophages even in the presence of triptolide [2,11].
Taken together, our data showed that the cytotoxicity of triptolide toward macrophages was regulated by the LPS-signaling cascade through both LPS-tolerance and LPS/TLR4, implying that triptolide might be useful to cause macrophages to undergo apoptotic cell death for the prevention of excessive processes of harmful inflammation caused by LPS both in vivo and in vitro.
Acknowledgements
This work was partly supported by JSPS KAKENHI Grant numbers 12672147(FA) and 26860072 and 17K15529 (AK).
Conflict of Interest
The authors declare no conflict of interest.
References
- Gutknecht MF, Bouton AH (2014) Functional significance of mononuclear phagocyte populations generated through adult hematopoiesis. J Leukocyte Biol 96: 969-980.
- Alexander P, Evans R (1971) Endotoxin and double-stranded RNA render macrophage cytotoxic. Nature New Biol 232: 76-78.
- Klimp AH, De Vries EGE, Scherphof GL, Daemen T (2002) A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol 44: 143-161.
- Amano F, Karahashi H (1996) A cytotoxic effect of lipopolysaccharide (LPS) on a macrophage-like cell line, J774.1, in the presence of cycloheximide. J Endotoxin Res 3: 415-423.
- Karahashi H, Amano F (1998) Apoptotic changes preceding necrosis in lipopolysaccharide-treated macrophages in the presence of cycloheximide. Exp Cell Res 241: 373-383.
- Karahashi H, Amano F (2000) Changes of caspase activities involved in apoptosis of a macrophage-like cell line J774.1/JA-4 treated with lipopolysaccharide (LPS) and cycloheximide. Biol Pharm Bull 23: 140-144.
- Koike A, Shibano M, Mori H, Kohama K, Fujimori K, et al. (2016) Simultaneous addition of Shikonin and its derivatives with lipopolysaccharide induces rapid macrophage death. Biol Pharm Bull 39: 969-976.
- Kupchan SM, Court WA, Dailey RG, Gilmore CJ, Bryan RF, et al. (1972) Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc 94: 7194-7195.
- Kohama K, Koike A, Amano F (2017) Cytotoxic effect of triptolide on LPS-treated macrophages through sustained phosphorylation of p38 MAP kinase and apoptosis. Intl Biology Rev.
- Karahashi H, Nagata K, Ishii K, Amano F (2000) A selective inhibitor of p38 MAP kinase, SB202190, induced apoptotic cell death of a lipopolysaccharide-treated macrophage-like cell line, J774.1. Biochim Biophys Acta 1502: 207-223.
- Amano F, Akamatsu Y (1991) A lipopolysaccharide (LPS)-resistant mutant isolated from a macrophage-like cell line, J774.1, exhibits an altered activated-macrophage phenotype in response to LPS. Infect Immun 59: 2166-2174.
- Karahashi H, Amano F (2003) Endotoxin-tolerance to the cytotoxicity toward a macrophage-like cell line, J774.1, induced by lipopolysaccharaide and cycloheximide: role of p38 MAPK in induction of the cytotoxicity. Biol Pharm Bull 26: 1249-1259.
- Karahashi H, Amano F (2000) Lipopolysaccharide (LPS)-induced cell death of C3H mouse peritoneal macrophages in the presence of cycloheximide: different susceptibilities of C3H/HeN and C3H/HeJ mice macrophages. J Endotoxin Res 6: 33-39.
- Liu Q (2011) Triptolide and its expanding multiple pharmacological functions. Intl Immunopharmacol 11: 377-383.
- Matta R, Wang X, Ge H, Ray W, Nelin LD, et al. (2009) Triptolide induces anti-inflammatory cellular responses. Am J Transl Res 1: 267-282.
- Zhao G, Vaszar LT, Qiu D, Shin L, Kao PN, et al. (2000) Anti-inflammatory effcts of triptolide in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 279: 1958-1966.
- Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF, et al. (2012) Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett 319: 203-213.
- Zhang C, Cui GH, Liu F, Wu QL, Chen Y, et al. (2006) Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol Sin 27: 1438-1446.
- Mengozzi M, Ghezzi P (1993) Cytokine down-regulation in endotoxin tolerance. Eur Cytokine Netw 4: 89-98.
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, et al. (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749-3752.
- Bao X, Cui J, Wu Y, Han X, Gao C, et al. (2007) The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J Mol Med (Berl) 85: 85-98.
- Wu Y, Cui J, Bao X, Chan S, Young DO, et al. (2006) Triptolide attenuates oxidative stress, NF-kappaB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int J Mol Med 17: 141-150.
- Choi YJ, Kim TG, Kim YH, Lee SH, Kwon YK, et al. (2003) Immunosuppressant PG490 (triptolide) induces apoptosis through the activation of caspase-3 and down-regulation of XIAP in U937 cells. Biochem Pharmacol 6: 273-280.
Citation: Kohama K, Koike A, Amano F (2017) Triptolide Induces Cell Damage in Lipopolysaccharide (LPS)-Treated Macrophages in an LPSSignaling Cascade-Dependent Manner. J Cytokine Biol 2: 117. DOI: 10.4172/2576-3881.1000117
Copyright: © 2017 Kohama K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9012
- [From(publication date): 0-2017 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 8048
- PDF downloads: 964