Review Article Open Access
Opportunities and Challenges of Therapeutic Monoclonal Antibodies as Medical Countermeasures for Biodefense
Wei-Gang Hu* and Les P Nagata
Defence Research and Development Canada, Suffield Research Centre, Alberta, Canada
- *Corresponding Author:
- Wei-Gang Hu
Defence Research and Development Canada, Suffield Research Centre, Alberta, Canada
Tel: +1403544-4674
E-mail : weigang. hu@drdc-rddc.gc.ca
Received date: July 22, 2016; Accepted date: August 01, 2016; Published date: August 07, 2016
Citation: Hu WG, Nagata LP (2016) Opportunities and Challenges of Therapeutic Monoclonal Antibodies as Medical Countermeasures for Biodefense. J Bioterror Biodef 7:149. doi:10.4172/2157-2526.1000149
Copyright: © 2016 Hu WG, et al.. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
Antibodies, naturally produced in the body as part of the immune response to infectious agents, can also be introduced artificially to treat infectious diseases. Advances in biotechnology in the last decades have made human or humanized monoclonal antibodies (mAbs) as therapeutics possible. These therapeutic mAbs currently enjoy unprecedented success and recognition of their potential. Unlike vaccines, therapeutic mAbs can confer instant and consistent protection against bio-threat agents when administered regardless of the recipient’s immune status. Therapeutic mAbs can be administered in higher levels than those elicited by vaccines, and thus provide a higher level of protection or treatment that is necessary in a biological attack where people are exposed to a higher exposure of agent concentration than that found in nature. Furthermore, therapeutic mAbs have substantial advantages over antimicrobial drugs, such as high specificity, low systemic toxicity, relatively long half-life, and no concerns over disrupting the body’s microbiome. Therapeutic mAbs can be used for both pre- and post-exposure protection; therefore, they have great value as effective medical countermeasures (MedCMs) against bio-threat agents. However, there are still some challenges to be overcome before therapeutic mAbs become ideal MedCMs against bio-threat agents. In this review, both opportunities and challenges in development of therapeutic mAbs are discussed.
Keywords
Therapeutic; Monoclonal antibodies; Biodefense
Introduction
A bio-threat agent is a biological pathogen or toxin that can be used purposefully as a weapon in bioterrorism to spread life-threatening diseases on a large scale in order to cause fear, disorganization, and panic in the public. Numerous potentially weaponizable bio-threat agents have been described and studied. It is impossible to predict when and where, a bioterrorist attack is going to happen, let alone predict which agent would be used.
Vaccination can induce protective immune responses and then protect the vaccinated population against specific bio-threat attacks. Unfortunately, vaccines require time (weeks or months) to take effect. The required time period is usually longer than that between bio-threat exposure and onset of diseases. Moreover, vaccination does not guarantee that all the vaccine recipients would mount protective responses. It is estimated that around 10% of recipients do not amount any protective responses after vaccination [1]. These drawbacks of vaccines would limit their usefulness during an emergency response to a potential bio-threat scenario. Besides, it is also controversial to vaccinate a whole population or large number of individuals for preexposure prophylaxis against an uncertain and unpredictable biological attack [2].
Antimicrobial drugs are very effective against various bacteria, but obviously ineffective at eliminating viral infections. Antimicrobial drugs can provide protection when administered after exposure, however they have a short serum half-life (hours) and resistance often emerges after repeated use [3]. More than 70% of hospital-acquired bacterial infections are resistant to at least one of the drugs typically used to combat them [4]. Resistance to multiple drugs is increasing. In addition, most antibiotics are broad-spectral, killing commensal bacteria and leading to imbalanced gut micro flora in the body. Furthermore, there is also a concern regarding the intentional bioengineering of anti-microbial resistance in potential bio-threat agents. While clearly there is a critical need for new antibiotics and antiviral drugs not only for biodefense, but also to combat naturally occurring infectious diseases, unfortunately, new therapeutics are years from being developed and approved [5].
Antibodies, naturally produced in the body, are highly versatile immune-defense molecules to counteract pathogens (bacteria, viruses, toxins, etc.). As a matter of fact, antibodies have been used as MedCMs against infectious diseases since the late 19th century [6,7].
Animal anti-serum
Antibody therapy against infectious diseases was discovered by the German physiologist, Emil Adolf von Behring a century ago [7,8]. His work led to the first instance of industrial production of protective serum from horses in 1893 and to his receipt in 1901 of the first Nobel Prize in medicine. Following Behring’s discovery, animal immune serum (anti-serum) was used to treat bacterial, viral, and toxin-mediated diseases where a protective immune response could be induced in the animal host by vaccination. However, the use of animal anti-serum was not entirely safe. Due to its foreignness to humans, its use gave rise to anaphylaxis (serum sickness). Therefore, the use of animal anti-serum was largely abandoned by the 1930s.
Human anti-serum
Replacing animal anti-serum, human anti-serum from convalescent or immunized subjects came into use. Despite unquestioned efficacy in clinics, human anti-serum therapy suffered from a number of drawbacks, including high cost, limited source, cumbersomeness, batch-to-batch variation, low content of specific antibodies (only 1% of the total antibodies), uncertain dosing, and a risk of infection from blood borne products. Subsequently, human anti-serum therapy against bacterial infections was largely abandoned in the 1940s when antibiotics came into clinical practice. However, human anti-serum therapy for viral and toxin-mediated diseases for which there are no alternative therapeutic options has continued to develop. Recently, the serum in anti-serum therapy has been replaced by serum-derived immunoglobulin (polyclonal antibodies) from pooled human donors. The risk from adventitious agents has been reduced by better screening of donors and new production methods which inactivate or remove viruses. These hyper immune human serum-derived immunoglobulin products approved by the U.S. Food and Drug Administration (FDA), are mainly used for viruses (such as cytomegalovirus, respiratory syncytial virus, hepatitis A virus, hepatitis B virus, rabies virus, vaccinia virus, varicella-zoster, and measles virus) underscoring the fact that antibody therapy remains an effective means of treatment against viruses [9-12].
Monoclonal antibodies (mAbs)
In 1975, the development of mAbs by murine hybridoma technology opened a new era in antibody therapy. In contrast to polyclonal antibodies (serum-derived immunoglobulin), mAbs are derived from a single cell line and are thus identical in binding sites and binding affinities [13]. Using this technology, it has been possible to produce large amounts of antibodies of the desired specificity. At the time, it was widely believed that mAbs would be the “magic bullets” for therapy. However, the early excitement was rapidly replaced by disappointment when it was found that mAbs, like animal anti-sera, had serious side effects in humans mainly due to its “foreignness” to humans [14], such as rapid clearance of mAbs from humans and potentially dangerous anaphylactic response. Furthermore, the mouse antibody constant region does not interact properly with the human immune system and results in inefficiency at exerting therapeutic effects [15]. A tremendous effort was devoted to make human mAbs. However, it was soon found that hybridoma technology was unable to produce human mAbs.
Chimeric, humanized, and human mAbs
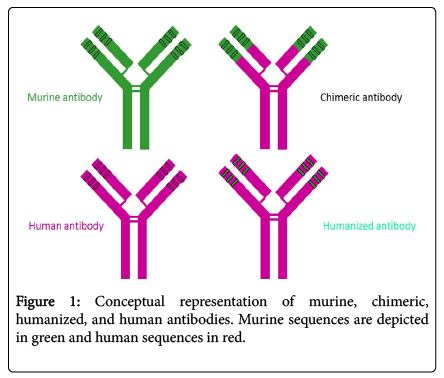
As molecular biology technology advanced in the mid-1980s, two approaches were developed to minimize the potential of non-human sequence for unwanted and potentially dangerous immune responses in humans and enhancement of therapeutic effects of wellcharacterized murine mAbs. This was accomplished by replacing some parts of the murine antibody with human antibody counterparts: chimerization and humanization as shown in (Figure 1) [16-22].
