Research Article Open Access
Effects of The β-Carotene on the Growth Performance and Skin Pigmentation of Rainbow Trout (Oncorhynchus mykiss, W. 1792)
Kelestemur GT* and Çoban OE
Fisheries Faculty, Firat University, Department of Aquaculture, Elazig, Turkey
- *Corresponding Author:
- Kelestemur GT
Fisheries Faculty
Firat University
Department of Aquaculture
Elazig, Turkey
Tel: +904242370000
E-mail: gkelestemur@firat.edu.tr
Received Date: December 07, 2015; Accepted Date: January 19, 2016; Published Date: January 25, 2016
Citation: Kelestemur GT, Çoban OE (2016) Effects of The ß-Carotene on the Growth Performance and Skin Pigmentation of Rainbow Trout (Oncorhynchus mykiss, W. 1792). J Fisheries Livest Prod 4:164. doi:10.4172/2332-2608.1000164
Copyright: © 2016 Kelestemur GT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
In this study, the effect of dietary supplementation of β-carotene (beta carotene) on growth and skin pigmentation of rainbow trout. Fish were fed with diets containing 30 and 70 mg β-carotene kg-1, and without supplemented basal diet for 12 weeks. Weight gain (WG), specific growth rate (SGR) and survival rate (SUR) in the C group was significantly lower (p<0.05) than beta carotene supplemented diet groups. Feed concervation ratio (FCR) in the C was significantly lower (p<0.05) than β-carotene supplemented diet groups. But PER of fish did not differ among the diet groups (p>0.05). Crude protein value of fish meat was higher in beta-carotene supplemented diet groups (p<0.05) than control diet group. But, crude lipid and ash were not statistically different among the groups (p>0.05). The lowest carotenoid concentration levels in the lateral and tail regions of the fishes in the experimental groups have been obtained in the C (control) group (lateral region: 0.263 ± 0.021 ug/g; tail ragion: 0.009 ± 0.002 ug/g) while the highest cumulative values have been determined in the fishes of β70 groups (lateral region: 0.643 ± 0.46 ug/g; tail region: 0.124 ± 0.015 μg/g).
Keywords
Rainbow trout; Growth performance; β-carotene; Pigmentation
Introduction
Skin color is important factor in aquaculture influencing the commercial value of fish, mostly in those species sold live or fresh. Most studies of fish skin color in aquaculture have focused particularly on the effects of diet [1,2]. Synthetic carotenoids are of increasing importance for the pigmentation of farmed fish [3].
Carotenoids are synthesized by plants and microorganisms. They are present as micro-components in fruits and vegetables and are responsible for their yellow, orange and red colors. In recent years the antioxidant properties of carotenoids has been the major focus of research [4-6]. Animals are able to absorb carotenoids from their diet, and deposit them in the unesterified from [7]. They are absorbed intake by the mucosal cells and appear unchanged in the circulation and tissues. Carotenoids are absorbed differentially by different tissues. Little is known about the mechanisms of tissue absorption of carotenoids at this time. The major site of tissue storage of carotenoids is the adipose tissue [5,6]. However, the pigmentation of rainbow trout change throughout life. Generally, fingerlings have limited capacity for carotenoid deposition in the flesh, while significant amounts are deposited in the skin [8]. The decrease in total carotenoid content in fish flesh after reaching a maximum value may also have originated from initiation of maturation of fish gonads. Fish undergoing sexual maturation are known to mobilize carotenoids from muscles and transfer them to their gonads and skin [9].
Beta carotene one of the carotenoids responsible for the orange and red pigmentation of fish. Aquatic animals cannot synthesize carotene and therefore it must be supplemented in the diet. As well as being a pigment, beta-carotene has been shown to have other biological and nutritional functions essential for fish growth and health. This study was therefore undertaken to find out if synthetic beta carotene at two different dietary concentrations would affect survival, growth, and skin pigmentation of juvenile rainbow trout.
Materials and Methods
Experimental disayn
This trial was conducted in Keban Dam Lake (Elazig, Keban) third hunting ground in special facility. Three cages were used in the research. Each cage was stocked 300 rainbow trout (Oncorhynchus mykiss). Fish (initial average weight, 60.3 ± 0.27 g; initial length, 17.24 ± 0.04 cm were distributed into three round cage (mesh size: 1.8 mm, bag depth: 4 m, diameter: 9 m). Cages were placed approximately away from 100 m from shore. Dissolved oxygen and temperature values were recorded with a portable YSI Probe (55 Model 51/12) duration of the research. The pH of the water in the tanks where the fish were situated was determined by a portable Checker brand pH meter.
Diet
Composition of the experimental diets are shown in Table 1. Experimental diets supplemented with 30, 70 mg kg-1 propolis (respectively; β30, β70), and basal diet (C) (not supplemented diet of beta carotene) were prepared [3,10].
| Ingredient | Percent of dry weigth |
|---|---|
| Fish (anchovy) meal | 50 |
| Soybean meal | 23.1 |
| Wheat flour | 19.8 |
| Sunflower oil | 6 |
| Antibiotica | 0,10 |
| Vitamin premixb | 0.9 |
| Mineral premixc | 0.1 |
| Proximate composition | |
| Crude protein | 44.55 |
| Crude fat | 8.46 |
| Crude fibre | 4.12 |
| Crude ash | 13.43 |
aAntioxidant (mg/kg dry diet): Butilen Hydroxytoluene (BHT); 125.000 mg/kg.
bVitamin premix (IU or mg/kg dry diet): Menadion 3.000, Riboflavin 6.000, Pridoxin
5.000, Cobalamin 15, Niasin 25.000, Biotin 40, Folic acid 1.000, Colin Chloride
300, Calcium, D-pantothenat 8.000, Calsiferol 2.000.000, Vitamin A 1000, Vitamin
E 1.000.000 IU. Ascorbic Acid, 150.000
cMineral premix (mg/kg dry diet): Mn 80.000, Fe 35.000, Zn 50.000, Cu 5.000, I
2.000, Co 400, Se 150.
Table 1: Composition and proximate analysis of the experimental diets.
All experimental fish were acclimated to the basal diet for two week prior to start of the trial. The experimental fish were fed three times a day for 4 weeks. Daily feed allowance was 3% body weight per day. The feeding trial was conducted for 12 weeks. Before the fish were anesthesia (15 mg l-1 Quinaldin), these body weights were measured one every 2 weeks. The prepared pellets were dried in 24 hours at 45°C and during the trial were stored at +4°C. Proximate composition of diets were assayed according to AOAC methods [11]. Water content was calculated by drying in an oven at 105°C until achieving constant weight, and ash were assayed by combustion in a muffle furnace (550°C for 16 h). The determination of crude protein after acid digestion (N×6.25) was analysed by the kjeldhal method and lipids were extracted with diethyl ether by the soxhlet method.
Carotenoid determination
A 1.5-2 g sample (diet and tissue) was homogenized in the presence of Na2SO4 and extracted with acetone. The exctract was filtred with chloroform into a round-bottomed flask and evaporeted to dryness with a rotary evaporator at bath temparatures between 30-35°C. The residue was saponified with 1 ml 50% KOH and 10 ml methanol in a flask that fillet with nitrogen before being left for 2 h in the dark. Thereafter, the mixture was transferred to a seperating funnel with 40 ml each of ether and distilled water and extracted twices. The pooled ether extract were filtered through Na2SO4 to remove residual moisture, and evaporated to dryness under vacum. The residue was dissolve in hexan [7,12]. Jast after the pruduction of solution was determined in 472 nm. Carotenoid concantration was calculated according to this formula:
C=Absorbance × 10000/2100
where C is concentration (μg/g for tissue) 2100 is E (1%, 1 cm)=the extinction coefficent of the carotenoids in hexan at 472 nm; 10000 is the scale factor.
Statistical methods
All the values were presented as mean ± S.E. Differences between group means were assessed by a one-way analysis of variance (ANOVA) and post-hoc Duncan test used by SPSS/PC computer program (SPSS, 1999). Results with P<0.05 were considered statistically significant.
Results
Growth performance
WG, SGR, FCR, PER and SUR values of rainbow trout at the end of the experiment are presented in Table 2. The highest WG, SGR and SUR were obtained in the β-carotene supplemented groups (p<0.05), while the lowest WG, SGR and SUR were obtained in the C group (p<0.05). The FCR improved in β-carotene supplemented diet groups compared to without supplemented control diet group. On the other hand, PER values were not different among all diets groups. Additionally, crude protein values was found significantly higher in the β-carotene supplemented diet groups than C groups (p<0.05). However, crude lipid and ash were not statistically different among the groups (p>0.05) (Table 2).
| Growth Parameters | C | β 30 | β 70 |
|---|---|---|---|
| WG1 | 23.82 ± 0.24 b | 37.29 ± 0.45 a | 39.13 ± 0.19 a |
| SGR2 | 1.43 ± 0.04b | 1.76 ± 0.02a | 1.79 ± 0.02a |
| FCR3 | 3.87 ± 0.24a | 2.12 ± 0.11b | 2.17 ± 0.21b |
| PER4 | 1.56 ± 0.09 | 1.60 ±0.13 | 1.59 ± 0.15 |
| SUR5 | 82.72 b | 90.15 a | 92.34 a |
| Body Composition (%) | |||
| Crude protein | 47.46 b | 55.67 a | 54.83 a |
| Crude lipid | 9.32 | 10.45 | 10.67 |
| Crude Ash | 25.67 | 27.34 | 26.26 |
a-bMeans in the same column with different supscript are significantly (ANOVA,
P<0.05). 1 FR: Flow rate
1Weigth gain (WG): (final wt - initial wt)
2Specific growth rate (SGR) (%): [(loge final wt-loge initial wt)/duration in days] ×
100
3Feed conservation ratio (FCR): Duration in days consumed feed/(final wt-initial wt)
4Protein efficiency ratio (PER): (final wt-initial wt)/feed consumption (g) × feed in the diet
5Survival Rate (SUR): 100 × Final fish number/Initial fish number (wt: Weigth)
Table 2: Experimental groups of WG, SGR (specific growth rate), FCR (feed conversation ratio), PER (protein efficiency ratio), SUR (live rate) values and body composition of fish.
Skin carotenoid concentration
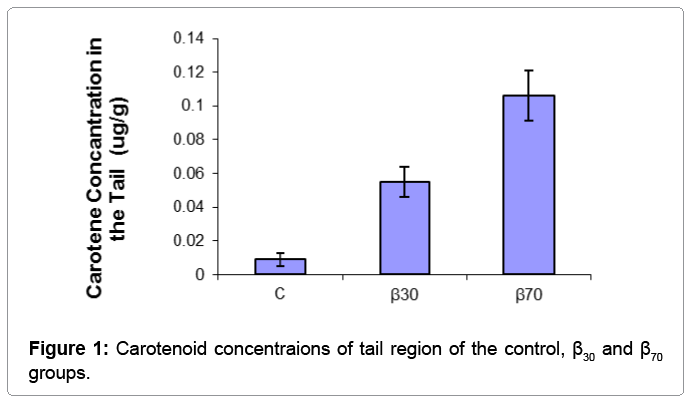
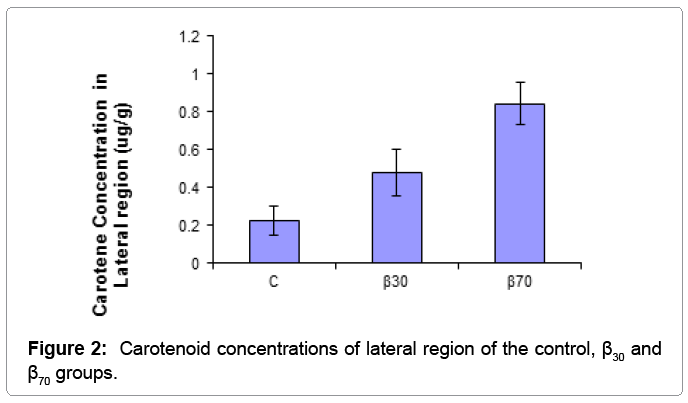
Skin carotene concentrations lateral and tail region of fish fed with the diets are presented in Table 3.
| Skin Carotene Concentrations (ug/g) | C | β30 | β70 | P |
|---|---|---|---|---|
| Tail Region | 0.009 ± 0.0038c | 0.055 ± 0.009b | 0.106 ± 0.015a | ** |
| Lateral Region | 0.223 ± 0.078c | 0.476 ± 0.122b | 0.843 ± 0.113a | * |
a-c Means in the same column with different supscript are significantly. (ANOVA, *P<0.05, **P<0.01)
Table 3: Carotenoid concentrations of lateral and tail regions of the experimental groups determined with the spectrophotometric method (μg/g).
The results of one-way ANOVA test showed that carotene concentration of fish skin were positively affected by dietary supplementation of beta carotene. β-carotene levels in lateral region of fish were found to be significantly higher in the β70 group than other groups (p<0.01), followed by the β30 group. The lowest carotenoid concentrations in the tail regions was obtained in the C group than other groups (p<0.05). Skin carotenoid concentraions of tail and lateral region of the experimantal groups were showed (Figures 1 and 2). For salmonids and trouts, increasing worldwide production and pricing pressures have focused attention on flesh quality issues to satisfy market preferences. Although there is no simple definition of flesh quality factors, of particular importance are the nutritional value, safety, flavour, colour, preservation and processing characteristics of the fillets [13]. Carotenoids, particularly those that are vitamin A precursors, have received increasing attention in recent years due to their reported health benefits. Already, the effects of carotenoids on aquatic animals are multi-faceted: they enhance larval growth and survival, improve the performance of broodstock and nauplii quality, as well as increase resistance to diseases [12]. Carotenoids also have excellent antioxidative characteristics. Cold-water fishes, like salmon, have a high level of polyunsaturated fat in their membranes, and protection of lipid tissue from peroxidation seems to be a metabolic function [14]. Some researchers found that canta supplementation in diet had a growth promoting effect in Atlantic salmon fry [7,14]. Hu et al. growth of fish fed highest level (200 mg) of β-carotene reduced to similar WG as those fed diet supplemented with 15 mg β-carotene migth be harmful to tilapia [15]. High beta-carotene thus migth build up relatively high amount of oxidized producs in animal body. Hu et al. reported that beta-carotene needed for normal growth of tilapia was 26.6-44.3 mg/kg [15]. In our study, it was observed that growth performance of fish were positively affected by dietary supplementation of beta carotene. These results indicated that beta carotene supplementation in diet has the growth stimulating action for juvenile rainbow trout. But, in contrast another previous experiments reported that various sources of did not affect growth and survival of various fish species [16,17]. Exting differences in water temperature, feeding regime, diet formulation and size of fish migth be responsible for the observed differences.
In this study, β-carotene dietary supplementations increased regional coloration in juvenile rainbow trout. Similarly, previous experiments reported that there was increased in the concentration of carotenoids in fish flesh as the duration of feeding of fish on pigmented diets was increased [8].
In conclusion, using β-carotene supplementation in juvenile rainbow trout diets improved growth performance and skin carotene concentration positively affected. However, 30 mg/kg β-carotene supplementation in juvenile rainbow trout diet was determined to be sufficent on growth performance. In addition it was determined that 70 mg/kg β-carotene supplementation was more effective on skin carotene concentration.
References
- Zeng BV, Li Z, Ye S, Xie S, Liu J, Zhang T, Duan M (2010) Effects of stocking density on growth and skin color of juvenile darkbarbel catfish (Pelteobagrus vachelli, Richardson). J Appl Ichthyol 26: 925-929.
- Baron M, Davies S, Alexander L, Snellgrove D, Sloman KA (2008) The effect of dietary pigments on the coloration and behaviour of flame-red dwarf gourami, Colisa lalia. Anim Behav 75: 1041-1051.
- Çoban EO, Kelestemur TG (2011) Effect of synthetic β-carotene at different rates on the levels of lipid Peroxidation and muscle carotenoid stability of the rainbow trout (Oncorhynchus mykiss, W. 1792) fillets. Veterinary J Health Sci25:17-2.
- Erdman JW, Bierer TL, Gugger ET (1993) Absorption and transport of carotenoids. Ann NY Acad Sci 691: 76-85.
- Parker RS (1996) Absorption, metabolism and transport of carotenoids. FASEB Journal 10: 542-51.
- Rao AV, Rao L (2007) Carotenoids and Human Health. Pharmacology Res 55: 207-216.
- Metusalach J, Brown A, Shahidi F (1997) Effects of stocking density on colour characteristics and deposition of carotenoids in cultured arctic charr (Salvelinus alpinus). Food Chem 59: 107-114.
- Merhabi Y, Zeraatkish Y, Abdalhy H (2010) Study the retention of plant carotenoids in flesh of rainbow trout fingerling. World Journal of Zoology 5: 71-74.
- No HK, Storebakken T (1992) Pigmentation of rainbow trout with astaxanthin and canthaxanthin in fresh water and sea water. Aquaculture 101: 123-134.
- Kelestemur TG (2012) Effects of Dietary Supplementation β-Carotene on Tissue MDA Level of Juvenile Rainbow Trout (Oncorhynchus mykiss). Firat Univ Vet Journal of Health Sci 26: 61-64.
- AOAC (1990) Official Methods of Analysis Association of Agricultural. Washington Acedemy Press.
- Amara EC, KironV, Satoha S, Watanabe T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) associated with dietary intake of carotenoids from natural products. Fish & Shellfish Immunology 16: 527-537.
- Johnston IA, Alderson R, Sandham C, Dingwall A,Mitchell D et al. (2000) Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L). Aquaculture 189: 335-349.
- Torrissen OJ, Naevdal G (1984)Pigmentation of salmonids: Genetical variation in carotenoid deposition in rainbow trout. Aquaculture 38: 59-66.
- Hu C, Chen MS, Pan HC,Huanga CH (2006) Effect of dietary vitamin A or ß- carotene concentrations on growth of juvenile hybrid tilapia, Oreohcromis niloticus x O. Aureus. Aquaculture 253: 602-607.
- Wang Y, Chien Y, Pan H (2006) Effect of dietary supplementation of caretenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon Callistus. Aquaculture 261: 641-648.
- Page G, Davies SJ (2002) Astaxanthin and canthaxanthin do not induce liver or kidney xenobiotic-metabolizing enzymes in rainbow trout (Oncorhynchus mykiss,Walbaum).CompBiochem Physiol C Toxicol Pharmacol 133: 443-451.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 13039
- [From(publication date):
March-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11880
- PDF downloads : 1159