Review Article Open Access
Integrating Immunobased Detection and Identification Methods for Ricin Analysis: An Overview
Payal Puri and Om Kumar*
Division of Pharmacology and Toxicology, Defence Research and Development Establishment, Jhansi Road, Gwalior, India
- *Corresponding Author:
- Om Kumar
Defence Research and
Development Establishment
Jhansi Road, Gwalior
Tel: +91-0751-2233489
E-mail: omkumar63@rediffmail.com
Received Date: September 22, 2011; Accepted Date: December 10, 2011; Published December 14, 2011
Citation: Puri P, Kumar O (2011) Integrating Immunobased Detection and Identification Methods for Ricin Analysis: An Overview. J Bioterr Biodef S2:007. doi: 10.4172/2157-2526.S2-007
Copyright: © 2011 Puri P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
Ricin is one of the most potent naturally occuring toxins and is among the deadliest poisons available. It gained fame by its use in the so-called ‘umbrella murder’ to kill the Bulgarian dissident Georgi Markov in 1978. Ricin is considered as a potential bioterrorism agent due to its toxicity, easy availability of raw materials, inexpensive and simple production of large quantities, and an extensive history of use as a bioweapon. In addition, ricin is soluble in water and stable under heat and a wide pH range. Therefore, there is a potential for ricin to be intentionally added into food or water. For environmental detection in bioterrorism attack as well as for medical treatment purpose, there is a need for a rapid and sensitive detection and quantitation method on trace amount of ricin. This review gives an overview of the different detection methods available for ricin-a biothreat agent. The aim of this review is to give an overall picture of the past and present trend in the detection methodologies and to integrate them for ricin analysis. The objective of this paper is focused on the detection of ricin that might be used as a biological weapon.
Keywords
Ricin; Toxin; Toxicity; Biothreat; Detection; Quantitation
Introduction
Toxins are specific poisons made by living organisms. They are highly effective due to their specificity. Toxins generally consist of an amino acid chain of several hundred to a thousand in molecular weight. They can be low-molecular organic carbons as well. Bacteria, fungi, algae and plants all produce toxins. These toxins can be very poisonous, some have toxicity orders of magnitude greater than nerve agents. When the potential weapons for bioterrorism are reviewed for needed characteristics, the biological activity/toxicity attracts special attention. A number of selected toxins and their toxicity are presented in Table 1. One example of such toxins is ricin, which is listed as a Category B bioterrorism agent according to the Centers for Disease Control and Prevention. The potency of ricin, and ease with which it can be produced makes it a potential weapon of mass terror.
| Biological Toxins | |||||||
|---|---|---|---|---|---|---|---|
| Disease Agent | Fatal dose (μg/ kg) | Onset (hours) | Antitoxin Availability | Intervention (hours) | Stability | Fatality Risk | Treatability |
| Botulinum | 0.1 | 1-72 | Yes | 0.5-1 | Low | High | No |
| Staphylococcal Enterotoxin B | 1-10 | 1-72 | No | 0.5-1 | Moderate | Low | No |
| Perfringens | 10-500 | 6-24 | No | 1-6 | Moderate | Low to high | No |
| Ricin | 100 | 8-12 | In development | 0.5-1 | Moderate | High | No |
| Saxitoxin | 800 | 0.1-2 | No | 0.2 | Low | Low | No |
| Tetrodotoxin | 800 | 0.1-8 | No | 0.1 | Low | Moderate | No |
| Aflatoxin | 100,000 | Very long | No | 6 | High | Long term | No |
Table 1: Likely Biological Agents and Their Characteristics.
