Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
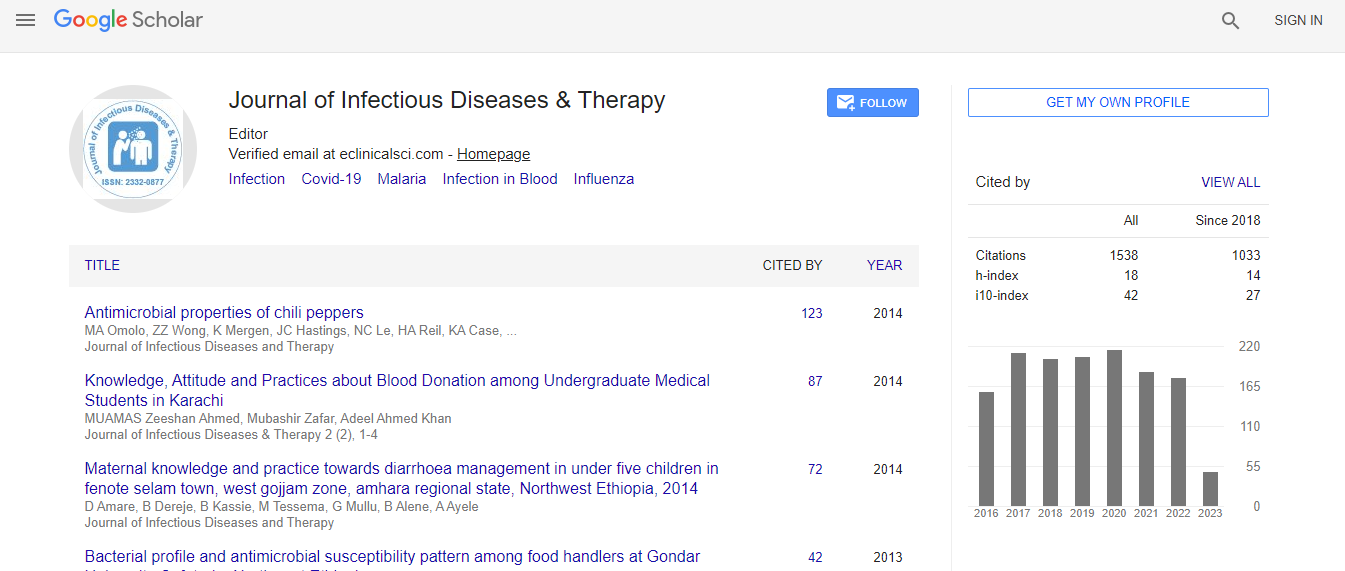
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Modifications improving effectiveness of DNA vaccine against H5N1
International Conference on Influenza
R�?³�?¼a Sawicka, Patrycja Redkiewicz , Anna G�?³ra-Sochacka and Agnieszka Sirko
Polish Academy of Sciences, Poland
Posters-Accepted Abstracts: J Infect Dis Ther
Abstract
Injection of plasmid or linear form of DNA encoding vaccine antigen can lead to the induction of immune response. This approach offers a number of potential advantages over traditional approaches, including the stimulation of both humoral and cell-mediated responses, the relative ease of large-scale manufacture and improves vaccine stability enabling withdrawal from restrictive and very expensive cold chain transport. The most important disadvantage is low level of antigen expression in-vivo and hence low immunogenicity of vaccine. Several strategies can be used to overcome this difficulty. Our studies are focused on DNA vaccine against H5N1. To improve vaccine immunogenicity and reduce its cost of production we evaluated different strategies of modification of basic vaccine variant prepared. Basal H5 HA DNA vaccine was modified in the following ways: i - proteolytic cleavage site was removed, ii ��? codon usage was change using three different algorithm, iii ��? expression cassette was modified to permit DNA transport to nucleus increasing gene expression level, iv ��? RNA-OUT vector was used allowing selection without antibiotics and enabling in vivo expressed proteins transport to different cell compartments: Endosome or secrete outside the cell. All vaccine variants were tested in mice and vaccine efficacy was measured by ELISA assay. Our results showed that tested doses of DNA vaccine elicit HA- dependent humoral response. The level of immunological response depended on the tested vaccine variant and the best modification was chosen for further studies.Biography
Ró�?¼a Sawicka graduated as biologist, specialization in biochemistry in 2009 at Maria Curie-Sk�?�?odowska University in Lublin. She completed the Post-graduate studies in 2012 as Research Project Management and Commercialization of Research Results at University of Technology in �?ód�?º. Since 2009 she was associated with Institute of Biochemistry and Biophysics Polish Academy of Sciences (Warsaw, Poland), where she worked as biologist in project concerning veterinary DNA vaccine against influenza and now she is working in this interesting area as a PhD student.
Email: rozasawicka@ibb.waw.pl

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi