Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
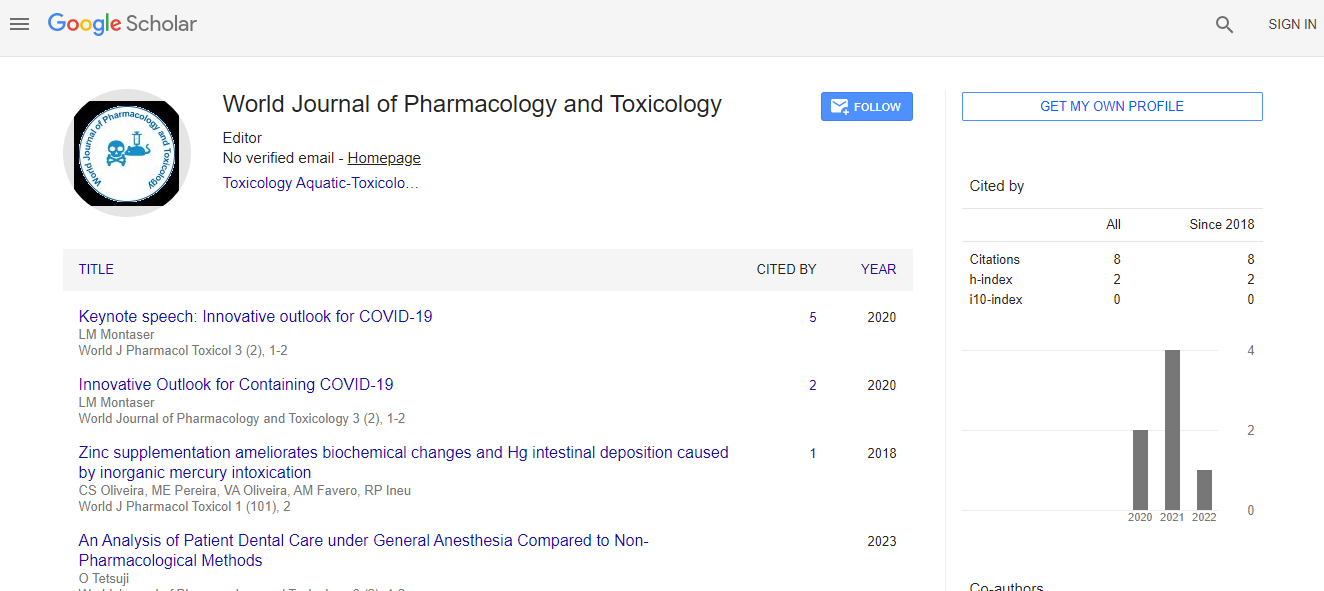
Google Scholar citation report
Citations : 8
World Journal of Pharmacology and Toxicology received 8 citations as per Google Scholar report
Indexed In
- Google Scholar
- ICMJE
Useful Links
Share This Page
William Reed Kelce

William Reed Kelce
PAREXEL International
United States of America
Biography
Dr. Kelce leads drug development programs from discovery to approval, provides gap analyses and grant writing support, and authors Investigational New Drug (IND) and New Drug Application (NDA) (or equivalent) regulatory submissions worldwide. He has led development programs in, ophthalmology, metabolic disease, dermatology, wound healing, cancer, gastroenterology, pain and inflammation, neuropharmacology, cardiology, medical countermeasures using the animal rule, and medical devices. Dr. Kelce has published 13 book chapters; 52 original research articles and has been invited to speak at scientific conferences worldwide including the United States, Europe, Japan and Australia. He currently is Associate Editor of Toxicology and Applied Pharmacology and was past Chairman of the Board of Publications for the Society of Toxicology (2007-2008). He was elected to the Academy of Toxicological Sciences (ATS) in 1999, the Pharmacia Science Fellow Program in 2002, and was awarded the US EPA Gold Medal for scientific research on environmental endocrine disruptors.
Research Interest
• Orphan drug applications
• FDA expedited development program applications
• Nonclinical (CTA, IMPD)/NDA (BLA, MAA) regulatory submissions
• IND (CTA, IMPD)/NDA (BLA, MAA) gap analyses
• Animal rule approval strategies
• Development of small and large molecules
• Development and strategy for cellular and gene product therapies

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi