Asthma in HIV-Infected Population: A Review of Respiratory Symptoms, Pulmonary Function Abnormalities and Pathophysiology
Received: 27-May-2014 / Accepted Date: 09-Jul-2014 / Published Date: 16-Jul-2014 DOI: 10.4172/2161-1165.1000164
Abstract
Asthma is prevalent around the world, requiring frequent hospital admissions and resulting in significant morbidity. National surveillance for asthma in the United States estimated the prevalence of asthma from 2006-2008 to be approximately 7.3% in adults, with the prevalence being higher in females (8.9%) than males (5.5%). The pathophysiology of asthma is characterized by inflammation and hyper-responsiveness of lung bronchioles. There is emerging evidence indicating an association between asthma and human immunodeficiency virus (HIV) infection, but much remains to be learned. The article will review the prevalence of respiratory symptoms, pulmonary function abnormalities and asthma in the HIV-infected population and summarize recent research focusing on potential mechanisms linking the two disease processes. In addition, we review important asthma treatment considerations in HIV-infected individuals.
Keywords: Asthma; Lung bronchioles; Human Immunodeficiency Virus
161744Respiratory Symptoms and Pulmonary Function in HIV-Infected Individuals
Several studies have investigated prevalence of respiratory symptoms in the HIV-infected population (Table 1). Before anti-retroviral therapy (ART), there was a high prevalence of dyspnea and cough in this population [2]. These studies showed low CD4 cell counts, history of smoking and intravenous drug use, and previous history of pneumonia were associated with increased risk of dyspnea and cough symptoms. More recent studies in the post-ART era show that respiratory symptoms continue to be common in HIV-infected individuals, with the prevalence of cough ranging from 23-37% and prevalence of dyspnea ranging from 16-44% [3,4]. In these studies, significant predictors of respiratory symptoms include age, history of intravenous drug use, smoking history, higher HIV RNA levels and history of pneumonia. In addition George et al.found that forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) was significantly lower in participants with respiratory symptoms [3]. In conclusion, respiratory symptoms are common in the HIV-infected population, and individuals with intravenous drug use, history of smoking and pneumonia appear to be at high risk.
| Author and Year | Design and Methods | Major Results |
|---|---|---|
| Diaz et al.[38] | 327 HIV-infected and 52 HIV-uninfected controls were given the ATS-DLD questionnaire. Majority of individuals were white males. | HIV-infected individuals had a greater prevalence of respiratory symptoms, including dyspnea (41.6% vs. 7.7%), cough (40% vs. 25%), and phlegm production (41.0% vs. 23.1%). Smoking was consistently associated with respiratory symptoms. |
| George et al.[3] | Cross-sectional study of 234 HIV-infected individuals at an outpatient clinic with mean age of 44.1 years. Majority of subjects were male, had smoking history and were being treated with ART. Subjects answered questions about respiratory symptoms. | Prevalence of any respiratory symptom was 31.5%, with 23% of patients having cough and 16% having dyspnea on exertion. Respiratory symptoms positively associated with age, smoking history, lower FEV1/FVC, and higher HIV RNA levels. |
| Gingo et al.[4] | Cohort of 167 HIV-infected individuals with mean age of 46 years. Majority of subjects were male and 49.7% were African Americans. Eight-one percent were on ART, and the majority were current or prior smokers.Modified SGRQ used to assess respiratory symptoms. | 63.5% of patients had respiratory symptoms, and 27.5% reported inhaler use. Dyspnea (43.7%) and cough (37.1%) were the most common symptoms. Individuals with symptoms were more likely to be ever smokers (OR 2.40, 95% CI 1.16 to 4.16, p=0.02) or ever use intravenous drugs (OR 3.64, 95% CI 1.32 to 10.06, p=0.01) |
| Cui et al.[39] | Cohort of 120 HIV-infected participants with mean age of 43.4. Majority of participants were male, Caucasian and on ART. Participants answered a questionnaire about respiratory symptoms, including cough, sputum production or breathlessness. | 53% of patients had at least one respiratory symptom. Smokers had a higher likelihood of having at least one respiratory symptom (OR=4.9, 95% CI 2.0 to 11.8, p<0.0001) after controlling for ART. Smoking was a significant risk factor for each symptom. |
| Leung et al. [40] | Cohort of 199 HIV-infected males with mean age 49.3±10.1 years. 52% smokers and majority on ART. All participants were administered the SGRQ. | Worse SGRQ scores associated with history of viral load > 100,000 copies/ml (p=0.043), lower nadir CD4 count (p=0.040), and current CD4 count ≤ 350 cells/μL (p=0.005). |
Table 1: Studies of respiratory symptoms in HIV-infected individuals in the post-ART era
In addition to respiratory symptoms in HIV-infected individuals, investigators have also long noted an association between HIV and obstructive lung disease (Table 2). Two recent studies found an association between low FEV1/FVC and age, pack year history of smoking, history of bacterial pneumonia, and use of ART [3,4]. Although these studies find that smoking and ART use is associated with worse obstruction, there is also evidence that indicates an association of obstructive lung disease with poor HIV control. In a cohort of urban intravenous drug users, HIV-infected participants with HIV viral loads ≥ 200,000 copies/ml were at significantly higher risk of obstructive lung disease (OR 3.41, 95% CI 1.24 to 9.39, p=0.02) compared to HIV-uninfected participants [5]. Analysis of pulmonary function over time in this cohort showed that participants with an HIV viral load >75,000 copies/ml had a greater annual decline in FEV1 and FVC compared to HIV-uninfected participants and HIV participants with viral load ≤ 75,000 copies/ml [6]. A similar association was found between CD4 cell count and pulmonary function, and participants with CD4 counts< 100 cells/mm3were found to have the most rapid decline in FEV1 and FVC compared to HIV-uninfected participants. Overall, there is a high prevalence of obstructive lung disease in the HIV-infected population, and risk factors for obstructive lung disease in this population include intravenous drug use, history of smoking, history of pneumonia, and poor HIV control.
