Research Article Open Access
Depolymerizing Activities of Aromatic Hydrocarbon Degrading Phyllosphere Fungi in Sri Lanka
Kannangara S*, Undugoda L, Rajapaksha N and Abeywickrama K
Department of Botany, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka
- *Corresponding Author:
- S Kannangara
Department of Botany
University of Kelaniya, Kelaniya, Sri Lanka
Tel: +0112914480
E-mail: sagarikadpk@kln.ac.lk
Received date: October 05, 2016; Accepted date: October 20, 2016; Published date: October 22, 2016
Citation: Kannangara S, Undugoda L, Rajapaksha N, Abeywickrama K (2016) Depolymerizing Activities of Aromatic Hydrocarbon Degrading Phyllosphere Fungi in Sri Lanka. J Bioremediat Biodegrad 7:372. doi: 10.4172/2155-6199.1000372
Copyright: © 2016 Kannangara S, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Application of aromatic hydrocarbon degrading fungi to bioremediate aromatic hydrocarbonic (AH) pollutants is a current trend and many research on the use of such fungi to remediate aromatic hydrocarbonic pollutants in temperate situations have been reported. Bioremediation of these hydrocarbons is through an array of lignolitic and non lignolitic extra cellular enzymes. Therefore, the present investigation attempts to assess lignolytic and non lignolytic enzyme activities of selected phyllosphere aromatic hydrocarbon degrading fungi during the aromatic hydrocarbon degradation. In a previous research aromatic hydrocarbon degrading fungi were isolated from ornamental leaf samples collected from highly urbanized and industrialized areas of Sri Lanka. These fungal species were then selected to evaluate their enzyme activities when degrading aromatic hydrocarbons. They were screened for their manganese dependent peroxidases (Mnp), Lignin peroxidases (Lip) and laccases enzyme activities. Most efficient naphthalene degrading fungi showed Mnp and Lip enzyme activities. The best naphthalene degrader, Penicillium oxalicum showed significantly higher Mnp (26 Uml-1 min-1) activity during naphthalene degradation process. However, phenanthrene degrading phyllosphere fungal strains showed higher laccase activities. Penicillium oxalicum showed significantly higher laccase activity during the phenanthrene degradation showing the same fungal species had different enzyme predominant pathways for different xenobiotics. Same fungal species performed differently for different AH substrates. Mnp was the predominantly used enzyme in the most efficient naphthalene degrading fungal species and phenanthrene degradation of them was manipulated by laccases. The promising results of the present investigation will broaden the perspective of ecofriendly practical application of the above fungal strains at environmental sites where contamination is caused by AHs especially, phenanthrene, naphthalene, toluene and xylene. Also this opens many avenues for conducting future research in the field of bioremediation and biodegradation.
Keywords
Phyllosphere; Penicillium oxalicum; Phenanthrene; Air pollution naphthalene; Biodegradation
Introduction
Air pollution is one of the major problems arising in the modern world and it can be due to many anthropogenic chemical compounds. Out of these air pollutants, aromatic hydrocarbons (AH) play a special role as hazardous chemicals due to high carcinogenicity and genotoxicity to all living beings [1]. Main source of these chemicals to the air are vehicular emission, industrial processes and oil refinery processes which are increasing day by day as a consequence of urbanization and industrialization [2]. These AH pollutants in the ambient air deposit on the ground level, mainly leaf surfaces soil and other substrates through wet and dry depositions. As reported in literature, many of the microorganisms inhabiting the polluted soil and leaf surfaces have special ability to degrade such kind of AH pollutants [3]. Especially, lignolytic and non-lignolytic fungi have special ability to degrade AH pollutants into the non-polluted level using various types of enzymes like oxidasese and peroxidases.
Many studies have focused on the ability of litter-decomposing fungi [4-6] and non-lignolytic fungi [7], which could also metabolize a variety of PAHs to polar metabolites. Capability of wood decaying fungi to detoxify polycyclic aromatic hydrocarbons (PAHs) has been studied extensively [8] and the majority of these studies have focused on white rot fungi [9-14]. Particularly Phanerochaete chrysosporium [15-17]. Phanerochaete chrysosporium, are wood decomposing basidiomycetes that can degrade not only lignin but also a broad spectrum of recalcitrant environmental organopollutants, including PAHs that are not readily degraded by other microorganisms [11]. This degradative activity is due to the lignin-degrading systems of these fungi, which is regulated by array of enzymes such as lignin peroxidase, manganese peroxidase and laccase [18].
Lignin peroxidase (Lip) is a haem containing peroxidase with an unusually high redox potential. It is highly glycosylated as most enzymes are secreted for extracellular action. Most but not all white rot species produce it [19]. LiP is able to oxidize aromatic compounds with redox potentials higher than 1.4V (NHE) by single-electron abstraction, but the exact redox mechanism is still poorly understood [20]. Manganese peroxidase (Mnp) is also haem containing and is generally considered to be essential for lignin degradation. MnP is an extracellular heme enzyme which is common in lignin-degrading basidiomycetes fungi. Other than basidiomycetes some ascomycetes are also able to degrade aromatic hydrocarbons using these lignolitic enzymes. In fact, Trichoderma hazianum isolated from three different sources, soil, compost and rotting wood from Poland demonstrated the potential in degrading aromatic hydrocarbonic compounds using lignolitic enzymes [21]. Even though most of the fungal species belong to basidiomycota and Ascomycota have amylases, lignases, pectinases and cellulases enzymes which degrade mostly many polypeptides they also involve in degradation of aromatic pollutants deposited on the ground level [12]. Many studies on Fusarium spp. have shown their capability to degrade high molecular-weight organic compounds such as coal cellulose, xylan, pectin and different hydrocarbons [22] as well as PAHs [23].
Micro fungi inhabiting the plant surfaces especially the phyllosphere are able to degrade hazardous AH pollutants into non-toxic forms as much as lignolytic degraders. Ref. [24] revealed that nonlignolytic fungi, Penicillium oxalicum, Aspergilllus aculeatus, Aspergillus oryzea and Colletrotrichum siamense which inhabit the phyllosphere of ornamental plants collected from polluted sites in Sri Lanka were able to degrade xylene, toluene, phenanthrene and naphthalene.
Leaf phyllosphere is one of the major exposing surfaces to these hazardous pollutants. The phyllosphere represents the largest biosphere-atmosphere inter-phase on the earth and is dominated by leaves. Like other biological surfaces, the phyllosphere harbors a large number of diverse microorganisms, including bacteria, yeasts, oomycetes, fungi, and algae [25].
As stated by Ref. [24] many of leaf phyllosphere bacteria as well as fungi showed in vitro degradation of aromatic hydrocarbons. As per literature, little has been known about the AHs degradation by phyllosphere fungi for tropical situations compared to temperate situations. Also the enzymes involved in degrading these aromatic hydrocarbons have not been studied extensively. Therefore, the objective of the present research was to characterize the extracellular enzymes involve in the naphthalene, phenanthrene, xylene and toluene degradation by selected AH degrading phyllosphere fungi that inhabit the phyllosphere of four ornamental plants (Ixora chinensis, Ervatamia divaricata, Hibiscus rosa-sinensis and Amaranthus cruentus) grown in the five polluted sites (Colombo Fort, Orugodawattha, Maradana, Panchikawattha and Sapugaskanda) in Sri Lanka.
Methods and Materials
Leaf sample collection
Leaf samples were collected from the ornamental plants, Ixora chinensis, Ervatamia divaricata, Hibiscus rosa-sinensis and Amaranthus cruentus which are highly abundant in roadsides of five urbanized areas, Colombo Fort, Orugodawattha, Maradana, Panchikawattha and Sapugaskanda in Sri Lanka.
Isolation of phyllosphere fungi and the screening of AH degradation
Isolation of AH degrading phyllosphere fungi was carried out as described in Ref. [24]. Potential of the isolated AH degrading fungi were evaluated using plate assays, colorimetric methods and HPLC analysis.
The best aromatic hydrocarbon degraders were then identified upto species level using molecular techniques and they were identified as Penicillium oxalicum (KP863926), Coletritichum ciamense (KP863925), Nygrospora oryzea (KT737384), Trichoderma asperillum (KX452094), Aspergillus aculeatus (KP863927), Aspergillus oryzea (KP791804), Cylindrocarpon sp, Cladosporium sp. and Mucor sp. [24]. In the present investigation their enzyme activities in degrading AHs as well as other polymers were screened as follows.
Manganese-dependent peroxidases (MnPs) lignin peroxidases (LiPs) and laccases enzyme activity of AH degrading fungi
Erlynmeyer flasks (100 ml) containing BBH broth medium (50 ml) with pure aromatic hydrocarbons; naphthalene, phenanthrene, xylene and toluene (5 gl-1) were separately autoclaved at 121°C for 15 minutes. Two agar plugs (1 cm2) from the periphery of 7 days old pure cultures of each fungal species grown on Czapek-Dox agar were inoculated into the sterilized flasks with the broth medium. The flasks were then incubated at room temperature (28 ± 2°C) for 10 days. Then the content was filtered using a filter paper (Watman No.4) and filtrate was obtained. This filtrate was then used for measuring the extracellular lignolytic enzyme activities (Mnp, Lip and laccases). This experiment was carried out in six replicates [26-28].
Manganese-dependent peroxidases (MnPs) activity
Mnp enzyme activity was assayed using a spectrophotometer at a wave length of 270 nm (εmax=11,590 mol-1 cm-1) at room temperature (28 ± 2°C). The Mn+3 malonate forming reaction was performed in 1 ml volumes containing sodium malonate buffer (70 mmoll-1, pH= 4.5), MnSO4 (2 mmoll-1), H2O2 (6 mmoll-1) and 25 μl of enzyme extract. One unit of MnPs was defined as 1 μmol of product formed per milliliter per minute under the assay conditions [27].
Lignin peroxidases (LiPs) activity
Lignin peroxidase activity was determined by monitoring the oxidation of veratryl alcohol to veratraldehyde at 37°C by an increase in absorbance at 310 nm (εmax=9300 mol-1 cm-1). The reaction mixture (2.5 ml) contained sodium tartarate buffer (10 × 10-3 moll-1, pH=3.0), veratryl alcohol solution (10 × 10-3 moll-1), H2O2 (2 × 10-3 moll-1) and enzyme extract (500 μl). One unit of enzyme activity was defined as the amount of enzyme oxidizing 1 μmol of substrate per minute under the assay conditions [28].
Laccases activity
Laccases (Phenol oxidases) activity was determined at 470 nm (εmax=6,740 mol-1cm-1) using guaiacol as the substrate. The enzyme reaction was performed in 1 ml volumes containing acetate buffer (pH=5.0, 10 × 10-3 moll-1), guaiacol (2 × 10-3 moll-1) and properly diluted enzyme solution (0.1 ml) and then the mixture was incubated at 50°C for 30 minutes. In the blank, guaiacol was replaced with acetate buffer. One unit of enzyme activity was defined as the amount of enzyme oxidizing 1 μmol of substrate per minute under the assay conditions [28].
Calculation of enzyme (MnPs, LiPs and Laccases) activities
The following equation was used to express the enzyme activities in μmol of substrate oxidized per milliliter per minute.
U ml-1 min-1=ΔAbs (106) (εRT)-1
U=μmol of the of the substrate oxidized, ΔAbs=difference between final and initial absorbances, ε=extinction coefficient of each enzyme product (LiPs - ε310=9300 mol-1 cm-1, MnPs - ε270=11,590 mol-1cm-1, Laccases - ε450=65 000 mol-1 cm-1), R=volume in milliliters of supernatant, T=reaction time in minutes [26].
The enzyme activities of amylases, lignases, pectinases and cellulases responsible for the biodegradation of starch, lignin, pectin and celluloses
The pectin, starch, cellulose and lignin degradation ability of isolated fungi using the fungal enzymes in the presence of AHs in the medium was observed following the methods given in Ref. [29,30].
Species were tested for their substrate utilization abilities using pure substrates of starch, cellulose, pectin and lignin. The substrate utilization ability of the individual fungal species was observed separately as described below. Each experiment was carried out in six replicates.
Cellulase activity
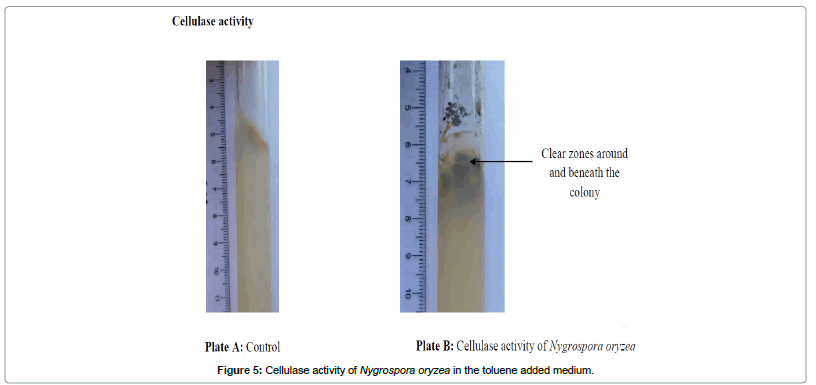
The isolated phyllosphere fungal species were grown on basal medium of Ref. [31] supplemented with 1% cellulose powder and 1% AH (phenanthrene, naphthalene, xylene and toluene). Subsequently, fungi inoculated test tubes were incubated at room temperature (28 ± 2°C) for a month and then the observations were taken at weekly intervals. Cellulose utilization of the fungal species was detected using the clear zones around the actively growing colonies [30,31].
Amylolytic activity
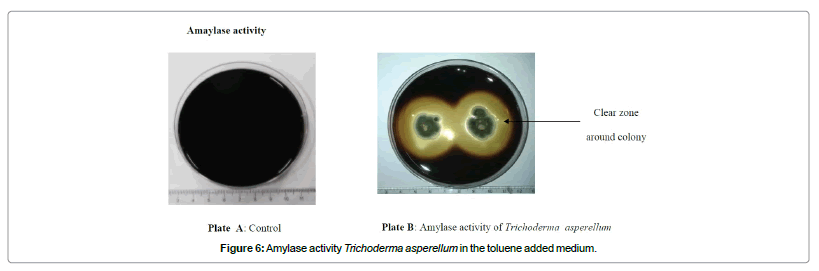
Starch degradation through the amylase activity was assessed using Difco nutrient agar supplemented with 5% starch and 1% AH (phenanthrene, naphthalene, xylene and toluene) [29]. These starch agar plates were inoculated separately with isolated fungal species and incubated at room temperature (28 ± 2°C) for five days. After the incubation period test plates were flooded with 1% KI/I2 solution to observe the amylolytic activity indicated by the production of clear zones around the active growing colonies [30].
Polygalacturonase and pectatelyase activities
Pectin degradation of isolated fungi using two enzymes; polygalacturonase and pectatelyase was assessed using mineral nutrient agar plates which contained the basal medium of Ref. [31], 0.5% of liquid citrus pectin and 1% AH (phenanthrene, naphthalene, xylene and toluene) [30]. Two sets of plates were prepared, one set of plates was adjusted to pH 5 and the other pH 7 to observe optimal polygalacturonase activity (at pH 5) and pectatelyase activity (at pH 7) using a pH meter (Model- Eutech Cyber Scan PC 510). Plates of each type of pectin agar were then inoculated separately with each isolated phyllosphere fungus. After 7 days’ incubation period at room temperature (28 ± 2°C), all the plates were flooded with 1% cetavlon solution (cetyltrimethyl ammonium bromide) which precipitated the intact pectin in the medium. Clear zones around the active colonies indicated pectin utilization ability of selected fungal strains [30].
Laccase, peroxidase and tyrosinase activities
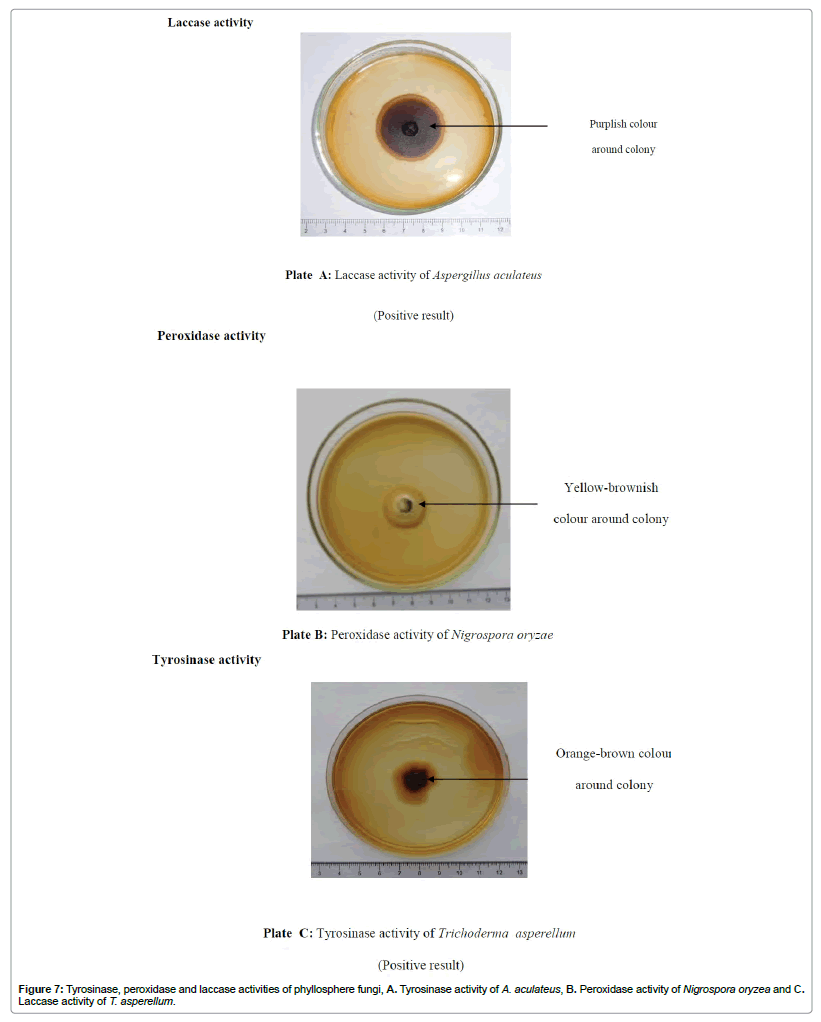
The degradation of lignin by three enzymes, laccase, peroxidase and tyrosinase, was assessed by growing isolated fungi on malt extract agar (pH 7) supplemented with 1% AH (phenanthrene, naphthalene, xylene and toluene). After the 7 days’ incubation period at room temperature (28 ± 2°C), observations were taken using the drop tests [32]. Six replicates were carried out for each fungal species. The methods for the drop tests were given below.
Production of lacccase
A test solution of 0.1M Napthol was prepared in 96% ethanol. Drops from this solution were tested on the active marginal hyphae of each of the test fungal species using a pasture pipette. Diffusion of purplish coloration into the medium indicated the presence of laccase [33].
Production of peroxidase
Drops of freshly prepared 0.4% H2O2 and 1% pyrogallol in water (50 ml H2O2: 50 ml 1% pyrogallol) were tested on the marginal hyphae of each of the test fungi to observe the production of peroxidase. Appearance of a yellow-brownish colour indicated the production of peroxidase by the test fungus [30,32].