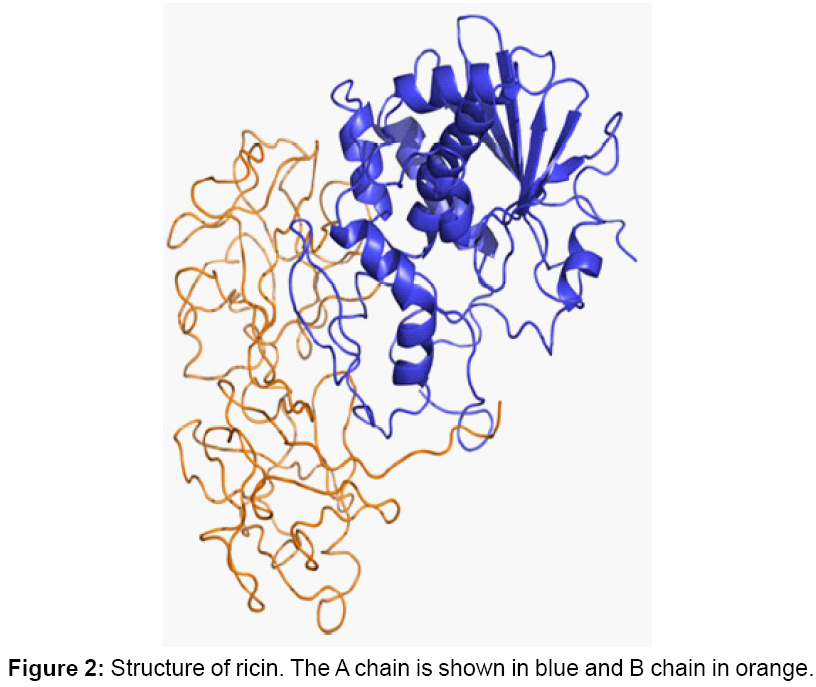
Ricin, also known as Ricinus communis agglutinin II, is a protein toxin naturally present in the castor bean seeds (Figure 1). The ricin molecule is composed of 2 chains, A and B, (Figure 2) both of which are needed to achieve the toxic effects in vivo [1]. The A chain is responsible for the enzymatic activity of the toxin. It removes an adenine from the 28S RNA of ribosome and leads to the inhibition of the protein synthesis. The B chain is responsible for delivering the A chain into the cell. It is estimated that the LD50 for ricin in humans is approximately 5 to 10 µg/kg through inhalation and 1 to 20 mg/kg body weight or 8 castor beans through ingestion [2-5]. Currently, there is no effective treatment available for ricin intoxication. Detection and identification of this toxic agent is important for medical diagnostics, food/water safety testing, and biological warfare defense. Methods to detect ricin include immunoassay, quantitative PCR, sensors, mass spectrometry and genetic analysis. Because of the high bioterrorism risk concern over ricin, more and more recent research efforts have been directed to the design of sensitive methods for the detection of this toxin in both buffer and complex food matrices.
Ricin was first considered for use as a biological weapon during World War I by several nations, including the United States, which gave the toxin the code name, “Compound W”. During World War II, the U.S. and Great Britain worked together to develop a “W bomb”. The story about a Bulgarain dissident, Georgi Markov, who was killed by ricin fired from an altered umbrella in London 1978, is one of the more well-known caese of homicide worldwide [6]. Recently the toxin was illegally used and mail contaminated with it was sent to the US senate and the White House [7].
Growing awareness and concern about ricin as a possible terrorist weapon has neccessiated a comprehensive review of different detection technologies available for this poison. This highlights the need to develop a rapid, efficient detection and quantitation method for biotoxins detection as a part of the global mission against terrorism. Ideally, these methods should be both fast and sensitive to allow a rapid detection of minute amounts of the suspected toxin and should represent an important tool to prevent, or deal with the consequences of, contamination and intoxication.
Detection Methods for Ricin: Analytical Aspects
Sensitive methods for detecting ricin are needed for clinical diagnosis and forensic use. Beside the detection in biological fluids or tissues, the determination of ricin in environmental samples, beverages or food matrices is of interest when it is partially purified or refined into a terrorist or warfare agent. In the literature, various qualitative and quantitative methods are described for the detection of ricin. Most of them are immunology-based detection assay including enzyme-linked immunosorbent assay [8,9], radioimmunoassay [10], chemiluminescence immuno-sorbent assay [11], immunochromatography technique (ICT)-based assay [12], array biosensors [13], hydrogel-based protein microchips [14], immuno-polymerase chain reaction [15] and molecularly impinting technology [16].
Immunoassay
One of the earliest detection techniques for low concentrations is radioimmunoassay (RIA), which could be used to quantify amounts as low as 100 pg of ricin based on rabbit antiserum in buffer [10,17]. This buffer consists of 0.1% sodium azide and 0.1% bovine serum albumin in 0.05M sodium phosphate buffer. Even though it has a good sensitivity, its major drawbacks are lengthy incubation time and difficulties in handling and disposing of radioisotopes. These limitations have made it less preferred compared to enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunosorbent assay (ELISA) involves shorter assay time and has the advantage that it does not require handling of, and exposure to, radioisotopes. The principle of ELISA is based on antibody-antigen interaction. It can be in a direct, competitive or sandwich format. A sandwich ELISA using rabbit anti-ricin antibody was developed with a limit of detection (LOD) of 40 ng/ml for ricin in body fluids through colorimetric measurement [18]. Later enhanced colorimetric and chemiluminescence ELISA were explored with affinity-purified goat polyclonal antibodies in a sandwich format giving a LOD of 100 pg/ml for ricin in buffer as well as in human urine and serum [19]. Colorimetric measurement was made based on avidin-linked alkaline phosphatase (AP) at optical density of 405 nm. The enhancement on colorimetric assay was achieved by an increment in biotinylated antibody content and a reduction in dilution ratio of avidin-linked AP, giving a detection of 100 pg/ml for ricin in phosphate-buffered saline (PBS), human urine and human serum. This sandwich assay could also be detected based on chemi-luminescence but the quantitative work was limited to the range of 0.1–1 ng/ml and was subjected to greater variability compared to colorimetric assay. Colorimetric assay was performed in a sandwich format with affinity purified anti-ricin B chain MAb and anti-ricin A chain MAb that extracted ricin to give a detection at optical density of 450 nm [8]. The detection limit of ricin in phosphate-buffered saline, human urine and human serum was 5 ng/ml. Pradhan et al. [20], reported fluorescence immunoassay for ricin detection at concentration of 10 fg/ml in assay buffer and 20 fg/ml in plasma and red blood cells. Through coupling primary antibody with carboxylated latex particles Kumar et al. [21], reported a sensitized latex agglutination test for ricin to give a detection limit of 200 ng.