| Author and Year | Design and Methods | Major Results |
|---|---|---|
| George et al.[3] | Cross-sectional study of 234 HIV-infected individuals at an outpatient clinic with mean age of 44.1 years. Pre-bronchodilator spirometry according to the ATS guidelines done. |
6.8% had OLD, of these 62.5% were GOLD stage I, 37.5% GOLD stage II. Lower FEV1/FVC had a positive independent association with age, more pack-years smoking and history of bacterial pneumonia. ART had an independent association with lower FEV1/FVC. |
| Gingo et al. [4] | Cohort of 167 HIV-infected individuals with mean age of 46 years. 26.4% were female, 49.7% African-Americans. All participants performed pre- and post-bronchodilator spirometry and measurements of diffusion capacity. |
21% had irreversible airway obstruction (FEV1/FVC<0.70). Airway obstruction more common in ever-smokers than never smokers (26.8% vs. 2.5%, p=0.001) Irreversible airway obstruction independently associated with intravenous drug use, increased pack years smoked, and use of ART. |
| Hirani et al. [41] | Cohort of 98 HIV-infected participants with mean age of 45 years. Majority of participants were males and had current or prior smoking history. Eightly-eight percent on ART. All participants underwent spirometry and were given the SGRQ. |
OLD prevalence in non-smokers was 13.6% vs. 18.5% in smokers based on spirometry criteria. SGRQ scores were higher in those with spirometry defined OLD. |
| Drummond et al. [5] | 1077 participants with intravenous drug use in the Baltimore area with median age 48±8 years. Majority were male, and African-American with a smoking history.303 were HIV-infected.Pre- and post-bronchodilator performed according to ATS guidelines. | 16% patients had spirometry-defined OLD. HIV-infected participants with viral load > 200,000 copies/ml had a 3.4-fold higher risk of OLD (OR 3.41, 95% CI 1.24 to 9.39, p=0.02). Association remained after controlling for ART use. No relationship found between CD4 counts and OLD. Each 10-year increase in age, history of smoking and bacterial pneumonia had a positive independent association with OLD. |
| Drummond et al. [6] | 1064 participants who reported intravenous drug use in the Baltimore area with mean age 48±7 years. Majority were male, smokers and African-American. 316 were HIV-infected. Pre-bronchodilator spirometry obtained over the course of follow up, median follow up of 2.75 years | HIV infection independently associated with lower absolute FEV1 (-154ml, 95% CI -221 to -86, p<0.001) and FVC (-210ml, 95% CI -288 to -132 ml, p<0.001) Compared to HIV-uninfected participants, HIV-infected participants with a viral load >75,000 copies/ml had a greater adjusted annual FEV1 decline (-99.1 vs. -23.5 ml/year, p=0.004) and a greater adjusted annual FVC decline (-74.0 vs. 8.24 ml/year, p=0.008) Individuals with lowest CD4 counts < 100 cells/mm3 experienced the most rapid decline in FEV1 and FVC compared to HIV-uninfected participants. |
Table 2: Studies of pulmonary function testing in HIV-infected cohorts during the post-ART eraSGRQ = St. George Respiratory Questionnaire.
Association between Asthma and HIV
The association between asthma and HIV has not been studied as well as the association between HIV and airway obstruction in general. In the pre-ART era, studies investigated an association between HIV and bronchial hyper-responsiveness, an important feature of the pathophysiology of asthma (Table 3). A small study of 66 HIV-infected and matched HIV-uninfected controls showed that airway hyper-responsiveness was not more common in HIV-infected subjects compared to HIV-uninfected controls (19.3% vs. 12.9%, p>0.1) [7]. This study used methacholine challenge testing as the means to detect airway hyper-responsiveness, which was defined as a decline of ≥ 20% in FEV1 from the post-diluent value after inhalation of ≤125 cumulative breath units. In contrast, another early study with 105 individuals with Acquired Immunodeficiency Syndrome (AIDS) found that 33% of participants had decreased airflow rates and 31% had significant response to bronchodilators [8]. Poirier et al. did the largest study of bronchial hyper-responsiveness in 248 male HIV-infected and 236 HIV-uninfected participants [9].
| Author and Year | Design and Methods | Major Results |
|---|---|---|
| Wallace et al. [7] | Cohort of 66 HIV-infected and matched HIV-uninfected participants. Methacholine challenge testing performed on participants. AHR defined as ≥ 20% decline in FEV1 from post-dilutent value after inhalation of ≤ 125 cumulative breath units. |
AHR not more common in HIV-infected participants compared to HIV-uninfected participants (19.3%vs.12.9%, p>0.1). Participants with AHR were more likely to have an episode of pneumonia in 2-years (46% vs. 9%, p<0.01), ≥1 episode of asthma during the study period (39% vs. 1%, p<0.001), and wheeze noted during clinic visits (62% vs. 17%, p<0.01). |
| Poirier et al. [9] | Cross-sectional study of prevalence of asthma in HIV-infected population. 248 male HIV-infected and 238 HIV-uninfected participants selected randomly. Answered ECRHQ for asthma and asthma-like conditions. Spirometry obtained according to ATS guidelines. Methacholine administered to a maximum dose of 2mg and testing stopped with a 20% fall in FEV1. |
HIV-infected males were likely to be older, currently smoke, have BHR (26% vs. 14.4%) and have an elevated IgE level (37.8% vs. 25.7%) BHR was significant only in smokers when comparing the two arms (p<0.005). In HIV-infected males, independent predictors of BHR were lower FEV1/FVC (p=0.0001) and more likely to have elevated IgE (p=0.0035). |
| Crothers et al. [10] | Data used from Veterans Aging Cohort Study Virtual Cohort with 33,420 HIV-infected subjects and 66,840 HIV-uninfected participants median age 45 years. Majority of participants were male smokers and 40% African-American ICD-9 codes used to determine pulmonary conditions. |
In both HIV-infected and HIV-uninfected participants, asthma was the second most common pulmonary diagnosis with similar incidence of 5.6 (95% CI, 5.2 to 5.9) and 5.6 (95% CI, 5.4 to 5.8) per 1,000 person years (p=0.6) HIV was an independent risk factor for all pulmonary conditions except asthma. |
| Gingo et al. [4] | Cohort of 223 HIV-infected participants. Participants answered questions about whether they had ever been diagnosed with asthma and when. Pre- and post-bronchodilator (with 480 μg of albuterol) were done on all participants according to ATS guidelines. Bronchodilator reversibility noted if there was an improvement in either the FEV1 or FVC of at least 200ml or 12%. |
20.6% of participants reported doctor-diagnosed asthma. BDR noted in 9% of cohort. Individuals with doctor-diagnosed asthma were likely to be younger, female, have history of bacterial or Pneumocystis pneumonia, be current smokers and less likely to be on ART currently. They were also more likely to have worse spirometry but no more likely to have BDR. Prevalence of doctor-diagnosed asthma was significantly greater in the highest BMI quartile, and prevalence increased with each quartile. High sputum eosinophil count more common with doctor-diagnosed asthma. Doctor-diagnosed asthma was more common in participants with high sputum IL-4 (27% vs. 10.5%, p=0.02) and RANTES (26.6% vs. 9.8%, p=0.02). BDR was more common in those with high plasma MIP-1α (26.3% vs. 4.8%, p=0.002), and high sputum MIP-1β (26.1% vs. 4.3%, p=0.001) |
| Kendall et al. [12] | Validated algorithm used to identify HIV-infected participants (n=14,005) in Ontario. HIV-uninfected arm (n=71,410) selected randomly. HIV-infected were more likely to be male and less likely to be in youngest (18-35yo) or oldest (>65yo) age group. Previously validated algorithms used to determine the status of various comorbidities in the participants. |
Prevalence of asthma in the HIV-infected population was 12.7% (95% CI 12.2% to13.3%) with the prevalence ratio in the two arms was 1.31 (95% CI 1.20 to 1.43). Prevalence of multi-morbidity (≥ 2 chronic conditions) in HIV-infected cohort was 10.8% (95% CI 10.3% to 11.3%), and the prevalence ratio was 1.30 (95% CI 1.18 to 1.44) |
Table 3: Overview of asthma studies in HIV-infected cohorts
There was a statistically significant greater prevalence of current wheezing in the HIV-infected participants compared to HIV-uninfected participants (54.4%, vs. 21.2%, p<0.001). The prevalence of bronchial hyper-responsiveness was only statistically significant in HIV-infected smokers vs. HIV-uninfected smokers (30.1% vs. 13.3%, p<0.05). HIV-infected participants with bronchial hyper-responsiveness were more likely have a reduced FEV1/FVC (0.76 vs. 0.81, p=0.001) and elevated IgE levels > 100IU/ml (43.1 vs. 23.5, p=0.005), and they tended to be more likely to have a positive skin allergy test (63.1 vs. 49.7, p=0.06). Overall, in the pre-ART era, bronchial hyper-responsiveness appeared to be common in HIV-infected individuals, especially those with a history of smoking.
In the era of ART, only a few studies have examined asthma in HIV. A large study focused on pulmonary disease in patients in the Veterans Health system and used a cohort of HIV-infected (n=33,420) and HIV-uninfected (n=66,840) individuals in the Veterans Aging Cohort [10]. The median age of the cohort was 45 years with most participants being male and smokers. In both groups asthma was the second most common ICD-9 diagnosis with the incidence being similar in both groups (5.6 per 1000 person years in HIV-infected cohort vs. 5.6 per 1000 person years in HIV-uninfected cohort, p=0.6).
In this study, HIV was not an independent risk factor for asthma, but pulmonary testing was not performed. Incident pulmonary diseases, including asthma, COPD and pulmonary infections were less likely in participants with lower HIV RNA levels and use of ART at baseline. In another study with a cohort of 223 HIV-infected participants who performed spirometry, 9% had a bronchodilator response [11]. Participants with a bronchodilator response were more likely to have abnormal spirometry and wheezing. This study also found the prevalence of doctor-diagnosed asthma was 20.6%, much higher compared to a recent estimate of 7.3% in the general population, although it is difficult to make direct comparisons [1].
Risk factors for doctor-diagnosed asthma in the cohort including parental history of asthma, female sex, obesity, current smoking, history of Pneumocystis pneumonia, and not being on ART. Another recent study compared prevalence of comorbidities in an HIV-infected population (n=14,005) and their age and sex-matched controls using administrative data from the Canadian health care system in Ontario [12]. The study population in this analysis was mostly males living in an urban setting with an average age of 45.36 years. HIV-infected participants had a high prevalence of asthma (12.7%, 95% CI 12.2% to 13.3%), and the prevalence ratio of asthma in HIV-infected vs. HIV-uninfected individuals was 1.31 (95% CI 1.20 to 1.43). In the same study, chronic obstructive pulmonary disease (COPD) was also a common comorbidity with a prevalence in HIV-infected persons of 7.9% (95% CI 7.5% to 8.3%), which was about 38% less prevalent than asthma. In conclusion, HIV-infected individuals appear to have a higher prevalence of asthma and bronchial hyper-responsiveness compared to the general population, but large studies specifically testing for asthma in the HIV-infected population and appropriate control groups are lacking.
Updates in Asthma Pathophysiology
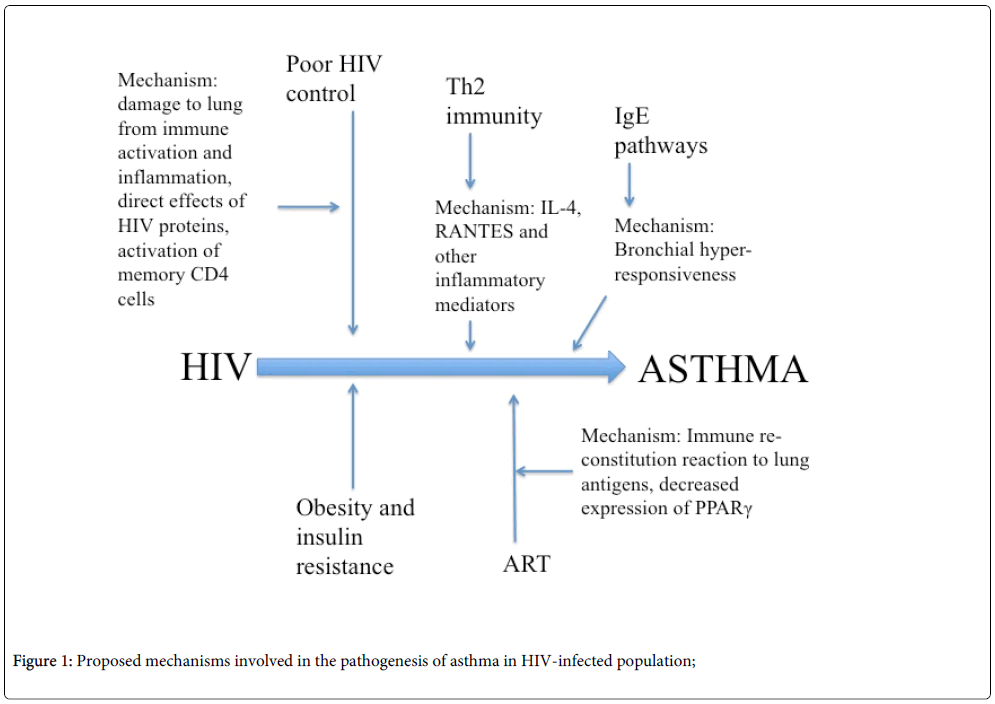
Specific mechanisms resulting in asthma in the HIV-infected population are not well-understood. Investigators have theorized about potential roles of ART, inflammation, and obesity in the pathogenesis of asthma in this population (Figure 1), but definitive studies are lacking. Causes of asthma may be similar to those in the HIV-uninfected population, but there may be mechanisms unique to HIV infection as well. Potential HIV-related mechanisms leading to asthma include damage to the lung from immune activation and inflammation, direct effect of HIV proteins, or activation of memory CD4 T cells [3]. Mechanisms may also vary by asthma “phenotype” as is seen in the HIV-uninfected population. Recent research shows there are different phenotypes of asthma based on the severity of symptoms, key inflammatory biomarkers and corticosteroid response [13]. Whether such phenotypes exist in HIV-infected individuals is not known.
One factor to consider in the pathogenesis of asthma in HIV-infected individuals is the role of HIV virus. As noted above, some studies indicate that higher viral loads and poor HIV control are associated with worse lung function [5,6]. In addition, some studies have shown that higher HIV RNA levels are predictors of respiratory symptoms [3]. These studies would indicate that the virus itself may be important in pathways that can lead to asthma. Unfortunately, research studying the mechanisms involving the virus itself leading to asthma is lacking.
Interestingly, ART itself has been associated with the pathogenesis of asthma in HIV-infected individuals, but data are conflicting. Evidence suggesting a detrimental relationship with ART comes from pediatric literature. A large study compared asthma medication use in HIV-infected children on ART to medication use in HIV-infected children not on ART [14]. Based on a time dependent Cox model, researchers found that HIV-infected participants on ART were more likely to be on asthma medication compared to HIV-infected participants not on ART (hazard ratio=3.34, p=0.01). In addition, the study analyzed asthma medication use at follow up in participants born in pre-ART era. The prevalence of asthma medication use at 11 years of age in this group was higher in HIV-infected participants on ART compared to those not on ART (OR 3.38, p=0.02). The researchers hypothesized that loss of CD4 T cells in untreated HIV-infected children is protective against asthma, and ART acts as a potential risk factor for asthma due to immune-reconstitution of CD4 T cells. Similar results were seen in an earlier smaller study of 136 HIV-infected adult participants in a community clinic showed that a recent CD4 count of ≥ 200 cells/dL was positively associated with current asthma (p=0.01) [15]. In addition, the mean CD4 count in participants with asthma was significantly higher than other participants (log CD4 cells/μ l5.63±0.73 vs. 4.92±1.38, p=0.01). Potential mechanisms involving ART in the pathogenesis of asthma include auto-immune reaction or an immune reconstitution-like reaction to antigens in lungs and direct effects of ART. Overall, the role of ART in asthma pathogenesis in the HIV-infected population is unclear and may differ in children and adults, possibly due to varying underlying causes of asthma.
Certain inflammatory pathways and cytokines may be important in the pathogenesis of asthma in the HIV-infected population. Immunoglobulin E (IgE) is a well-known mediator of allergy and asthma. Early studies showed significantly higher IgE levels in the HIV-infected population with CD4 cell count ≤ 200 cells/mm3, and there was an inverse relationship between IgE level and both helper T cell and suppressor/cytotoxic T cell numbers [16]. Another study showed IgE levels were elevated in HIV-infected vs. HIV-uninfected participants (log IgE IU/ml 4.40±0.48 vs. 3.60±0.18, p<0.05), with the difference being especially strong when comparing participants with AIDS (log IgE IU/ml 4.96±0.23 vs. 3.60±0.18, p<0.005) [17]. The study also showed that IgE levels were important markers of disease progression and survival. The exact etiology of elevated IgE levels is not well-understood, but T helper cells may play a role. HIV leads to decreases and alterations in T helper cells, which are critical in IgE synthesis [2]. More recently, IgE levels have been shown to be positively associated with sputum eosinophil count in a cohort of 223 HIV-infected participants [11]. An older study found elevated IgE levels to be independent predictors of bronchial hyper-responsiveness based on methacholine challenge (p=0.0035) [9]. Thus, the clinical significance of elevated IgE levels in HIV-infected individuals needs to be further investigated, along with the relationship between elevated IgE levels and asthma in HIV. Future research focusing on correlation between elevated IgE levels and pulmonary function, and interventions to decrease the IgE levels in HIV-infected individuals could provide further insight into the role of IgE in this population.
Recent studies also provide evidence for involvement of other inflammatory mediators in the development of asthma in HIV. In the cohort of 223 HIV-infected participants discussed above, doctor-diagnosed asthma appeared to be more common in participants with high sputum interleukin (IL-4) (27% with asthma if high IL-4 vs. 10.5% with asthma if low IL-4, p=0.02), and high regulated on activation, normal T cell expressed and secreted (RANTES) (26% vs. 9.8%, p=0.02) [11]. The association between IL-4 and RANTES with doctor-diagnosed asthma indicates a role of type 2 T helper (Th2) cells in the pathogenesis of asthma in the HIV-infected population. Bronchodilator response was associated with high plasma macrophage inflammatory protein (MIP)-1α (26.3% with asthma if high MIP-1α vs. 4.8% with asthma if low MIP-1α, p=0.002) and high sputum MIP-1β (4.6% vs. 1.0%, p<0.001). RANTES, MIP-1α and MIP-1β are chemokines that suppress HIV and are released by CD8 T cells during chronic HIV infection [18]. These studies suggest that there is a complex role of inflammation in the pathogenesis of asthma in HIV-infected individuals.
One proposed mechanism that could link ART and asthma involves the peroxisome-proliferator activated receptor γ (PPARγ), which has been shown to be involved in pathogenesis of lipodystrophy in HIV-infected individuals likely secondary to decreased PPARγ expression due to ART [19]. PPARγ is produced by adipocytes and is regulates genes associated with growth, insulin sensitivity and immunity. PPARγ also regulates production and secretion of adipokines such as leptin and adiponectin, which have been shown to be associated with asthma, as discussed below. Given the metabolic effects of PPARγ in the general population, the role of PPARγ in HIV-uninfected asthmatics is also being studied. In a study of 34 non-smoker asthmatic participants, Benayoun et al.showed that PPARγ expression was increased in bronchial submucosa, bronchial epithelium and airway smooth muscle in steroid-untreated asthmatics [20]. In comparison, steroid-treated asthmatics had significantly lower PPARγ levels, especially in bronchial submucosa and airway smooth muscle. The researchers also noted that degree of PPARγ expression negatively correlated with FEV1. In contrast, Spears et al.showed that PPARγ agonist rosiglitazone improved FEV1 (183 ml, p=0.051) in asthmatic smokers [21]. Since PPARγ plays a role in asthma pathogenesis and HIV-related lipodystrophy, it may be an important factor in obesity related HIV-associated asthma.
In the general population, there are emerging links between obesity and asthma. Several studies of adults and children reported an association between elevated body mass index (BMI) and prevalence of asthma. A recent meta-analysis showed that BMI in the overweight and obese range increased risk of 1 year incidence asthma diagnosis (OR 1.51, 95% CI 1.27 to 1.80) [22]. Researchers also noted a dose-response effect of high BMI on asthma diagnosis. Associations between obesity and asthma in HIV-infected population have recently been investigated in a cohort of 1833 HIV-infected participants researchers, finding that obesity was a risk factor for multi-morbidity compared to HIV-infected individuals with normal BMI (OR 1.52, 95% CI 1.15 to 2.00) [23]. In HIV-infected individuals, the odds of doctor-diagnosed asthma was 2.3 times greater in obese compared to non-obese participants [11]. These studies indicate that obesity phenotype asthma is prevalent in HIV-infected population. One potential mechanism linking obesity and asthma is inflammation, and studies in this area have focused on adipokines produced by adipocytes [24-26]. Leptin is a proinflammatory adipokine that increases production of inflammatory cytokines. In contrast, adiponectin is an adipokine that downregulates the production of inflammatory cytokines. Both leptin and adiponectin have been associated with asthma in mouse models, but data on the effects of these adipokines in human samples are lacking. The mechanisms behind the effect of role of these adipokines in development of asthma are not well understood. Evidence does show that these adipokines affect eosinophil chemotaxis in lung tissue. It is also hypothesized that increased production of inflammatory cytokines such has leptin, IL-4 and TNF-α leads to decreased activity of regulatory T-lymphocytes and increased production of IL-10 by macrophages and adipocytes, thereby shifting the balance of immune status towards a Th2 phenotype and increasing the risk of allergic disease, but there is no clear evidence to support this hypothesis thus far [27]. Therefore, the role of inflammatory cytokines and adipokines in obese HIV-infected asthmatics needs to be further investigated.
Another link to obesity and asthma is oxidative stress, and the role of oxidized low-density lipoprotein (ox-LDL) in pulmonary disease has gathered attention. Scichilone et al.studied LDL subclasses in 24 asthmatics and 24 healthy matched controls [28]. The results showed that in asthmatics, the least pro-inflammatory LDL (LDL-1 and LDL-2) appear to be lower and the most pro-inflammatory LDL (LDL-3 and 4) appear to be higher. Also, LDL-1 (least pro-inflammatory) positively correlated with lung function. LDL-3 (most pro-inflammatory) negatively correlated with lung function suggesting that LDL is a potent trigger for lung inflammation. Thus, based on early evidence, LDL-ox might be an important mediator in the pathogenesis of inflammatory lung diseases like asthma. It is important to note that several studies have shown that obese asthmatics are more prone have poor therapeutic response to traditional asthmatic treatments like glucocorticoids [26], and the treatment options for these patients are limited. Early studies on small samples show that weight loss does result in improvement in lung function. Thus obesity may be linked to asthma through several pathways but there is still limited understanding of these mechanisms. Further investigations need to focus on the inflammatory pathways related to adiponectins and oxidized LDL to understand these mechanisms in order to develop therapeutic options, especially in HIV-infected population where obesity associated asthma phenotype is prevalent.
Similarly, recent evidence also implicates the role of insulin resistance in the pathogenesis of asthma with Theusen et al.finding an association between insulin resistance wheezing (OR 1.87, 95% CI 1.38 to 2.54, p=0.002) and asthma-like symptoms (OR 1.61, 95% CI 1.23 to 2.10, p=0.001) [29]. Further evidence for a link between insulin resistance and asthma comes from a study in which clinical measures of insulin resistance and obesity were correlated with airway hyper-responsiveness (AHR) based on methacholine challenge test [30]. Researchers found that in men, FEV1 significantly correlated with fasting insulin level and insulin resistance based on homeostasis model of assessment of insulin resistance (HOMA-IR). After adjustment for age, HOMA-IR was a statistically significant risk factor for AHR, but became non-significant after adjustment for age and BMI. Similarly, in women, the HOMA-IR and BMI were statistically significant risk factors for AHR after adjustment for age. The relationship between HOMA-IR and AHR again became insignificant after adjustment for age and BMI. Insulin resistance is important in the HIV-infected population because of the high prevalence of metabolic changes in this population. Recent estimates of diabetes mellitus is ~3% in ART-naïve HIV-infected individuals and ~10% in individuals on highly active ART [31]. In addition, metabolic changes that occur in HIV infection have been known to cause lipohypertrophy likely secondary to chronic inflammation [32]. Biron et al.found a prevalence of metabolic syndrome of 18.2% in cross-sectional study of 269 HIV-infected individuals started on ART with a median duration of 30 months of treatment [33]. The researchers concluded that HIV-infected individuals on ART are at high risk of complications like cardiovascular disease. Thus, HIV-infected individuals with obesity and insulin resistance may be at risk for asthma. Future studies in this area need to explore the relationship between insulin resistance, and asthma in HIV-infected individuals, including investigating prevalence of AHR in HIV-infected population with insulin response and their response to treatment.
Treatment of Asthma in HIV Infection
Current asthma treatments used in the general population have not been tested in the HIV-infected population. If HIV-infected individuals have different mechanisms of asthma than the general population, then current therapies may be less effective, particularly if HIV or ART are involved in pathogenesis. In addition, there are several interactions of concern in the HIV-infected population including increased complications of inhaled corticosteroids (ICS) such as candidiasis, tuberculosis, and bacterial pneumonia [34]. The TORCH (towards a revolution in COPD health) study in COPD treatment studied adverse effects of combination therapy with salmeterol and fluticasone compared to the placebo group [35]. The study found that probability of developing bacterial pneumonia during the 3 year follow-up was greater in combination therapy group than the placebo group (19.6% vs. 12.3%, p<0.001). Tuberculosis risk may also be increased. In a study of 853,439 participants with inhaled respiratory medicine use, the use of ICS was associated with an increased rate of TB diagnosis (adjusted OR 1.20. 95% CI 1.08-1.34, p<0.001) [36]. The HIV-infected population is likely at increased risk of such complications with ICS therapy. Furthermore, ART can lead to systemic side effects of ICS. Pharmacologic studies and case reports provide evidence that combination of ritonavir and fluticasone can lead to symptoms of Cushing’s syndrome and adrenal insufficiency, and the combination treatment is contraindicated in HIV-infected individuals [37]. The interaction between these medications is likely secondary to the CYP3A4 system, and other inhaled corticosteroids also are substrates of CYP3A4, which is inhibited by ritonavir. It is possible that there are interactions between ritonavir and other inhaled corticosteroids at higher doses. These important treatment considerations should be remembered in the HIV-infected population and further research needs to determine efficacy and safety of current asthma treatments in this population.
Conclusion
In summary, pulmonary diseases including asthma appear to be highly prevalent in the HIV population, and they are increasingly becoming an important cause of morbidity and mortality. Asthma is one of the most common pulmonary diseases in the HIV-infected population, and recent evidence suggests a higher rate of bronchial hyper-responsiveness in HIV-infected individuals. Smoking, in particular, appears to be a strong risk factor in development of respiratory symptoms and obstructive pathophysiology. Newer research points to several inflammatory cytokines, metabolic diseases such as obesity, and ART in the pathogenesis of asthma in HIV patients. Future research needs to focus on understanding the mechanisms that lead to development of asthma in HIV to improve prevention and treatment.
References
- Moorman JE, Zahran H, Truman BI, Molla MT; Centers for Disease Control and Prevention (CDC) (2011) Current asthma prevalence - United States, 2006-2008. MMWR SurveillSumm 60 Suppl: 84-86.
- Kynyk JA, Parsons JP, Para MF, Koletar SL, Diaz PT, et al. (2012) HIV and asthma, is there an association? Respir Med 106: 493-499.
- George MP, Kannass M, Huang L, Sciurba FC, Morris A (2009) Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One 4: e6328.
- Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, et al. (2010) Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J RespirCrit Care Med 182: 790-796.
- Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, et al. (2012) Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax 67: 309-314.
- Drummond MB, Merlo CA, Astemborski J, Kalmin MM, Kisalu A, et al. (2013) The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS 27: 1303-1311.
- Wallace JM, Stone GS, Browdy BL, Tashkin DP, Hopewell PC, et al. (1997) Nonspecific airway hyperresponsiveness in HIV disease. Pulmonary Complications of HIV Infection Study Group. Chest 111: 121-127.