Production of tyrosinase
A solution of p-cresol (0.1M) in 96% ethanol was prepared in order to observe the production of tyrosinase. Drops from this solution were tested on the marginal hyphae of each of the test fungal species using a Pasteur pipette. The appearance of an orange-brown colour diffusing into the medium indicated the production of tyrosinase [30,32].
Statistical analysis
Data presented for enzyme activities were the means of six replicates. The SPSS 16.0 software was used in determination of standard error (SE) and least significant difference (LSD), ANOVA.
Results
Manganese-dependent peroxidases (MnPs) lignin peroxidases (LiPs) and laccases enzyme activity of PAH degrading fungi
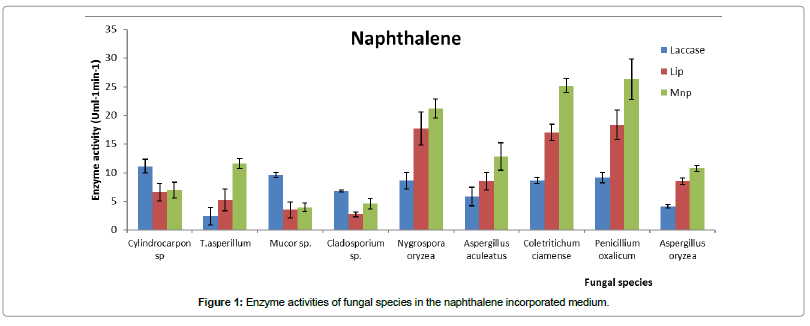
Most of the higher naphthalene degrading fungal species (Penicillium oxalicum, Coletritichum ciamense, Nygrospora oryzea, Trichoderma asperillum, Aspergillus aculeatus and Aspergillus oryzea) showed higher Mnp and Lip enzyme activities compared to Laccase during the naphthalene degradation (Figure 1). In fact, according to the HPLC results [24], the best naphthalene degrader Penicillium oxalicum showed higher Mnp and Lip activity compared to Laccase. Further, its Mnp activity (26 Uml-1min-1) was significantly higher than Lip activity (18.4 Uml-1min-1) (Figure 1). But, low naphthalene degrading fungal species Cylindocarpon sp., Mucor sp. and Clostridium sp. showed higher laccase activity when they degraded naphthalene (Figure 1).
Most of the higher phenanthrene degrading phyllosphere fungal strains (Penicillium oxalicum, Coletritichum ciamense, Nygrospora oryzea, Trichoderma asperillum, Aspergillus aculeatus) showed higher laccase activity compared to the other two oxidoreductases (Figure 2). As an example laccase activity (22 Uml-1min-1) of the best phenanthrene degrader Penicillium oxalicum was high during the phenanthrene degradation (Figure 2). But low phenanthrene degraders showed high Mnp level compared to laccase when phenanthrene was in the medium compared to the high degraders. In fact, the low phenanthrene degraders, Clostridium sp., Mucor sp. and Cylindrocarpon sp. showed significantly high activity for Mnp compared to the other two oxidoreductasees.
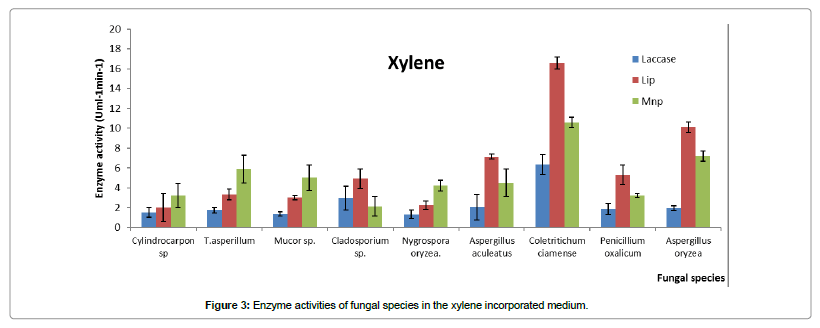
When xylene was in the medium, xylene degraders (Penicillium oxalicum, Coletritichum ciamense Aspergillus niger, and Aspergillus aculeatus) showed significantly higher lipase activity compared to the other tested two oxidoreductases. As an example the best xylene degrader, Coletritichum ciamense manifested significantly higher lipase enzyme activity during the xylene degradation (Figure 3). Its Mnp activity was significantly higher than its laccase activity. As an example the low phenanthrene degraders, Nygrospora oryzea, Cylindrocarpon sp. 1 and Trichoderma asperellum showed higher Mnp activities during xylene degradation.
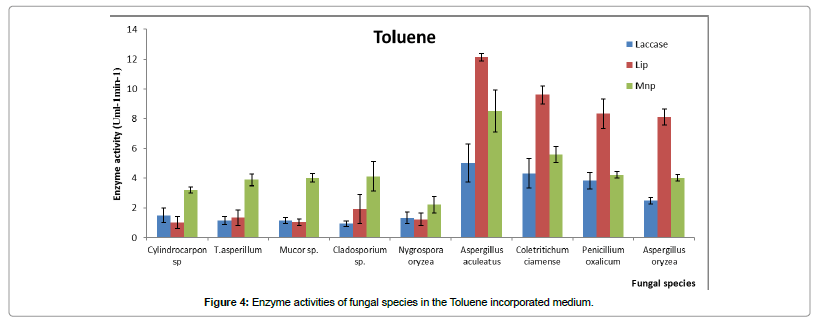
Enzyme activity results revealed, when medium has toluene as a substrate, lipase enzyme activity in some fungi (Penicillium oxalicum, Coletritichum ciamense, Aspergillus aculeatus and Aspergillus niger) was quite high (Figure 4). Further, Mnp enzyme activity also showed a significantly higher activity compared to the laccase activity. According to the HPLC results [24] Aspergillus aculeatus is the best toluene degrading phyllosphere fungal species. Present investigation indicated that there is an increase in Mnp enzyme activity when certain fungi degrade toluene, compared to the other two oxidoreductase. Nygrospora oryzea, Trichoderma asperillum and Cylindrocarpon sp.1 which were considered as poor toluene degraders showed higher Mnp enzyme activities, during the toluene degradation process.
The enzyme activities of amylases, lignases, pectinases and cellulases responsible for the biodegradation of starch, lignin, pectin and celluloses
Present study clearly showed the monoaromatic hydrocarbon degraders also had the potential to secrete cellulose and starch degrading enzymes cellulases (Figure 5) and amylases (Figure 6) respectively. But the fungi (Penicillium oxalicum, Colletritichum ciamense and Aspergillus aculateas) that could degrade phenanthrene and naphthalene lack any of these enzymes whereas both of the polyaromatic and monoaromatic hydrocarbon degraders had the ability in secreting pectin degrading polygalacturanase and pectatelyase like enzymes. Further were able to degrade lignin using lignin degrading enzymes as laccase, peroxidase and tyrosinase (Table 1; Figure 7).
| Phyllosphere fungal species | Cellulose | Starch | Pectin | Lignin | |||
|---|---|---|---|---|---|---|---|
| Polygalacturanase | Pectate lyase | Laccase | Peroxidase | Tyrosinase | |||
| Nigrospora oryzae | + | + | - | - | + | + | + |
| Aspergillus aculeatus | - | - | + | + | + | + | + |
| Cylindrocarpon sp. 1 | + | + | - | - | + | - | - |
| Trichodermaasperellum | + | + | - | - | + | + | + |
| Cladosporium sp.1 | + | + | - | - | + | + | + |
| Penicillium oxalicum | - | - | + | + | + | + | + |
| Colletotrichum siamense | - | - | + | + | + | + | + |
| Mucor sp. | - | + | - | - | + | + | + |
| Aspergillus oryzea | - | - | + | + | + | + | + |
Table 1: Cellulose, starch, pectin and lignin degradation ability of phyllosphere fungal strains.
Discussion
Microorganisms have evolved in such a way for several enzymes capable for degrading the different polymers. These enzymes include cellulases (for degrading the cellulose), xylanases (for degrading the hemicellulose), and ligninolytic enzymes (for degrading the lignin). There are two major families of ligninolytic enzymes which are involved in lignolysis: peroxidases and laccases [21,34-36]. Out of these lignolitic enzymes, Mnp, Lip and laccase enzymes are predominant in aromatic hydocarbonic degradation activity of fungal species [35]. As per the HPLC results of a research carried out by Ref. [24] Penicillium oxalicum, Coletritichum ciamense and Aspergillus aculateus were the best aromatic hydrocarbon degraders and during the aromatic hydrocarbon degradation process all these fungal strains demonstrated significant Mnp, Lip and laccase enzyme activities in the medium.
These results revealed the involvement of lignolitic enzymes in the process of aromatic hydrocarbon degradation.
Manganese peroxidase production is far more superior to lignin peroxidases the most efficient naphthalene degraders and the observations are supported with the results of Ref. [37]. Higher MnP enzyme activity detected as compared to Lip and laccase may be due to the presence of Mn-dependent peroxidase in the medium if they contain enough Mn2+ to initiate MnP catalyzed reactions. As per literature Mnp plays a crucial role in the degradation of PAHs [5,17,36,38,39]. However, the enzyme activity of laccase was far greater than that of Mnp, suggesting that laccase might be the predominating enzyme during phenanthrene degradation. Therefore, laccase was the major enzyme involved in the phenanthrene degradation. The results of the present investigation revealed though Penicillium oxalicum was able to degrade naphthalene and phenanthrene efficiently, it has two different types of enzyme pathways for naphthalene and phenanthrene degradation. Naphthalene pathway was based on Lip enzyme activity and phenanthrene degradation was mainly depended on the laccase enzyme activity. Further, while laccase was predominant enzyme in higher phenanthrene degraders (Penicillium oxalicum, Coletritichum ciamense Nygrospora oryzea, Trichoderma asperillum and Aspergillus aculeatus), Mnp was the predominant enzyme in low phenanthrene degraders (Clostridium sp., Mucor sp. and Cylindrocarpon sp.). These results highlights that the presence of two different types of enzyme pathways in low phenanthrene degrading and high phenanthrene degrading phyllosphere fungi. Such kind of phenomenon was also described by Ref. [40], who has reported that laccase was the only extracellular enzyme detected during the benzo[a]pyrene degradation by Fusarium solani and Mnp was predominant in Fusarium oxysporum. Ref. [41] stated that the oxidative-related enzyme system is independent of species and not a species-specific characterization.
Since xylene is a more complex structure compared to toluene, lipase enzyme activity is significantly higher in xylene incorporated medium compared to a medium containing toluene. In fact, lipase enzyme activity of Colletrotrichum ciamense was higher than that in Aspergillus aculeatus degrade toluene.
Many studies on Fusarium spp. have shown their capability to degrade highmolecular-weight organic compounds such as coal cellulose, xylan, pectin, different hydrocarbons [22] as well as PAHs [23]. As investigated in the present research monoaromatic fungal degraders also demonstrated amylase and cellulase activities more than the lignolytic enzymes. Many of the polyaromatic degraders lack such kind of enzymes. However, they had pectin degrading enzymes at a significant level. The reason for this may be, due to their adaptations to degrade mostly the AHs like complex structures using lignolitic and pectin degrading enzymes rather than the degradation of comparatively simple substrates such as cellulose and starch.
In summary, the present investigation can be considered as the first reported experiment conducted in Sri Lanka which explored the enzyme activities especially Mnp, Lip and laccases activities of phyllosphere fungal strains during the biodegradation of AHs (phenanthrene, naphthalene, toluene and xylene). Findings of the research also opens several avenues for further experiments in the relevant field and also indicate the possibility of the use of the efficient AH degraders in remediating the aromatic hydrocarbon pollutants from environmental sites where high contamination prevails.
Acknowledgements
Author’s acknowledge the financial assistance provided by the HETC Window QIG3 Grant.
Conflict of Interest
Authors have no conflict of interests of any nature to state in this section.
References
- Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P, et al. (2015) Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pacific Journal of Tropical Biomedicine 5: 182-189.
- Harvey RG(1991)Dihydrodiols. In: polycylic aromatic: chemistry and carcinogenicity. Cambridge university press, New York, USA,pp:306-329.
- Waight K, Pinyakong O, Luepromchai E(2007)Degradation of phenanthrene on plant leaves by Phyllosphere bacteria. Thejournal of applied microbiology53: 265-272.
- Steffen KT,Hatakka A,Hofrichter M (2002) Removal and mineralization of polycyclic aromatic hydrocarbons by litter-decomposing basidiomycetous fungi. The journal of applied microbiology and biotechnology60: 212-217.
- Steffen KT, Hatakka A, Hofrichter M (2003) Degradation of Benzo[a]pyrene by the litter-decomposing basidiomycete Strophariacoronilla: role of manganese peroxidase. The journal of applied microbiology and biotechnology 69: 3957-3964
- Steffen KT, Schubert S, Tuomela M, Hatakka A, Hofrichter M (2007) Enhancement of bioconversion of high-molecular mass polycyclic aromatic hydrocarbons in contaminated non-sterile soil by litter-decomposing fungi. The journal of biodegradation18: 359-369.
- Cerniglia CE (1997) Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. The microbiology and biotechnology 19: 324-333
- Sutherland JB, Rafii F, Khan AA,Cerniglia CE (1995) Mechanism of polycyclic aromatic hydrocarbon degradation. In: Microbial transformation and degradation of toxic organic chemicals. Wiley-Liss,pp: 269-306.
- Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3: 351-368
- Bezalel L, Hadar Y, Fu PP, Freeman JP, Cerniglia CE (1996) Metabolism of phenanthrene by the white rot fungus Pleurotusostreatus. The journal of applied environmental microbiology62: 2547-2553.
- Magan N, Mswaka AY (1998) Wood degradation, and cellulase and ligninase production, by Trametes and other wood-inhabiting basidiomycetes from indigenous forests of Zimbabwe. The journal of mycology102: 1399-1404.
- Kim MS, Huh EJ, Kim HK, Moon KW (1998) Degradation of polycyclic aromatic hydrocarbons by selected white-rot fungi and the influence of lignin peroxidase. The journal of microbiology and biotechnology8: 129-133.
- Mori T, Kitano S, Kondo R (2003) Biodegradation of chloronaphthalenes and polycyclic aromatic hydrocarbons by the white-rot fungus Phlebialindtneri.The journal of applied microbiology and biotechnology 61: 380-383.
- Han MJ, Choi HT, Song HG (2004) Degradation of phenanthrene by Trametes versicolor and its laccase. The journal of microbiology42: 94-98.
- Dhawale SW, Dhawale SS, Dean-Ross D (1992) Degradation of phenanthrene by Phanerochaetechrysosporium occurs under ligninolytic as well as nonligninolytic conditions. The journal of applied environmental microbiology58: 3000-3006.
