Research Article Open Access
Global Warming can be Protected by Promotion of Plankton CO2 Assimilation
Shoichiro Ozaki*The Institute of Physical and Chemical Research, 2-1 Hirosawa, Wakoshi Saitama, Japan
- *Corresponding Author:
- Shoichiro Ozaki
The Institute of Physical and Chemical
Research 2-1 Hirosawa, Wakoshi Saitama, Japan
Tel: +81046767099
E-mail: ozaki-0991@jcom.zaq.ne.jp
Received date: September 14, 2016; Accepted date: October 17, 2016; Published date: October 26, 2016
Citation: Ozaki S (2016) Global Warming can be Protected by Promotion of Plankton CO2 Assimilation. J Marine Sci Res Dev 6:213. doi: 10.4172/2155-9910.1000213
Copyright: © 2016 Ozaki S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
The earth is warmed up by the heat evolved by the burning of fossil fuel releasing CO2. CO2 assimilation reaction is the reaction of CO2 with water to produce carbohydrate absorbing heat. Burning reaction is reverse reaction of CO2 assimilation. If we can compensate the generation of CO2 and heart of burning with the absorption of CO2 and heart by CO2 assimilation, global warming can be protected. Plankton CO2 assimilation reduced 95% CO2 in Precambrian Eon to 400 ppm now in 35 billion years. It is said that 70% CO2 assimilation is carried out at sea. Supply of nutrient N and P to sea is most important. Large amount of NOx is produced when fossil fuel is burned. This NOx should be released without elimination procedure as it is. Large amount of N and P is contained in drainage. The drainage should be released as it is. Deep sea water contain much nutrient N and P. Shallow sea water contain very little nutrient. Agitation of deep sea water with shallow sea water increases the plankton growth. These three points are effective methods to increase the plankton growth, to increase fish production and reduce CO2 production and to protect earth warming.
Keywords
NOx; Carbon dioxide; Carbon dioxide assimilation; Global warming; Plankton
Introduction
The plant is growing by absorbing CO2 and water making carbohydrate and oxygen absorbing energy. This reaction is called CO2 assimilation and fix CO2 and absorb heat. The earth is warmed up by the heat evolved by the burning of fossil fuels. Global warming can be protected by promotion of CO2 assimilation, by promotion of plant growth, by promotion of CO2 fixing and heat absorption by providing enough N and P [1-5].
70% of CO2 assimilation is said to be carried out at sea. Plankton photosynthesis are studied by many investigators [6-67]. It is estimated that between 50%-85% of the world’s oxygen is produced via plankton photosynthesis [9,10]. The rest is produced via photosynthesis on land by plants [10]. Furthermore, plankton photosynthesis has controlled the atmospheric CO2/O2 balance since the early Precambrian Eon [11]. The growth of plankton populations is dependent on light levels and nutrient availability. Supplies of nutrients are expected to have important impacts on future plankton productivity [20].
The agitation of deep sea water (rich N,P) with poor nutrient sallow sea water, and effective uses of NOx in burned gas and P in drainage are powerful support for the promotion of plankton CO2 assimilation.
In this paper I will describe these two methods to protect global warming by increase of nutrients N and P in the sea.
Carbon dioxide assimilation
CO2 assimilation produces carbohydrate (glucose) and oxygen absorbing heat 114 kcal.
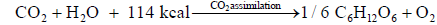

When burning reaction, left going reaction <--------- is predominant, earth warming is predominant. When CO2 assimilation reaction, right going reaction ------> is predominant earth cooling is predominant by enough absorption of CO2 and heat by CO2 assimilation, earth can be cooled down [68-70].
CO2 concentrations increase annually, in 1950 278 ppm, in 1986 350 ppm, in 1996 357 ppm, in 2000 372 ppm, in 2010 390 ppm, in 2014 387 ppm, in 2015 400 ppm. This is due to the predominant production of CO2. We must increase the absorption of CO2 by CO2 assimilation. To increase the CO2 assimilation we must increase the concentration of nutrient nitrogen N and nutrient phosphorous P [71].
(A) At laboratory: The reaction of CO2 1 mole 44 g and H2O 1 mole 18 g absorbing 114 kcal giving glucose 1/6 mole 30 g and oxygen O2 1 mole 32 g, 22.4 L, is called as CO2 assimilation. This reaction is most important reaction for our all living biology. By this reaction, all biology could live for 50 billion years.
(B) At rice field: Rice plants grow by eating CO2. How much amount of CO2 is eaten at 0.1 hectare (1000 m2, 300 Tsubo). Rice 430 kg is produced at 0.1 hectare. Plant 1 tone, 1000 kg is produced including rice straw in one year. To make 1 tone of plant, CO2 1 tone×44/30=1.47 tone are necessary. Heat 38×106 kcal is absorbed. At the under of big tree, we fool cool. This is due to the absorption of heat by assimilation.
(C) At Setoinland sea: Area of Setoinland sea (sea between Shikoku and Chugoku in Japan) is 47000 km2. 4.7 million times wider than 1 hectare. If assimilation efficiency is same as rice field, 1.47 t×47×105=69×106 t of CO2 will be absorbed and 114×47×106=5.3×1012 kcal heat will be absorbed. And 47×106 t of fish will be expected.
(D) At earth: Fossil fuel 1.4×1010 t was burned at whole world in 2010 and about 4.4×1010 t CO2 was released and about 4×1010 t CO2 was fixed and 7.4×1015 kcal is absorbed.
(E) At forest: Total wood weight in the world is 700×108 tones. Tree grows 1-2% annually then annual CO2 absorption is 7×108 tones. 1.6×1013 kcal is absorbed.
(F) At land: Annual CO2 fix by land plant in the world is 10×108 tone and 5.7×1013 kcal is absorbed.
(G) At sea: Annual CO2 fix by ocean plant in the world is 20×108 tones. And 5.7×1013 kcal was absorbed.
(H) At sea: Annual CO2 fix by ocean plankton is 2×1010 tones. And 1.1×1014 kcal is absorbed.
(I) Prof Matsunaga, Tokyo Agriculture University studied the fixing of CO2. Sea weed can grow 4320 g/m2/day, if enough N and P are provided [72].
70% of CO2 assimilation is said to be carried out at sea. Assimilation is carried out by sea weed and plankton. Sea weed and plankton are growing under ice at arctic and Antarctic ocean, eating much CO2, absorbing much heat and giving much food for whales, penguin and earless seals. When we consider the fact that oil is fossil of plankton and coal is a fossil of tree. We astonish the magnitude, greatness and contribution of plankton assimilation.
The reason why earth is warmed up is due to the heart evolved by the burning of fossil fuels. CO2 assimilation is a reverse reaction. By absorption of heat by CO2 assimilation, earth can be cooled down.
Fossil fuel 1.4×1010 t was burned at whole world in 2010 and about 4.4×1010 t CO2 was produced and 2.5×1015 kcal is produced. By doing reverse reaction, CO2 assimilation, and by absorption of same amount of CO2 and heart, the equilibrium of CO2 and heart will be possible.
Nitrogen oxide NOx is natural fertilizer for ten thousand years
Best method to protect global warming is the promotion of growth of plant and plankton. To promote the growth of plant, the supply of nutrient nitrogen and phosphorous is most important [1-5].
Nature has natural systems to change N2 to nutrient nitrogen. By the high temperature at fire place for cooking, warming up of room by burning of wood, by thunder storm, by forest fire, by forest burning, by bonfire, following reactions proceed.
1/2 N2+1/2 O2 ----------> NO- 21.6 kcal
2NO+O2 ----------> 2 NO2+13.5 kcal
3 NO2 +H2O ----------> 2 HNO3+NO
NOx (Mixture of 90% NO and 10% NO2) is produced and dissolved in rain water, giving nutrient nitric acid ion NO3- produced. NO3 - ions is a natural nitrogen fertilizer and promote the growth of plant and plankton.
In 1 liter rain water, 0.8 mg ammonium ion and 0.44 mg nitric acid nitrogen, total 1.2 mg of nitrogen is contained in 1970. As 1200 mm water fall in one year, 120 liter of rain fall in 1 m2 in Japan, 15 kg nitrogen in 1 hectare area are given as fertilizer to all area irrespective to mountain, field or sea. Old agriculture such as rice production was carried out without synthetic fertilizer using this natural fertilizer. Old proverb says that many thunder storm year gives good harvest.
In such way, use of nitrogen and recycle of nitrogen are done for 10 thousand years. The equilibrium of burning of plant (CO2 generation) and×plant (CO2 absorption), and the equilibrium of heat generation and heat absorption were maintained.
As civilization advances, people use fossil fuel like oil, natural gas and coal. Large amount of CO2 was released. Large amount of NOx is liberated in the process of burning of fossil fuels. In 2010 fossil 1.4×1010 t was burned and CO2 4.4×1010 t was produced. As C/N ratio of plant is around 5/1-50/1(average 25/1) [73,74]. NOx 1.4×1010 t×1/50=2.8×108 t were produced. Plant is growing by eating CO2 and nutrient N by the ratio of CO2/N 5/1-50/1 (average 25/1). One N can fix 5-50 (average 25) CO2.
The CO2 assimilation is promoted by the increase of nutrient N and P. The most easily available nutrient N is NOx. Therefore we should use all NOx produced by burning as it is produced. We should not eliminate NOx. Nature look likes set up the amount of NOx to balance the loss of burning material (fossil) and increase of burning material (plant) by promoting the growth of plant by produced NOx.
Fossil fuel 1.4×1010 t was burned at whole world in 2010 and about 4.4×1010 t CO2 was produced and 2.5×1015 kcal is produced and NOx 2×109 t is estimated to be produced. NOx produced by exhaust gas is 0.4×108 tones. If we use these all NOx 2.4×109 t, we can fix CO2 5×1010 t (25×2.4×109 t). This amount is almost same as 4.4×1010 t (CO2 produced in 2010). We can protect global warming by promotion of CO2 assimilation by using NOx.
Toxicity of NOx
NOx is hated as pollution gas and is eliminating using much fossil. But no report as to the sickness caused by NOx is reported.
Low doses of inhaled nitric oxide have been reported to be clinically effective, and most current dosing recommendation does not exceed 40 ppm. At this dose, the little measurable short term toxicity. Indeed, it is noteworthy that in the large randomized trials of inhaled nitric oxide, major clinical toxicity (e.g., methemolobinemia) was observed only at dose>80 ppm [75,76]. NOx is released at exhaust gas at power station and does not exceed 40 ppm in respiratory tract.
Phosphorous is essential for fixing CO2
Phosphorous P is important atom constituent of plants and animals. Phytic acid (Inositol Hexaphosphate) calcium salt is contained in every surface of grain such as rice, wheat and corn about 30%. Plant makes glucose by photosynthesis from CO2 and water. Some of glucose is converted to inositol. Inositol is converted to phosphoinositides (PIP2) and phytic acid. PIP2 is converted to IP3 and diacylglycerol. These two compounds are essential for signal transduction of plant [77]. Plants make phytic acid as storage of phosphorous. Phosphorous is an essential atoms as fertilizer, because it is an essential atom to make DNA. The seed store phosphorous atoms as a store so that even when seed germinate at no phosphorous land. To make this phytic acid, plants absorb corresponding phosphorous at harvest time. Lack of phosphorous gives poor harvest [3,78].
How phosphorous is supplied?
There are three routes to supply phosphorous to plant.
1. Tripolyphosphate: As laundry detergent, 60 thousand tones were used in 1984 in Japan. By using this tripolyphosphate, 60 thousand×25=1500 thousand tone CO2 was fixed. CO2/P ratio would be similar to CO2/N ratio, 1000 thousand tone plankton is produced and fish 490 thousand tone was produced.
2. Phosphorous in drainage: About 60 thousand tone phosphorous was contained in drainage in Japan. By using this phosphate, 60 thousand×25=1500 thousand tone CO2 can be fixed. And 1000 thousand tone plankton can be produced and fish 490 thousand tone will be produced. Animal eat food containing P and exclude excreta containing P. When toilet disposal and drainage are sent to excreta disposal treatment plant. P in water was made to water insoluble mass, mixed with cement and made to concrete and buried in soil. Plant cannot use P any more. This process use huge electricity and consume much fossil fuel. Around two hundred thousand tone fossil and producing five hundred thousand tones CO2. For the elimination of one phosphorous, about 2 carbon fossil is used and about 2 CO2 is produced. One phosphorous can fix 25 CO2. The phosphorous elimination process should be avoided. Because excreta is best food for plant. Ocean dumping, field dumping and forest dumping of excreta are recommended to increase the concentration of nutrient phosphorous.
3. Phosphorous: 88 μg is in 1 little sea water. Annual CO2 fix by ocean plankton in the world is 2×1910 tones. This amount is more than half of CO2 generated in the world. Therefore fixing of CO2 by plankton at sea is most important.
Method to Reduce CO2 Production
CO2 production can be reduced 20% by stopping NOx and nutrient P elimination process.
Stopping NOx elimination process
The facility like power station has NOx elimination equipment. Flue gas is reacted with ammonia and NO is converted to N2 gas.
4 NO+4 NH3+O2 ---------> 4 N2+6 H2O
Equivalent molar amount of ammonia is required to eliminate NO.
The production of nitrogen oxide by persons operation in Japan is two million tones. If destroy NOx by ammonia, 1.13 million tone ammonia is necessary. This amount is 2 times of nitrogen fertilizer used in Japan. To make ammonia 1.13 million tone, 0.2 million tone hydrogen gas is required. To make 0.2 million tone hydrogen, butane 0.64 million tones is required. As the result, 1.76 million t CO2 is released. This is a huge promotion of global warming.
C4H10+8 H2O ----> 9 H2+4 CO2
N2+3 H2 ----> 2 NH3
By stopping this NOx elimination equipment, we can reduce 1.76 million tones CO2 production. Japan eliminates NOx completely. Therefore electricity price in Japan is two times higher than that of Korea. Because construction cost plus fossil cost are added for elimination of NOx. Many industrial factories are built at outside of Japan.
Stopping of elimination process of nutrient nitrogen and phosphorous in drainage. About 60 thousand tones phosphorous are present in drainage in Japan. We can reduce the production of 2 million tones CO2 production by stopping of elimination process, corresponding 1.5 % of total CO2 production. Two million tones NOx is produced in Japan and about two hundred million tones NOx is produced in the world About 60 thousand tones P are present in drainage in Japan, and 6 billion tones P in the world. We should use these N and P to increase the concentration of N and P at the surface of sea.
Elimination of nutrient N and P resulted in the elimination of plankton growth and fish industry and CO2 fix
Two news about the red sea (red plankton grow) at near hatchery fish plants at Kagawa prefecture, Japan and much water weed grow at Biwako lake were reported. These were special event at special district. But Japan Government established Environment Ministry. This Ministry established very strict rules to eliminate all N and P to inhibit the growth of all plankton effective for all over Japan by eliminating all N and P in air and water. This policy destroyed Japan fish industry. Sea changed dramatically. Sea weed do not grow. Plankton does not grow. Nori growing plant stopped. At all Japan sea area where Kuroshio (poor N, P nutrient sea current) is running: Rocky-shore denudation is seen. Fish decreased especially Pacific saury (sanma), Tuna (maguro), Bonite (katsuo), Sardin (iwashi) decreased, Bream (tai), Eel (unagi), Sea eel (anago), Shell fish like Oyster (kaki), Basket clam (shizimi), Short-neck clam (asari) decreased. Fisherman decreased. Fish price increased. Japan must buy Mackerel and Salmon from Norway, Octopus from Morocco, Tuna (maguro) from Croatia, and Shrimp from Vietnam. All these fish were cheap than meat before 1970. Japanese can live longest by eating these fish [70-78]. But now fish is much more expensive than meat since nutrient N and P elimination rule. We Japanese may lose long life record. In Japan, CO2 fix and absorption of heat by plankton CO2 assimilation decreased.