- O'Donnell CR, Bader MB, Zibrak JD, Jensen WA, Rose RM (1988) Abnormal airway function in individuals with the acquired immunodeficiency syndrome. Chest 94: 945-948.
- Poirier CD, Inhaber N, Lalonde RG, Ernst P (2001) Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J RespirCrit Care Med 164: 542-545.
- Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, et al. (2011) HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J RespirCrit Care Med 183: 388-395.
- Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, et al. (2012) Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy ClinImmunol 129: 708-714.
- Kendall CE, Wong J, Taljaard M, Glazier RH, Hogg W, et al. (2014) A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 14: 161.
- Wenzel SE (2012) Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716-725.
- Foster SB, McIntosh K, Thompson B, Lu M, Yin W, et al. (2008) Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy ClinImmunol 122:159-65.
- Lin RY , Lazarus TS (1995) Asthma and related atopic disorders in outpatients attending an urban HIV clinic. Ann Allergy Asthma Immunol 74: 510-515.
- Wright DN, Nelson RP Jr, Ledford DK, Fernandez-Caldas E, Trudeau WL, et al. (1990) Serum IgE and human immunodeficiency virus (HIV) infection. J Allergy ClinImmunol 85: 445-452.
- Israël-Biet D, Labrousse F, Tourani JM, Sors H, Andrieu JM, et al. (1992) Elevation of IgE in HIV-infected subjects: a marker of poor prognosis. J Allergy ClinImmunol 89: 68-75.
- Cocchi F, DeVico AL, Yarchoan R, Redfield R, Cleghorn F, et al. (2000) Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. ProcNatlAcadSci U S A 97: 13812-13817.
- Caron M, Vigouroux C, Bastard JP, Capeau J (2009) Antiretroviral-related adipocyte dysfunction and lipodystrophy in HIV-infected patients: Alteration of the PPARγ-dependent pathways. PPAR Res 2009: 507141.
- Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, et al. (2001) Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J RespirCrit Care Med 164:1487-94.
- Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, et al. (2009) Bronchodilatory effect of the PPAR-gamma agonist rosiglitazone in smokers with asthma. ClinPharmacolTher 86: 49-53.
- Beuther DA, Sutherland ER (2007) Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J RespirCrit Care Med 175: 661-666.
- Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, et al. (2012) Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune DeficSyndr 61: 600-605.
- Beuther DA (2010) Recent insight into obesity and asthma. CurrOpinPulm Med 16: 64-70.
- Kim SH, Sutherland ER, Gelfand EW (2014) Is there a link between obesity and asthma? Allergy Asthma Immunol Res 6: 189-195.
- Sutherland ER (2014) Linking obesity and asthma. Ann N Y AcadSci 1311: 31-41.
- Hersoug LG, Linneberg A (2007) The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy 62: 1205-1213.
- Scichilone N, Rizzo M, Benfante A, Catania R, Giglio RV, et al. (2013) Serum low density lipoprotein subclasses in asthma. Respir Med 107: 1866-1872.
- Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A (2009) Insulin resistance as a predictor of incident asthma-like symptoms in adults. ClinExp Allergy 39: 700-707.
- Kim KM, Kim SS, Lee SH, Song WJ, Chang YS, et al. (2014) Association of insulin resistance with bronchial hyperreactivity. Asia Pac Allergy 4: 99-105.
- Lake JE1, Currier JS (2013) Metabolic disease in HIV infection. Lancet Infect Dis 13: 964-975.
- Willig AL, Overton ET (2014) Metabolic consequences of HIV: pathogenic insights. Curr HIV/AIDS Rep 11: 35-44.
- Biron A, Bobin-Dubigeon C, Volteau C, Piroth L, Perre P, et al. (2012) Metabolic syndrome in French HIV-infected patients: prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Res Hum Retroviruses 28:1672-8.
- Gingo MR, Morris A, Crothers K (2013) Human immunodeficiency virus-associated obstructive lung diseases. Clin Chest Med 34: 273-282.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775-789.
- Lee CH1, Kim K, Hyun MK, Jang EJ, Lee NR, et al. (2013) Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 68: 1105-1113.
- Foisy MM, Yakiwchuk EM, Chiu I, Singh AE (2008) Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med 9: 389-396.
- Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, et al. (2003) Respiratory symptoms among HIV-seropositive individuals. Chest 123: 1977-1982.
- Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, et al. (2010) Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther 7: 6.
- Leung JM, Liu JC, Mtambo A, Ngan D, Nashta N, et al. (2014) The determinants of poor respiratory health status in adults living with human immunodeficiency virus infection. AIDS Patient Care STDS 28: 240-247.
- Hirani A, Cavallazzi R, Vasu T, Pachinburavan M, Kraft WK, et al. (2011) Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med 105: 1655-1661.
Citation: Puri A, Gingo M, Morris A (2014) Asthma in HIV-Infected Population: A Review of Respiratory Symptoms, Pulmonary Function Abnormalities and Pathophysiology. Epidemiol 4:164. DOI: 10.4172/2161-1165.1000164
Copyright: © 2014 Gingo M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 21078
- [From(publication date): 9-2014 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 16276
- PDF downloads: 4802