- Paszczynski A, Crawford RL (1995)Potential for bioremediation of xenobiotic compounds by the white-rot fungus Phanerochaetechrysosporium. The journal of biotechnology11: 368-379.
- Zheng ZM, Obbard JP (2002) Oxidation of polycyclic aromatic hydrocarbons (PAH) by the white rot fungus, Phanerochaetechrysosporium. The journal of enzyme microbiology and technology31: 3-9.
- Hammel KE (1995) Mechanisms for polycyclic aromatic hydrocarbon degradation by lignolytic fungi. The journal of environmental health perspective103: 41-43.
- Hatakka A (1994)Lignin-modifying enzymes from selected white-rot fungi: production and role from in lignin degradation.The journal of FEMS microbiology13: 125-135.
- Piontek K, Smith AT, Blodig W(2001) Lignin peroxidase structure and function.The journal of biochemical society transactions29:111-116.
- Dominic WS (2009) Structure and action mechanism of ligninolytic enzymes. The journal of applied biochememistry and biotechnology157:174-209.
- KangZ, BuchenauerH (2000)Ultrastructural and Cytochemical Studies on Cellulose, Xylan and Pectin Degradation in Wheat Spikes Infected byFusarium culmorum. 148:263-275.
- Chulalaksananukul S, Geoffrey MG, Sangvanich P,Sihanonth P, Piapukiew J, et al.(2006)Biodegradation of benzo(a)pyrene by a newly isolatedFusariumsp. The journal of FEMS microbiology262: 99-106.
- Undugoda LJS, Kannangara S, Sirisena DM (2016) Aromatic hydrocarbon degrading fungi inhabiting the phyllosphere of ornamental plants on roadsides of urban areas in Sri Lanka. The journal of bioremediation and biodegradation7: 328.
- Lindow SE, Leveau JH (2002) Phyllosphere microbiology. CurrOpinBiotechnol 13: 238-243.
- Das P, Mukherjee S, Sen R (2008) Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. The journal of Chemosphere72: 1229-1234.
- Ledwozyw A, Michalak J, Stepien A, Kadziolka A (1986) The relationship between plasma tryglicerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. ClinChimActa 155: 275-284.
- Tien M, Kirk T (1988) Lignin peroxidase of Phanerochaetechrysosporium. The journal of enzymology161: 238-249.
- Kjoller A,Struwe S (1980) Microfungi of decomposing red alder leaves and their substrate utilization. The journal of soil biology and biochemistry12: 425-431.
- Kannangara BTSDP, Rajapaksha RSCG,Paranagama PA (2009) Nature and bioactivities of endolichenic fungi in Pseudocyphellariasp., Parmotremasp. and Usneasp. at Hakgala montane forest in Sri Lanka. The journal of letters in applied microbiology48:203-209.
- Eggins HOW, Pugh GJF (1961) Isolation of cellulose decomposing fungi from soil. The journal of Nature193: 94-95.
- Staplers JA (1978) Identiï¬cation of wood inhabiting a phyllophorales in pure culture. Institute of Royal Netherlands Academy of Arts and Science, Amsterdam, Netherlands, pp:10-11.
- Bourbonnais R, Leech D, Paice MG (1998) Electrochemical analysis of the interactions of laccase mediators with lignin model compounds.Biochimica et Biophysica Acta 1379:381-390.
- Tanka H, Koike K, Itakura S, Enoki A (2009)Degradation of wood and enzyme production by Ceriporiopsissubvermispora. The journal of enzyme and microbial technology45:384-390.
- Hatakka A, Hammel KE (2010) Fungal biodegradation of lignocelluloses: In Industrial Applications. 2nd edn.Mycota X,Hofrichter M (ed.), Springer-Verlag Berlin Heidelberg, USA.
- Hofrichter M, Ullrich R, Pecyna MJ, Liers C,Lundell T (2010) New and classic families of secreted fungal heme peroxidases. The journal of applied microbiol biotechnology87: 871-897.
- Liew CY, Husaini A, Hussain H, Muid S, Liew KC, et al. (2011) Lignin biodegradation and ligninolytic enzyme studies during biopulping of Acacia mangium wood chips by tropical white rot fungi. World journal of microbiology and biotechnology 27: 1457-1468.
- Bogan BW, Lamar RT (1996) Polycyclic aromatic hydrocarbon degrading capabilities of Phanerochaetelaevis HHB1625 and its extracellular ligninolytic enzymes. The journal of applied environmental microbiology62:1597-1603.
- Clemente AR, Anazawa TA, Durrant LR (2001) Biodegradation of polycyclic aromatic hydrocarbons by soil fungi. The journal of Brazilian microbiology32: 255-261.
- Verdin A, Sahraoui AL, Durand R (2004) Degradation of benzo[a]pyrene by mitosporic fungi and extracellular oxidative enzymes. The journal of biodetereoration and biodegeradation53: 65-70.
- Hammer E, Schauer F (1997) Fungal hydroxylation of dibenzo furan. The Journal of Mycology101: 433-436.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 12597
- [From(publication date):
November-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11554
- PDF downloads : 1043