The lengthy assay time – due to several washing steps – and the limited throughput of classical ELISA was the reason for the introduction of laser-induced fluorescence detection, with a LOD of 100 pg/ml in buffer and 1 ng/ml in river water and an assay time of 20 minutes. This is known as fluorescence-based fiber optic immunoassay [22]. This fluorescence-based assay enhanced its sensitivity through the improved antibody binding activity attributed from the avidin-biotin principle.
The use of colloidal gold particles further allowed an analysis time of less than 10 minutes with a LOD of 50 ng/ml ricin in a buffer based on monoclonal antibodies and could enhance further to 100 pg/ ml with the use of silver enhancer [16]. Kumar et al. [23], reported detection of ricin at concentration of 1 ng/ml in buffer by immunochromatography technique (ICT)-based assay. The advantages of these gold particles were the irreduced aggregation, superior mobility and commercial availability. Generally, ELISA shows high sensitivity. However, the major limitation of these assays is the detection of both functional and non-functional ricin.
Alternatives to immunoassays have been developed, such as a hydrogel-based protein microchip [14] or array biosensors, providing the possibility of analysing several toxins in the same sample [24,25]. A planar array immunosensor [26], equipped with a charge-coupled device (CCD) was reported to simultaneously analyze three toxins, namely ricin, SEB and Yersinia pestis. It was a simple and disposable sensing array coated with different antibodies detected through CCD. This planar array platform was able to give a detection limit of 25 ng/ ml ricin, 5 ng/ml SEB and 15 ng/ml Y. pestis based upon goat anti-ricin antibody, sheep anti-SEB and rabbit anti-Pestis respectively in PBS containing 0.05% (v/v) Tween-20. The advantages of this detection platform were that it allowed multiple samples’ analysis, minimum sample amount required and simultaneous analysis inclusive of controls. This array technology [27] was extended further to analyze simultaneously six toxic compounds namely, ricin, SEB, cholera toxin, botulinum toxoids, trinitrotoluene and mycotoxin fumonisin.
A flow-based microarray platform [28] was developed using micro-sized array where the surface was fitted to a flow module with six channels. This flow module containing the analytes’ solution would flow through the microarray of fluorescence-coated antibodies and could detect low amount of proteins and bacteria rapidly in 15 min, giving a detection limit of 10 ng/ml for ricin, 4 ng/ml for SEB, 8 ng/ml for cholera toxin and 6.2×104 cfu/ml for Bacillus globligi in PBS containing 0.05% (v/v) Tween-20 and 0.1% (w/v) bovine serum albumin (PBSTB). The major advantage of such micro-sized array immunoassay would be the massive number of spots on a single array surface, allowing multiple samples to run in parallel.
Antibody has been the crucial element for immunoassay technology. However, due to its sensitivity to high temperature that could cause denaturation, its storage life is short. This issue could be resolved by a change of the receptor to aptamer chips [29]. Aptamers are functional binding species obtained from oligomers of selected nucleotides, which can be chemically synthesized. The adaptions of aptamer receptor into a microarray platform allowed long storage life and ease of transportation due to its high stability, and could also be regenerated without any loss of activity. However, its sensitivity could only be achieved at 320 ng/ml in PBS containing 5mM MgCl2.
As described earlier, the identification of ricin in serum, urine, and tissues is typically by ELISA, RIA or by portable biosensors with LODs in the range of 0.05 to 10 ng/ml [8,10,19,27,30]. Recently, amperometric immunosensor based on screen-printed electrodes, graphite and carbon nanotube was reported which can detect ricin at the level of 40 ng/ml [30] and 0.625-25 ng/ml respectively [31]. Such devices would be very useful in the case of the mass exposure of a large population to a biotoxin weapon.
Using the principle of microchip incorporated with gel components containing proteins/antibodies, a protein gel-based microchip [14] was manufactured. This gel-based microchip was used in conjunction with various immunoassays and detected by fluorescence, chemiluminescence equipped with CCD and mass spectrometry (MS). The highest sensitivity of 0.1 ng/ml for ricin and 1 ng/ml for SEB in PBS containing 0.15% polyvinyl alcohol (PVA) and 0.15% polyvinyl pyrrolidone (PVP) was achieved by sandwich immunoassay format. However, when analyzed simultaneously with multiple toxins on a single microchip, its sensitivity was reduced to 0.7 ng/ml of ricin. This gel-based microchip had good storage stability of 6 months due to its hydrophilic nature in the gel matrix, ease of interaction due to its three-dimensional gel structure and no cross reaction possible once proper selection of the antibody pairs were made but its major flaw was that several hours of analysis was required. It was mentioned that the approach of MS is promising since it was a one-step procedure with no antibody labeling requirement.