In murine/human chimeric antibodies, the murine constant region (around 70% of total antibody sequence) is replaced by the human counterpart. Chimeric antibodies successfully retain the original murine antibody variable region (antigen-binding specificity) and possess a fully human antibody constant region, which make them considerably less immunogenic in humans and allows interaction with the human immune system to exert indirect effects. However, chimeric antibodies may still elicit an undesirable response to the murine antibody variable region [23].
In order to further reduce chimeric antibody immunogenicity, humanization was developed in 1988 to replace the murine antibody frameworks with those of selected human antibodies on chimeric antibodies [19]. The resulting “humanized” antibodies contained 90-95% human sequences [24-27]. Numerous clinical studies have confirmed that humanized antibodies are less immunogenic and more therapeutic in humans than murine or chimeric antibodies [28,29]. However, the process of humanization of murine antibodies is much more challenging than construction of murine-human chimeric antibodies. Humanization may result in a loss of antibody antigen binding activity. Nevertheless, humanization has played a fundamental role in the remarkable progress of antibodies as therapeutics.
An alternative to humanization is the derivation of fully human antibodies directly from in vitro phage display libraries of human antibodies, in which human antibody fragments are displayed on the surface of phages. Unlike the antibodies developed in vivo , these antibodies possess only limited antigen-binding affinity due to the absence of assistance from the in vivo immune system for affinity maturation by somatic hyper-mutation. Another approach has been to use transgenic mice modified to carry human antibody genes [30-32]. Immunization of these mice leads to the development of human antibodies, from which hybridomas that produce human antibodies can be generated. However, these mice cannot be used effectively when the targeted antigen is either toxic to mice or homologous with murine tissues. Because of these reasons, the majority of therapeutic mAbs approved by the FDA and in clinical trials are humanized mAbs. Therefore, humanization remains an attractive and proven strategy for switching well-characterized and highly specific murine antibodies into clinical therapeutics.
The technology breakthrough for making humanized or fully human mAbs has lessened the barrier for mAbs as therapeutics. Consequently, mAb therapy has had unprecedented success, growth in research and revenues, and recognition of its potential. This success is likely due to human/humanized mAbs being much better than human hyper immune immunoglobulin as therapeutics. It is undoubtedly revolutionizing the practice of medicine and truly realizing the earlier vision of “magic bullet” medicine. Since 2000, the therapeutic market for mAbs has grown rapidly and therapeutic mAbs have become the fastest growing class of therapeutic agents. Currently nearly all large pharmaceutical companies have at least one mAb licensed product and more candidates in their pipelines. As of 2014, there were around 50 therapeutic mAbs on the market and 300 more in clinical trials [33].
Highlights of human or humanized therapeutic mAbs as MedCMs for biodefense
Instant and consistent protection: Able to confer immediate protection to recipients regardless of recipient’s current immune status.
Higher than natural protection: Can be administered in higher level and then provide greater protective immunity than vaccination.
High specificity: Can be developed only to target specific components of bio-threat agents without risk of cross-reactivity with human tissue.
Low toxicity: Much more acceptable and tolerable than other types of MedCMs in humans due to their natural components within the body.
Reasonable duration: Because of the plasma half-life of 20 days, the protective immunity provided by passive mAbs can last for weeks after a single administration. It can be extended with additional administrations of mAbs, but it will not last for a lifetime of the recipient.
Double effects: Able to neutralize bio-threat agents by themselves and interact with host immune system to destroy bio-threat agents.
Short development time to licensure: Can be developed against most bio-threat agents, if not all within a shorter period of time than other types of MedCMs.
High regulatory approval rate: Higher approval rate than that of other drug classes due to the well-defined and understood pathway for licensure approval.
Scientific evidence on mAb effectiveness against many bio-threat agents [34-38].
Uniqueness in neutralizing toxins: Currently, no drugs are available that specifically counteract toxin-mediated intoxication, while toxin neutralization is a classical property of antibody-mediated protection.
Challenges
There are still certain challenges remaining for the human or humanized therapeutic mAbs as ideal MedCMs for biodefense.
Manufacturing cost
Therapeutic mAbs are among the most expensive drugs. The current production of therapeutic mAbs requires the use of very large cultures of mammalian cells followed by extensive purification steps, under Good Manufacturing Practice (GMP) conditions, leading to extremely high production costs ($300/gram) [39]. As a result, the FDA-approved anti-anthrax recombinant human antibody, Raxbacumab cost $5,100 for each dose when stockpiled. The high cost has adversely affected the development of mAbs as therapeutics.
The cost might be reduced by certain new technologies, such as plant-based mAb production. Plants can be used as bioreactors for large scale production of therapeutic antibodies at a low cost [40,41]. Significant progress has been made in the expression of mAbs in plants. In the first human trial of a mAb produced in transgenic tobacco plants, the mAb was demonstrated to provide protection against oral Streptococcus mutans colonization [42]. Preclinical studies of a humanized anti-herpes simplex virus (HSV) mAb produced in transgenic soybean plants showed that the mAb prevented transmission of HSV infection in mice [43]. Recently, the humanized anti-ricin therapeutic antibodies and anti-Venezuelan equine encephalitis virus (VEEV) therapeutic antibody were produced in plants at Defence Research and Development Canada, Suffield Research Centre. These two planted produced therapeutic antibodies have been confirmed to be comparable to their mammalian cellproduced counterparts in terms of anti-ricin and anti VEEV infection potency (publication in preparation). These results suggest promise for the use of this technology.
Plant systems have several advantages over mammalian cell culture. They are fast, efficient, highly versatile (for new product development), and easily scalable with significantly reduced manufacturing costs. In addition, they are free from contamination by mammalian pathogens. However, before plant-produced therapeutic mAbs come to the market, the plant production system for therapeutic mAbs has to meet the rigorous regulatory requirements and standards for pharmaceutical products.
Antibody efficacy
Antibody therapies are usually administered intravenously due to the large amount of antibody required. For example, the treatment regimen of raxibacumab (humanized mAb against inhalational anthrax, recently approved by the FDA) is 40 mg/kg [44]. Such an amount of mAb has to be administered by intravenous infusion, which takes at least 2 hours. Infusions on the order of hours require a specialized capability in a hospital environment. This approach would be impractical when large populations are exposed to a biological attack. To be effective at a large population-scale, delivery should be intramuscular (less volume), which requires higher potency of mAbs in the formulation than is currently available. The small amount of mAbs with higher efficacy could be supplied in self-injectable devices that allow victims to protect themselves upon notification of bio-threat attack. Two aspects should be taken into account in order to improve antibody efficacy:
Direct antibody effects: The antigen target (where the antibody binds) is crucial for the antibody to exert its neutralizing function. If the antibody binds to the pathogen or toxin at the right location, the antibody will efficiently block the entry of pathogen or toxin into cells; otherwise, the blockage will only be partial or null. For example, a recently developed anti-ricin mAb showed extremely high efficacy since it binds to the part of ricin which is responsible for binding to the cell surface to initiate ricin entry into cells [45]. Only a small amount of antibody (0.25 mg/kg) was required for complete protection against lethal ricin challenge [26,45-47].
Indirect antibody effects: The antibody constant region is responsible for interacting with immune system to recruit immune components to destroy the target cells. Engineering of constant region has demonstrated enhanced constant-region functionality (indirect effects) by 100 folds [48].
Cocktail of mAbs
Although specificity is the strength of mAbs, a bio-threat agent that may undergo rapid mutation to result in antigenic variation poses a significant hurdle for mAbs as therapeutics. For example, the high mutation rate of certain viruses enables them to escape neutralization [49]. This problem may be overcome by using mixtures of mAbs that target various areas of bio-threat agents. Several studies demonstrated that combination therapy with mAb cocktails could prevent escape variants for many viruses including influenza, coronavirus, and lymphocytic choriomeningitis virus [50-52]. Also, a cocktail of functional mAbs could provide more protection and target more microbial strains than a single one. The inclusion of multiple mAbs in any therapeutic formulation could be fraught with complex regulatory and licensing issues. However, just recently the FDA has allowed cocktails of mAbs to be clinically tested as one product due to the administration of a cocktail of mAbs required for many infectious disease indications [53].
Broad spectrum
Currently developed or developing anti-infective mAbs are only specific to one pathogen or toxin per antibody product. The new generation of therapeutic mAbs could be developed against multiple pathogens or toxins by a single mAb product. For this scenario to be realistic, there are two kinds of strategies:
Bio-threat agent-targeted mAbs: MAbs should be against certain conserved regions shared within a group of bio-threat agents. For example, antibodies may be developed against novel targets, such as those that regulate microbial growth and virulence factor expression, or are used by a subset of microbes to infect host cells [54,55]. To fight multiple drug resistance bacteria, some successful approaches have targeted the cellular efflux pumps responsible for antibiotic resistance [56]. These types of “broad-spectrum” mAbs are ideal therapeutics for MedCMs against unpredictable and uncertain future biological threats.
Host-targeted mAbs: Over the past years therapeutic antibodies have been developed to target pathogen or toxin components. The common cellular pathways used by a wide range of pathogens or toxins have been missed as therapeutic targets. Our understanding of hostpathogen or toxin cellular interactions involved in bio-threat agent pathogenesis remains minimal. The research on this field needs to be emphasized to develop the knowledge for identifying host targets and then host-targeted antibodies could be developed as broad-spectrum therapeutics. These host-targeted mAbs should be in a Fab or F(ab')2 format in order to eliminate potential adverse efforts of antibodydependent cellular or complement cytotoxicity via the Fc portion.
Pharmaceutical properties
Long-lasting: Despite the reasonably long half-life of therapeutic antibodies (around 20 days long serum half-life is much, for MedCMs against bio-threat agents, a desirable as it would decrease the need for repeat injections of mAbs to achieve a therapeutically relevant serum concentration. Recent studies showed that modification in the constant region of antibodies could extend up to four-fold their half-life while retaining efficacy [57,58].
An alternative approach is to take advantage of the body's natural ability to express transgenes to produce passive antibodies. This approach can be achieved by in vivo delivery of genes encoding biothreat agent-specific antibodies for biodefense applications [59]. Animal studies have shown that the expressed antibodies can be detected as early as day 3, reach peak levels at day 7, and maintain therapeutic levels in serum for more than seven months after a single administration via antibody gene delivery [60]. Therefore, antibody gene delivery in vivo might be a new approach for post-exposure prophylaxis or therapy and for pre-exposure prophylaxis of bio-threat agent-mediated diseases although there are still some problems to be overcome before this new approach could actually be used in humans.
Stem cells can be used as an antibody gene delivery platform to provide relatively long passive immunity against a pathogen. In a previous study, a single intramuscular injection of anti-VEEVengineered stem cells was demonstrated to generate and maintain higher circulating anti-VEEV antibody titers. Most critically, pretreatment with engineered stem cells significantly improved both survival and morbidity after exposure to a high lethal dose of highly virulent VEEV. Engineered stem cells maintained the protective anti- VEEV titers for up to 38 days after implantation into mice, and continued to secrete anti-VEEV antibody for over months [61]. Thus, engineered stem cells represent an attractive antibody delivery modality that improves the efficacy of already existing prophylactic countermeasures to infectious disease.
A single dose of antibody administration for post-exposure treatment is like one stone to kill two birds, that is, not only to provide immediate protection to the victim against infections/intoxications, but also quickly elicit a host immunity against infections/ intoxications that subsequently results in long term protection (5 months). In a murine ricin intoxication model, one dose of anti-ricin antibody administration 1 hour after 5 × LD50 ricin challenge rescued the mice. Nine days later, when the rescued mice received a second ricin challenge (5 × LD50), the mice still survived [62]. The experimental design excluded the possibility of residual passive antibody responsible for the protection against the second ricin challenge. Results confirmed that the active immunity against ricin in mice was induced quickly following the passive delivery of a single dose of anti-ricin antibody post-exposure. The mechanism for this phenomenon is that the ricin bound by the passive antibody can be captured and internalized efficiently by antigen-processing cells (APC) through the binding of passive antibody Fc portion to the Fc receptor on the APC and thereby dramatically elicit active anti-ricin immunity immediately.
Increasing stability
Another complication with antibodies is that they usually need to be kept cold to be stable. This is inconvenient for deploying an antibodybased strategy for biodefense or to any field operation. Technology advances in antibody engineering could allow development of stable preparations with their storage at room temperature as solutions. In a recent report, when some mutations were introduced, the antibody stability was improved dramatically [63].
Conclusions
Therapeutic mAbs have great value as effective MedCMs against bio-threat agents. Although there are still some challenges to be overcome before therapeutic mAbs become ideal MedCMs against biothreat agents. With continuous advances in antibody technology and in the understanding of infectivity and intoxication of bio-threat agents, a new generation of therapeutic mAbs with extraordinary efficacy could be developed and these therapeutic mAbs would be not only fastacting, but also cost-effective and long-lasting against unpredictable and uncertain future bio-threat agents for biodefense.
References
- Pirofski LA, Casadevall A (1998) Use of licensed vaccines for active immunization of the immuno compromised host. Clinical microbiology reviews 11:1-26.
- Evans G (2002) Bioterrorism watch. Anthrax aftermath: adverse drug reactions vaccine controversy undercut CDC extended treatment offer 27: 1-4.
- Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, et al. (2008) The epidemic of antibiotic resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 46: 155-164.
- Odonkor ST, Addo KK (2011) Bacteria resistance to antibiotics: recent trends and challenges. International Journal of Biological and Medical Research 2: 1204-1210.
- Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE (2004) Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 38: 1279-1286.
- Casadevall A, Dadachova E, Pirofski LA (2004) Passive antibody therapy for infectious diseases. Nature reviews Microbiology 2: 695-703.
- Behring EV (1890) EaSK: UeberZustandekommen der Diphtherie-Immunitat und der Tetanus-ImmunitatbeiThieren. S Dtsch Med Wochenschr 16: 1113-1116.
- Winau F, Winau R (2002) Microbes Infect 2002 4: 185-188.
- Loofbourow JC, Cabasso VJ, Roby RE, Anuskiewicz W (1971) Rabies immune globulin (human). Clinical trials and dose determination Jama 217: 1825-1831.
- Beasley RP, Hwang LY, Stevens CE, Lin CC, Hsieh FJ, et al. (1983) Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double blind, placebo controlled trial. Hepatology 3: 135-141.
- Snydman DR (2001) Historical overview of the use of cytomegalovirus hyperimmune globulin in organ transplantation. Transplant infectious disease: an official journal of the Transplantation Society 2: 6-13.
- Sawyer LA (2000) Antibodies for the prevention and treatment of viral diseases. Antiviral Res 47: 57-77.
- Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495-497.
- Khazaeli MB, Conry RM, LoBuglio AF (1994) Human immune response to monoclonal antibodies. J Immunother 15:42-52.
- Carter PJ (2006) Potent antibody therapeutics by design. Nature reviews Immunology 6: 343-357.