Agitation of deep sea water with shallow sea water promotes plankton growth and fish production
Annual CO2 fix by ocean plankton in the world is 2×1910 tones. Therefore fixing of CO2 by plankton at sea is most important. 88 μg of P is in 1 liter sea water. The Kuroshio current Japan (running water from south to north at east south coast of Japan) is clean and contain poor nutrition salt (phosphate salt, nitrate salt) and poor in plankton and fish. Oyashio Japan (running north of Japan near Hokkaido) is rich in nutrient salt and rich in plankton and fish. Cold current running west coast of United State is very rich in nutrient salt and is rich in plankton and fish.
Concentration of N and P of surface sea water at 100 km south of Muroto (South corner of Shikoku) is 1 μg/l, 0.3 μg/l respectively. N 33 μg/l, P 2.9 μg/l at 1000 m deep sea, water is 30 times and 10 times rich in nutrition than that of surface sea water. At Kumejima, Okinawa in Japan Plankton production and Kaki (Oyster) production using sea water pumped up from deep sea are now in production stage.
Agitation or stirring of sea water by using current power or wind power or construction of fence must be studied to increase the concentration of N and P at surface.
Coral bleaching is reported at sekisei reef lake at Okinawa, Japan in Sept 2016. Because no typhoon approach this year at this district, agitation was not enough to replace nutrient deep sea water (contain much nutrient nitrogen, phosphorous) with poor nutrient shallow sea water caused the no growth of zooxanthella. Coral bleaching is reported at Great Barrier Reef in 2016 June 6. They say coral bleaching is caused by too warm water by global warming. But I think this is caused by lack of nutrient N, P by insufficient agitation of nutrient rich deep sea water with poor nutrient surface sea water.
Global warming produce high temperature of sea water, evaporation of water and consequent many typhoon, hurricane. These typhoon and hurricane agitate surface sea water (poor nutrient) with deep sea water (rich nutrient). Plankton growth infinitely if enough nutrient N and P are present. Many hurricane attacking east south part of United State producing nutrient rich surface sea water and this Gulf current goes up to north producing much plankton and much CO2 and heat absorption and producing much fish.
Le Chatelier’s principle is working at Nature
High temperature is caused by global warming. Then evaporation of sea water causes hurricane and typhoon. Hurricane and typhoon agitate the sea water to give enough N and P to promote the CO2 assimilation, to promote the growth of plankton and promote CO2 absorption and heat absorption to cool down the earth. We must study and find out best method to agitate sea water. By the use of wind, sea current, the use of attraction of moon.
Amount of buried fossil and global warming
Since industrial revolution, mankind is using large amount of fossil for transportation and manufacturing of iron, aluminum, plastic and fertilizer. Global warming comes from over burning of fossil. Fossil fuels are fossil of plants made by CO2 assimilation from CO2 and water in 50 billion years. Mankind is now using up these fossils in 500 years. Estimated amount of buried fossil: oil is 42 years, natural gas is 60 years, coal is 132 years, and buried uranium is 124 years. When fossil is burned out, no global warming will happen. We must consider how we can live civilized life. How can we drive car, air plane, agriculture machine, fishing boat. How can we generate electricity? From what can we make plastic and solar cell module? We must consider how to use limited precious fossil. We should not use fossil for elimination of NOx and nutrient P. We should use produced NOx for promotion of CO2 assimilation. In 2010, fossil 1.4×1010 tones was burned and 4.4×1010 tone CO2 was produced. Estimated CO2 4×1010 tones was absorbed by plant and plankton. The difference is 10 percent. If we use NOx and nutrient P effectively, we can protect global warming.