Other than looking for better replacement of the antibody for sensitivity improvement, development on the solid phase surface of immunoassay technology was also made. In immunomagnetic microsphere [32] surface, it involved two-phase chemiluminescence based techniques, fluorogenic-chemiluminescence (FCL) and electrochemiluminescence (ECL) using magnetic microspheres which gave detection limit of 1000 pg/ml and 0.5 pg/ml, respectively for ricin in PBS containing SuperBlockTM blocking buffer. These ECL and FCL detectors could identify multiple samples like SEB, botulinum A, Bacillus anthracis, Bacillus subtillus and Escherichia coli with high sensitivity. Advantages of such microsphere were its large surface area leading to enhanced sensitivity, free moving microspheres coated with antibody sped up the reaction rates and reduced the assay time, and easily detected by using simple magnetic field. Thus, it allowed a direct separation of target analyte from a complex mixture. Both FCL and ECL had similar formats except that FCL used alkaline phosphatase as label and detected through measurement of fluorescence whereas ECL used ruthenium-tribipyridal as label and detected through photo emission. For magnetoelastic sensor, the detection technology behind was a sandwich immunoassay on the sensor surface and used biocatalytic precipitation to cause a change in mass, which resulted a change in the resonance frequency that allowed for quantitation of ricin at 5 ng/ml in aqueous media such as water, blood and serum. This magnetoelastic sensor had sensitivity comparable to ELISA but at a much lower cost, disposable and relative fast analysis.
Lubelli et al. [15], described the use of the immuno-polymerase chain reaction (IPCR) for the detection of ricin in buffer solution as well as in human serum samples. IPCR is a very sensitive antigen detection method combining the specificity of immunological analysis with the exponential amplification of PCR. As a result, the limit of detection (LOD) of an ELISA is generally enhanced 100 to 10,000- fold by the use of PCR as a signal amplification system. The assay allowed the detection of ricin in human serum with a LOD of 10 fg/ ml. Kumar et al. had reported detection of ricin in buffer at 50 fg/ml by IPCR [33]. In comparing IPCR with conventional immunological methods, the greater amount of time required to perform the assay due to PCR and post-PCR analysis, the use of more expensive reagents, and the enhanced reagent consumption make this technique less attractive. However, these limitations are counterbalanced by the higher sensitivity, enabling a broader range of applications. The LOD of IPCR, as described in this article, is 8 million times lower than that of conventional ELISA. The amount of time required for the assay (9 h for IPCR and 11 h for sandwich IPCR) is approximately twice the amount of time required for traditional assays; however, it has already been demonstrated that the use of real-time PCR technology dramatically reduces the time of the experiments [34]. Therefore, real-time IPCR will become a very useful tool for antigen detection, providing high sensitivity in a relatively short time period. A list of detection methods used to reveal and detect ricin is reported in Table 2.
| Detection method | Limit of detection (ng/ml) | References |
|---|---|---|
| Quartz crystal microbalance sensors | 5000 | [28] |
| Fluoroimmunoassay | 1000 | [36] |
| Aptamer microarray | 320 | [29] |
| Lateral flow devices | 250 | [36] |
| ELISA | 80 | [8] |
| Inhibition of lysozyme | 80 | [31] |
| Immunochromatographic assay | 50 | [12] |
| Immunocapture coupled with LC-MALDI-MS | 50 | [54] |
| ELISA with colorimetric measurement | 40 | [18] |
| Microarray biosensor assay | 10 | [28] |
| Immunoassay | 5 | [9] |
| Fluoroimmunoassay | 1 | [35] |
| Biosensor assay | 0.5 | [27] |
| Microelectromechanical sensors assay | 0.4 | [36] |
| Microarray biosensor assay | 0.18 | [37] |
| Surface Plasmon Resonance | 0.1 | [39] |
| Sandwich avidin/biotin ELISA | 0.2 | [40] |
| Immunoassay on gel-based microchips | 0.1 | [14] |
| Chemiluminescence ELISA | 0.1 | [19] |
| Protein array | 0.1 | [41] |
| Luciferase-based assay | 0.001 | [42] |
| Piezoelectric detection | 10 μg/crystal | [38] |
| IPCR | 0.00001 | [15] |
| Radioimmunoassay | 0.05-0.1 | [10] |
| Immunocapture coupled with LC-ESI-MS/MS | 0.1 | [53] |
| Multiplex assay | 10-100 | [43] |
Table 2: Overview of ricin detection methods based on Lubelli et al. [15] and Ler et al. [57] with slight modifications.
Chemical methods
The use of capillary electrophoresis (CE) allows both rapid separation and rapid purification of complex mixtures. It can detect 10mg/ml ricin in buffer by UV detector [44]. The attracting force towards the use of CE was its ability to do both separation and purification of complex mixtures rapidly. The incorporation of the high specificity of antibody from the immunoassay technology to the high separating efficiency of CE formed capillary electrophoresis-based immunoassay (CEIA) [45]. CEIA allowed more flexibility such as custom making of analytes assay, less sample and reagents requirement and yet enabling simultaneous multiple samples determination with ease of results interpretation. This CE technology was improvised into a hand-held device [46] based on two microchip separations namely, gel (CGE) and zone electrophoresis (CZE). Using a microfluidic principle on chip, it allowed detection of fluorescence-labeled toxin by a miniaturized laserinduced fluorescence detection module. This system was miniaturized for ease of logistics but its sensitivity was not optimized giving only 25nM for CZE and 5 nM for CGE in buffer.
Coupled with mass spectrometry (MS) CE allows characterization of and differentiation between various forms of ricin toxin [47,48]. It provides a more precise and efficient analysis of ricin. Most proteinaceous toxins are not amenable to gas chromatography mass spectrometry analysis (GC–MS) due to possibility of denaturation by GC’s high temperature requirement and thus, liquid chromatography (LC) based methods was used.
Methods that use mass spectrometry for ricin analysis have been developed in recent years. Tryptic digestion and mass spectrometry methods have been used to identify, though not quantify, peptides unique to the ricin protein. Mass spectrometers employed in proteomic analysis used are either matrix-assisted laser desorption/ionization (MALDI) or electrospray ionization (ESI) modes. However, ESI has been the standard ionization method [49] for LC–MS and LC–MS/MS where it could be apply to various form of quadrupoles systems and quadrupole ion trap systems, giving a moderate resolution. Darby et al. [50], described liquid chromatography-electrospray/ mass spectrometry (LC-ES/MS) and matrix-assisted laser desorption/ ionization timeof- flight (MALDI-TOF) MS methods for the identification of ricin toxin and an additional alkaloid marker, ricinine, from crude plant material. They developed a general protein identification scheme for size classification followed by the analysis of the tryptic digest.
Fredriksson et al. [51], demonstrated that ricin can be identified by LC-ES-MS/MS experiments with reduced, cysteine-derivatized, trypsin-digest material. It was also shown that MALDI MS can be used to prove the presence of intact ricin and to screen samples for ricin peptides. To improve the method’s sensitivity and efficiency, a few marker peptides from the A and B chain were selected to establish an LC-MS/MS screening method. The specific trypsin digest peptides T5, T7, T11, T12 and T13 from the A chain and T3, T5, T14, T19, and T20 from the B chain were chosen and this procedure allowed a single analysis run per sample at maximum instrument detection sensitivity. As described by Ostin et al. [52], the use of organic solvent to assist trypsin digestion dramatically shortens the sample preparation time from an overnight digestion to a 1 h digestion. The response time for an unambiguous answer regarding the presence or absence of ricin in a sample is shortened from 24 h to 2 h. Furthermore, this procedure leaves the disulfide bonds in the protein intact, which indicates the presence of an intact toxin and provides additional forensic evidence for the analytical results.
Becher et al. [53], described an approach based on immunocapture by anti B-chain antibodies coupled to MS determination of the release of adenine by the A chain, which also allowed the sensitive and specific determination of the entire active toxin with a LOD of 0.1 ng/ml. This was the first method specifically detecting functional ricin with sensitivity similar to that of immunoassays and easily applicable to environmental samples. The assay required 26 h, but a preliminary response can be given after 6 h with threefold lower sensitivity. Nevertheless, a false-positive result is still possible through the nonspecific extraction of other RIPs, especially when working on complex environmental samples.
Duriez et al. [54], also used immunocapture based on this procedure to isolate ricin from liquid-based samples. Magnetic beads coated with protein G were used to attach monoclonal antibodies, which were specifically directed against the B-chain of ricin. When the sample was passed over the beads, only ricin was attracted to the antibodies. Other components of the sample remained in the solution. Ricin was eluted with a weak solution of trifluoroacetic acid. The extract was neutralized, then ricin was digested with trypsin to produce a mixture of peptides. The digestion was not conducted under typical proteomics conditions but in a mixture of acetonitrile and water, the added organic solvent giving higher specificity of trypsin and a greater digestion yield. In contrast to the previous procedure, MALDI MS was used for the detection and mass spectra of the resulting peptide mixture revealed 20 peptides. From these a panel of three was selected for detection, corresponding to the 161-169, 150-60 and 233-248 residues of the protein. High selectivity and good detection sensitivity were demonstrated, without interference from other peptides in the mass spectrum. The sequences were subjected to similarity searches to ensure that they were specific to ricin. In order to ensure accurate detection and to allow for peptide quantification, synthetic stable isotope-labelled analogues of the three peptides were added to the eluted ricin before tryptic digestion. Under these conditions, a detection limit of 50 ng/ml was obtained in a buffer solution spiked with ricin. This is higher than for existing immunoassay or bioassay methods, but is sufficient to measure the levels of ricin in environmental and food samples. For ricin detection in milk, the presence of lactose might present a problem because it is known to bind strongly to the ricin B-chain. However, for semiskimmed and skimmed milk spiked with ricin, the mass spectrometric intensities of the three peptides were 85–110 and 110–125% of those observed in buffer, showing that lactose did not inhibit ricin binding to the antibody. The method was tested on the detection of ricin in castor beans from different varieties of the genus Ricinus communis or from different geographical origins (Spain, Tanzania, Pakistan, India, China). In all cases, the antibody recognized the B-chain and the three diagnostic peptides were observed consistently. This implies that there are no apparent differences between the peptide sequences of the ricins from the different origins or cultivars. The researchers claim that this is the first proteomics-based method for the analysis of ricin in environmental samples. The overall analysis time is about 5 h, which is regarded as adequate for bio-terrorism incidents. Table 3 summarizes comparison of different ricin detection technologies with their advantages and limitations.
| Method | Detection and Limit of Detection | Advantages | Limitations |
|---|---|---|---|
| Radioimmunoassay |
|
Good sensitivity |
|
| ELISA |
|
High sensitivity |
|
| Lateral Flow Assay |
|
Simple, fast and reliable | Qualitative only |
| PCR |
|
|
|
| ImmunoPCR |
|
High sensitivity |
|
| Mass Spectrometry |
|
|
|
Table 3: Comparison of ricin detection methods.
Ricin can be inactivated by heat; therefore, characterization of denatured ricin is essential to differentiate it from native ricin and to avoid any ambiguity in its identification. Kumar et al. [55], earlier reported that toxicity of native ricin is lost as it is heated at 100°C for different time intervals. They also demonstrated that all the immunological methods are able to detect native and denatured ricin but could not distinguish them. There is always a probability of denatured non- toxic ricin being confused with native (toxic) ricin to create unnecessary panic. Also denatured ricin can be used in place of native ricin as a biothreat. Therefore a highly sensitive and specific detection system is required which can distinguish between toxic and nontoxic form of toxins and will help to overcome man-made / terroristcreated havocs in future. Analytical techniques like MALDI-TOF/MS, LC-ESI/ MS and MS-MS can recognize ricin specific peptides and may distinguish native/ denatured ricin. Sehgal et al. [56], had presented the results on the thermal stability and detection of ricin when present alone or in food samples by MALDI-TOF/MS. In addition to this, they had developed a method that can detect denatured ricin in spiked food samples by using antibody-capture followed by tryptic digestion and mass spectrometry detection of ricin specific peptides. In their study they obtained one differentiating peptide maker for denatured ricin which could distinguish it from native ricin. The identification of such markers in heat-treated ricin samples is essential for differentiating native and denatured ricin. Preparation of marker peptide library by advanced analytical techniques LC-ESI/MS and MS-MS will provide immense potential to the study.
Conclusion
Ricin toxin can be used as an agent for bioterrorism, and its potential for future use is a major concern. Sensitive methods of ricin detection have to be developed. The advances made by all of the technologies discussed in this article have brought the notion that the mass spectrometry has emerged as a leading technology for ricin analysis and has many advantages over traditional molecular biology methods. Mass spectrometry provides a direct measure of the toxin itself, is not affected by interfering proteins, and has high sensitivity and selectivity. In addition, quantification can be achieved with high precision, even in the presence of complex matrices. One of the limitations of mass spectrometry is that it is devoid of high throughput screening. Therefore, an integrated diagnostic approach is required to recognize the biological threats of the future. No single technology is sufficient to definitively identify any biological threat; thus, diagnostic systems must be able to detect multiple biological markers. Future systems must use a combination of immunological, mass spectrometric, gene amplification and classical identification methods to identify important bio markers. Within the next few years, more of these technologies will hopefully transition into readily available, rapid, highly efficient and specific handheld devices for ricin detection, thereby further increasing their value.
Acknowledgment
This work has been supported by institutional funding of Defence R & D Organization, Ministry of Defence, Government of India.
References
- Olsnes S, Pihl A (1973) Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 12: 3121–3126.
- Balint GA (1974) Ricin: the toxic protein of castor oil seeds. Toxicol2: 77–102.
- Rauber A, Heard J (1985) Castor bean toxicity re-examined: a new perspective. Vet Hum Toxicol 27: 498–502.
- Challoner KR, McCarron M.M (1990) Castor bean intoxication. Ann Emerg Med 19: 1177–1183.
- Bradberry SM, Dickers KJ, Rice P, Griffiths GD, Vale JA (2003) Ricin poisoning. Toxicol Rev 22: 65–70.
- Crompton R, Gall D (1980) Georgi Markov-Death in a pellet. Medico-legal J 48: 48-62.
- Audi J, Belson M, Patel M, Schier J, Osterloh J, et al. (2005) Ricin Poisoning A Comprehensive Review. JAMA 294: 2342-2350.
- Griffith GD, Newman H, Gee DJ (1986). Identification and quantification of ricin toxin in animal tissues using ELISA. J Forensic Sci Soc 26: 349-358.
- Shyu HF, Chiao DJ, Liu HW, Tang SS (2002) Monoclonal antibody basedenzyme immunoassay for detection of ricin. Hybrid Hybridomics 21: 69–73.
- Godal A, Olsnes S, Pihl A (1981) Radioimmunoassay of abrin and ricin in blood. J Toxicol Environ Health 8: 409–417.
- Franz DR, Jaax NK (1997) Ricin Toxin. In: Medical Aspects of Chemical and Biological Warfare. R Zajtchur (ed.) Borden Institute Walter Reed Army Medical Center Washington DC 32: 631-642.
- Shyu RH, Shyu HF, Liu HW, Tang SS (2002) Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 40:255–258.
- Narang U, Anderson GP, Lifler FS, Burans J (1997) Fiber optic based biosensor for ricin. Biosens Bioelectron 12: 937-945.
- Rubina Ayu, Dyukova VI, Dementieva EI, Stomakhin AA, Nesmeyanov VA, et al. (2005) Quantitative immunoassay of biotoxins on hydrogel-basedprotein microchip. Anal Biochem 340: 317-329.
- Lubelli C, Chatgilialoglu A, Bolognesi A, Strocchi P, Colombatti M, et al. (2006) Detection of ricin and other ribosome-inactivating proteins by an immuno-polymerase chain reaction assay. Anal Biochem355: 102–109.
- Pradhan S, Boopathi M, Kumar O, Baghael A, Pandey P, et al. (2009) Molecularly imprinted nanopatterns for the recognition of biological warfare agent ricin. Biosens Bioelectron25: 592-598.
- Ramakrishnan S, Eagle MR, Houston LL (1982) Radioimmunoassay for ricin A and B chains applied to samples of ricin A-chain prepared by chromatofocussing and by DEAE Biogel A chromatography. Biochem Biophys Acta 719: 341-348.
- Koja N, Shibata T, Mochida K (1980). Enzyme-linked immunoassay of ricin. Toxicon 18: 611-618.
- Poli MA, Rivera VR, Hewetson JF, Merill GA (1994) Detection of ricin by colorimetric and chemiluminescence ELISA. Toxicon 32: 1371–1377.
- Pradhan S, Kumar O, Jatav PC, Singh S (2009) Detection of ricin by comparative indirect ELISA using fluroscence probe in blood and red blood cells. J Med CBRDef 7.
- Kumar O, Rai GP, Parida M., Vijayaraghavan R (2004). Rapid detection of ricin by sensitizing carboxylated latex particles by ricin antibodies. Defense Sci J 54: 57-63.
- Narang U, Anderson GP, Lifler FS, Burans J (1997) Fiber optic based biosensor for ricin. Biosens Bioelectron12: 937-945.
- Kumar O, Pradhan S, Vijayaraghavan R (2008) Indian patent filed for “ immunochromatography technique based simple, rapid and field detection system for ricin”. Ref No 831/DEL/2008.
- Meagher RJ, Hatch AV, Renzi RF, Singh AK (2008) An integrated micrfludic platform for sensitive and rapid detection of biological toxins. Lab Chip 8: 2046-2053.
- Suresh S, Kumar O, Kolhe P, Rao VK, Sekhar K (2007) Detection of ricin in water samples using disposable screen-printed electrodes. Defence Sci J 57: 839-844.
- Wadkins RM, Golden JP, Pritsiolas LM, Ligler FS (1998) Detection of multiple toxic agents using a planar array immunosensor. Biosens. Bioelectron. 13: 407-415.
- Ligler FS, Taitt CR, Shriver-Lake LC, Sapsford KE, Shubin Y, et al. (2003) Array biosensor for detection of toxins. Anal Bioanal Chem 377: 469–477.
- Delehanty JB, Ligler FS (2002) A microarray immunoassay for simultaneous detection of proteins and bacteria. Anal Chem 74: 5681-5687.
- Kirby R, Cho EJ, Gehrke B, Bayer T, Park YS, et al. (2004) Aptamer based sensor arrays for the detection and quantification of proteins. Anal Chem76: 4066-4075.
- Garber EA, Eppley RM, Stack ME, McLaughlin MA, Park DL (2005) Feasibility of immunodiagnostic devices for the detection of ricin, amanitin, and T-2 toxin in food. J Food Prot 68: 1294–301.
- Ghosh M, Bachhawat BK, Surolia A (1979) A rapid and sensitive assay for detection of nanogram quantities of castor bean ( Ricinus communis) lectins. Biochem J 183: 185-188.
- Ruan C, Varghese OK, Grimes CA, Zeng K, Yang X, et al. (2004) A magnetoelastic ricin immunosensor. Sens Lett 2: 138-144.
- Kumar O, Singh Y, Sehgal P, Vijayaraghavan R (2009) Indian Patent filed for “Development of an improved and sensitive Immuno-PCR for detection of ricin”.Ref No. 85/DEL/2009.
- Mackay IM (2004) Real-time PCR in the microbiology laboratory. Clin Microbiol Infect 10: 190–212.
- Wang L, Cole KD, Gaigalas AK, Zhang YZ (2005) Fluorescent nanometer microspheres as reporter for sensitive detection of stimulants of biological threats using multiplexed suspension arrays.Bioconjug Chem 16: 194-199.
- Ji HF, Yang X, Zhang J, Thundat T (2004) Molecular recognition of biowarfare agents using micromechanical sensors. Expert Rev Mol Diagn4: 859-866.
- Dill K, Montgomery DD, Ghindilis AL, Schwarzkopf KR, Ragsdale SR, et al. (2004) Immunoassays based on electrochemical detection using microelectrode arrays.Biosens Bioelectron 20: 736-742.
- Carter RM, Jacobs MB, Lubrano GJ, Guibault GG (1995) Piezoelectric detection of ricin and affinity purified goat anti-ricin antibody. Anal Lett 28: 1379-1386.
- Feltis BN, Sexton BA, Glenn FL, Best MJ, Wilkins J, et al. (2008) A hand-held surface plasmon resonance biosensor for the detection of ricin and other biological agents. Biosens Bioelectron23: 1131-1136.
- Leith AG, Griffiths GD, Green MA (1988) Quantification of ricin toxin using a highly sensitive avidin/biotin enzyme-linked immunosorbent assay. J Forensic Sci Soc28: 227-236.
- Huelseweh B, Ehricht R, Marschall HJ (2006) A simple and rapid protein assay based method for the simultaneous detection of biowarfare agents. Proteomics6: 2972-2981.
- Zhao L, Haslam DB (2005) A quantitative and highly sensitive luciferase based assay for bacterial toxins that inhibit protein synthesis. J Med Microbiol 54: 1023-1030.
- Garber EA, Venkateswaran KV, O'Brien TW (2010) Simultaneous multiplex detection and confirmation of the proteinaceous toxins abrin, ricin, botulinum toxins, and Staphylococcus enterotoxins A, B, and C in food. J Agric Food Chem58: 6600-6607.
- Hines HB, Breuggemann EE (1994) Factors affecting the capillary electrophoresis of ricin, a glycoprotein. J Chromatogr A 670: 199-208.
- Yeung WSB, Luo GA, Wang QG, Ou JP (2003) Capillary electrophoresis-based immunoassay. J Chromatogr B 797: 217-228.
- Fruetel JA, Renzi RF, Vandernoot VA, Stamps J, Horn BA et al. (2005) Microchip separations of protein biotoxins using an integrated hand-held device. Electrophoresis 26: 1144-1154.
- Despeyroux D, Walker N, Pearce M, Fisher M, Donnell MMc, et al. (2000) Characterization of ricin heterogeneity by electronspray mass spectrometry, capillary electrophoresis and resonant mirror. Anal Biochem 279: 23-36.
- Na DH, Cho CK, Youn YS, Choi Y, Lee KR, et al. (2004) Capillary electrophoresis to characterize ricin and its subunits with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Toxicon43: 329-335.
- Badwin MA (2004) Protein identification by Mass Spectrometry. Molecular and Cellular Proteomics 3: 1-9.
- Darby SM, Miller ML, Allen RO (2001) Forensic determination of ricin and the alkaloid marker ricinine from catsor castor bean extracts. J Forensic Sci46: 1033-1042.
- Fredriksson SA, Hulst AG, Artursson E, de Jong AL, Nilsson C, et al. (2005) Forensic identification of neat ricin and of ricin from crude castor bean extracts by mass spectrometry. Anal Chem 77: 1545-1555.
- Ostin A, Bergstrom T, Fredriksson SA, Nilsson C (2007) Solvent assisted trypsin digestion of ricin for forensic identification by LC-ESI MS/MS. Anal Chem 79: 6271–6278.
- Becher F, Duriez E, Vollard H, Tabet JC, Ezan E (2007) Detection of functional ricin by immunoaffinity and liquid chromatography-tandem mass spectrometry.Anal Chem 79: 659-665.
- Duriez E, Fenialle F, Tabet JC, Lamourette P, Hilarie D, et al. (2008) Detection of ricin in complex samples by immunocapture and matrix-assisted laser desorption/ionization of time-of-flight mass spectrometry. J Proteome Res7: 4154-4163.
- Kumar O, Pradhan S, Sehgal P, Singh Y, Vijayaraghavan R (2010) Denatured ricin can be detected as native ricin by immunological methods but non-toxic in vivo. J Forensic Sci 55: 801-807.
- Sehgal P, Rao MK, Kumar O, Vijayaraghavan R (2010) Characterization of Native and Denatured Ricin using MALDI-TOF/MS. Cell Mol Biol 56: 1385-1399.
- Cook DL, Jonathan D, Griffiths GD (2006) Retrospective identification of ricin in animal tissues following administration by pulmonary and oral routes. Toxicology 223: 61–70.
- Ler SG, Lee FK, Gopalakrishnakone P (2006) Trends in Detection of Warfare Agents. Detection Methods for Ricin, Staphylococcal Enterotoxin B and T-2 Toxin. J Chromatogr A 1133: 1–12.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 16055
- [From(publication date):
specialissue-2014 - Dec 21, 2025] - Breakdown by view type
- HTML page views : 11267
- PDF downloads : 4788