- Boulianne GL, Hozumi N, Shulman MJ (1984) Production of functional chimaeric mouse/human antibody. Nature 312: 643-646.
- Morrison SL, Johnson MJ, Herzenberg LA, Oi VT (1984) Chimeric human antibody molecules: mouse antigen binding domains with human constant region domains. ProcNatlAcadSci USA 81: 6851-6855.
- Neuberger MS, Williams GT, Fox RO (1984) Recombinant antibodies possessing novel effector functions. Nature 1984 312: 604-608.
- Verhoeyen M, Milstein C, Winter G (1988) Reshaping human antibodies: grafting an antilysozyme activity. Science 239: 1534-1536.
- Jones PT, Dear PH, Foote J, Neuberger MS, Winter G (1986) Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321: 522-525.
- Riechmann L, Clark M, Waldmann H, Winter G (1988) Reshaping human antibodies for therapy. Nature 332: 323-327.
- Hu WG, Yin JF, Chau D, Hu CC, Cherwonogrodzky JW (2014) Antibody humanization by a single cycle of CDR-grafting. United Arab Emirates Bentham Science.
- Bruggemann M, Winter G, Waldmann H, Neuberger MS (1989) The immunogenicity of chimeric antibodies. J Exp Med 170:2153-2157.
- Hu WG, Nagata LP (2013) Humanized anti-Venezuelan equine encephalitis virus recombinant antibodies. USA: Her Majesty the Queen in right of Canada as represented by The Ministry of National Defence.
- Hu WG, Phelps AL, Jager S, Chau D, Hu CC (2010) A recombinant humanized monoclonal antibody completely protects mice against lethal challenge with Venezuelan equine encephalitis virus. Vaccine 28: 5558-5564.
- Hu WG, Yin J, Chau D, Negrych LM, Cherwonogrodzky JW (2012) Humanization and characterization of an anti ricin neutralization monoclonal antibody. PLoS One 7: 45595.
- Rivera J, Zaragoza O, Casadevall A (2005) Antibody mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infection and immunity 73: 1141-1150.
- Hwang WY, Foote J (2005) Immunogenicity of engineered antibodies. Methods 36: 3-10.
- Tsurushita N, Hinton PR, Kumar S (2005) Design of humanized antibodies: from anti Tac to Zenapax. Methods 36: 69-83.
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348: 552-554.
- Hu WG, Jager S, Chau D, Mah D, Nagata LP (2010) Generation of a recombinant full length human antibody binding to botulinum neurotoxin A. ApplBiochemBiotechnol 160: 1206-1216.
- Green LL (1999) Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. Journal of immunological methods 231: 11-23.
- Ecker DM, Jones SD, Levine HL (2015) The therapeutic monoclonal antibody market. MAbs 7: 9-14.
- Bregenholt S, Haurum J (2004) Pathogen specific recombinant human polyclonal antibodies: biodefence applications. Expert OpinBiolTherpp: 387-396.
- Berry JD, Gaudet RG (2011) Antibodies in infectious diseases: polyclonals, monoclonals and niche biotechnology. New biotechnology 28: 489-501.
- Froude JW, Stiles B, Pelat T, Thullier P (2011) Antibodies for biodefense. MAbs 3: 517-527.
- Casadevall A (2002) Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerging infectious diseases 8: 833-841.
- Zhu Z, Dimitrov AS, Chakraborti S, Dimitrova D, Xiao X (2006) Development of human monoclonal antibodies against diseases caused by emerging and biodefense-related viruses. Expert review of anti-infective therapy 4: 57-66.
- Chadd HE, Chamow SM (2011) Therapeutic antibody expression technology. CurrOpinBiotechnol 12: 188-194.
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proceedings of the National Academy of Sciences of the United States of America 2103: 14701-14706.
- Graumann K, Premstaller A (2006) Manufacturing of recombinant therapeutic proteins in microbial systems. Biotechnology journal 1: 164-186.
- Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, et al. (1998) Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nature medicine 4: 601-606.
- Zeitlin L, Olmsted SS, Moench TR, Co MS, Martinell BJ, et al. (1998) A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nature biotechnology 16: 1361-1364.
- Kummerfeldt CE (2014) Raxibacumab: potential role in the treatment of inhalational anthrax. Infection and drug resistance 7: 101-109.
- Hu WG, Yin J, Chau D, Hu CC, Lillico D, et al. (2013) Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies. BioMed research international 471346.
- Hu WG, Yin JF, Chau D, Hu CC, Cherwonogrodzky JW (2014) Anti-ricin protective monoclonal antibodies. Sharjah, the United Arab Emirates: Bentham Science 14: 145-158.
- Hu WG, Negrych LM, Chau D, Yin JF, Jager SJ, et al. (2016) Anti-ricin antibodies and uses thereof. Edited by Office UP.
- Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, et al. (2007) Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumour expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res 67: 8882-8890.
- McKeating JA, Gow J, Goudsmit J, Pearl LH, Mulder C, et al. (1989) Characterization of HIV-1 neutralization escape mutants. AIDS 3: 777-784.
- Prabakaran M, Prabhu N, He F, Hongliang Q, Ho HT, et al. (2009) Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS One 4: e5672.
- TerMeulen J, Van den Brink EN, Poon LL, Marissen WE, Leung CS, et al. (2006) Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med 3: e237.
- Seiler P, Senn BM, Brundler MA, Zinkernagel RM, Hengartner H, et al. (1999) In vivo selection of neutralization-resistant virus variants but no evidence of B cell tolerance in lymphocytic choriomeningitis virus carrier mice expressing a transgenic virus neutralizing antibody. J Immunol 162: 4536-4541.
- Whaley KJ, Zeitlin L (2013) Antibody based concepts for multipurpose prevention technologies. Antiviral Res 100 Suppl: S48-53.
- Kaufmann GF, Park J, Janda KD (2008) Bacterial quorum sensing: a new target for anti-infective immunotherapy. Expert OpinBiolTher 8: 719-724.
- Pai JC, Sutherland JN, Maynard JA (2009) Progress towards recombinant anti-infective antibodies. Recent Pat Antiinfect Drug Discov 4: 1-17.
- Wigfield SM, Rigg GP, Kavari M, Webb AK, Matthews RC, et al. (2002) Identification of an immunodominant drug efflux pump in Burkholderiacepacia. The Journal of antimicrobial chemotherapy 49: 619-624.
- Spiegelberg HL (1974) Biological activities of immunoglobulins of different classes and subclasses. Advances in immunology 19: 259-294.
- Oganesyan V, Damschroder MM, Woods RM, Cook KE, Wu H, et al. (2009) Structural characterization of a human Fc fragment engineered for extended serum half life. Molecular immunology 46: 1750-1755.
- Hu WG, Nagata LP (2008) Antibody gene-based prophylaxis and therapy for biodefence. Human vaccines 4: 74-78.
- Jiang M, Shi W, Zhang Q, Wang X, Guo M, et al. (2006) Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin Cancer Res 12: 6179-6185.
- Braid LR, Hu WG, Davis JE, Nagata LP (2016) Engineered mesenchymal cells improve passive immune protection against lethal Venezuelan equine encephalitis virus exposure. Stem Cells Translational Medicine 5: 1026-1035.
- Hu CC, Yin J, Chau D, Cherwonogrodzky JW, Hu WG (2014) Active immunity induced by passive IgG post-exposure protection against ricin. Toxins 6: 380-393.
- Seeliger D, Schulz P, Litzenburger T, Spitz J, Hoerer S, et al. (2015) Boosting antibody develop ability through rational sequence optimization. mAbs 7: 505-515.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 15160
- [From(publication date):
September-2016 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 13959
- PDF downloads : 1201