Summary
Global warming can be protected by the promotion of plankton growth by the supply of nutrient N and P at sea by following three items to fit Paris agreement.
1. Stopping of NOx (at buned gas) elimination process.
2. Stopping of nutrient phosphorous and N elimination process in drainage.
3. Agitation of nutrient deep sea water (rich N, P) with poor nutrient sallow sea water.
References
- ShoichiroO (1993) Recycle of nitrogen and phosphorous for the increase offood production. New Food Industry35: 10 33-39.
- Ozaki ShoichiroMethods to protect global warming (2016) Adv Tech Biol Med 4:181.
- Ozaki S (2014) Chemical Approach to Signal Transduction by Inositol Triphosphate, J Bioengand Biome Sci 4: 133.
- ShoichiroO (2016) Methods to protect global warming, Food production increase way. New Food Industry 58: 47-52.
- Shoichiro O (2016) Global warming can be protected by promotion of CO2 assimilation using NOx.J of Climand Weather Forecast4: 171.
- Falkowski PG (1994)"The role of phytoplankton photosynthesis in global biogeochemical cycles". Photo synRes39: 235-258.
- Chisholm SW (2001) "Dis-crediting ocean fertilization". Sci 294: 309-310.
- Aumont O, Bopp L (2006) "Globalizing results from ocean in situ iron fertilization studies". Global BiogeoCy20: GB2017.
- (2015) How much do oceans add to world's oxygen?" Earth and Sky.
- Roach J (2004) "Source of Half Earth's Oxygen Gets Little Credit". Nat GeograNews.
- Tappan H (1968) "Primary production, isotopes, extinctions and the atmosphere". Palaeogeo, Palaeoclimatol, Palaeoecol4: 187-210.
- Wang G, Wang X, Liu X, Li Q (2012) Diversity and biogeochemical function of planktonic fungi in the ocean. In: C Raghukumar (ed), Biology of marine fungi. Springer Berlin Heidelberg p: 71-88.
- Emiliani C (1991)"Planktic/Planktonic,Nektic/Nektonic, Benthic/Benthonic". Jof Paleontol65: 329.
- Omori M, Ikeda T (1992) Methods in Marine Zooplankton Ecology. Malabar, USA: Krieger Publishing Company.
- Thurman HV (2007) Introductory Oceanography. Academic Internet Publishers.
- Ghosal R, Wray S (2011) "The Effects of Turbulence on Phytoplankton". Aerospace Technology Enterprise. NTRS.
- "NASA Satellite Detects Red Glow to Map Global Ocean Plant Health" NASA, 28 May 2009.
- "Satellite Sees Ocean Plants Increase, Coasts Greening". NASA 2 March 2005.
- Henson SA, Sarmiento JL, Dunne JP, Bopp L, Lima I, et al. (2010) "Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity". Biogeosci7: 621-40.
- Steinacher M, Joos F, Frölicher TL, Bopp L, CaduleP, et al. (2010) "Projected 21st century decrease in marine productivity: a multi-model analysis". Biogeosc7: 979-1005.
- Richtel M (2007) "Recruiting Plankton to Fight Global Warming". New York Times.
- Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) "Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate". Nature326: 655-61.
- Quinn PK, Bates TS (2011) "The case against climate regulation via oceanic phytoplankton sulphur emissions". Nature480: 51-6.
- Calbet A (2008) "The trophic roles of microzooplankton in marine systems". ICES J of Marine Sci65: 325-31.
- Redfield, Alfred C (1934) "On the Proportions of Organic Derivatives in Sea Water and their Relation to the Composition of Plankton". In Johnstone, James; Daniel, Richard Jellicoe. James Johnstone Memorial Volume. Liverpool: University Press of Liverpool P: 176-192.
- Arrigo KR (2005) "Marine microorganisms and global nutrient cycles". Nature 437: 349-355.
- Fanning KA (1989) "Influence of atmospheric pollution on nutrient limitation in the ocean". Nature339: 460-463
- Sterner, Warner R, Elser, James J (2002)Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton UnivPress.
- Klausmeier CA,Litchman E, Levin SA (2004) "Phytoplankton growth and stoichiometry under multiple nutrient limitation". Limnol and Oce49: 1463-1470.
- Klausmeier CA, LitchmanE, Daufresne T, Levin SA (2004) "Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton". Nature429: 171-174.
- Boyce DG, LewisMR, WormB (2010) "Global phytoplankton decline over the past century". Nature 466: 591-596.
- Schiermeier Q (2010) "Ocean greenery under warming stress". Nature p: 379.
- Mackas DL (2011) "Does blending of chlorophyll data bias temporal trend?".Nature472: E4-5; discussion E8-E9.
- Rykaczewski RR, Dunne JP (2011)"A measured look at ocean chlorophyll trends". Nature472: E5-6; discussion E8-9.
- McQuattersG, Abigail R, Philip C, Edwards M,Burkill PH, et al. (2011) "Is there a decline in marine phytoplankton?" Nature47: E6-7, discussion E8-9.
- Boyce DG, Dowd M, Lewis MR, WormB (2014) "Estimating global chlorophyll changes over the past century". Progress in Oceano22: 163-173.
- Antoine D (2005) "Bridging ocean color observations of the 1980s and 2000s in search of long-term trends". J of Geophy Res110: C6.
- Gregg WW, Conkright ME, GinouxP, O'Reilly JE, Casey NW (2003) "Ocean primary production and climate: Global decadal changes". Geophy Res Lettersp: 30.
- Gregg WW, ConkrightME (2002)"Decadal changes in global ocean chlorophyll". GeophyRes Letters. 29: 20-12-0-4.
- Raitsos DE(2005)"Extending the SeaWiFS chlorophyll data set back 50 years in the northeast Atlantic". Geophy ResLetters p: 32.
- Behrenfeld MJ, O’Malley RT, Siegel DA, McClain, Charles R, et al. (2006) "Climate-driven trends in contemporary ocean productivity". Nature444: 752-7555.
- Mace GM, Mora C, Chih-Lin W, Rollo A, Amaro T, et al. (2013)"Biotic and Human Vulnerability to Projected Changes in Ocean Biogeochemistry over the 21st Century". PLoSBiol11: e1001682.
- Cermeno P, Dutkiewicz S, Harris RP, Follows M, Schofield O, et al. (2008) "The role of nutricline depth in regulating the ocean carbon cycle". Proceedings of the National Acad of Sci105: 20344-20349.
- Cox PM, Betts RA, JonesCD, Spall SA, Totterdell IJ (2000) "Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model". Nature 40:184-187.
- Mincer TJ, Aicher AC (2016) "Methanol Production by a Broad Phylogenetic Array of Marine Phytoplankton". PLOS ONE11: e0150820.
- Sarmiento JL, Slater R, Barber R, Bopp L, Doney SC, et al. (2004). Response of ocean ecosystems to climate warming. Global Biogeochem Cycles 18.
- Taucher J, Oschlies A (2011) Can we predict the direction of marine primary production change under global warming?.GeophyRes Letters38.
- Tokoro T, Hosokawa ES, Miyoshi KT, Watanabe SK, Montani HK, et al. (2014) Net uptake ofatmospheric CO2014by coastal submerged aquatic vegetation. Global Change Bio20: 1873-1884.
- Kuwae T, Kanda J, Kubo A (2016) Blue carbon in human domunatedestrian and shallow coastal systems Anbio45: 290-301.
- Awaya(2004) International J of Remote Sensory 25: 1597-1613.
- Cai WJ (2011) Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Sci3: 123-145.
- Chambers JQ, Higuchi N, Tribuzy ES, Trumbore SR (2001) Carbon sinks for a century. Nature 410: 429.
- Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marba N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nature Clim Change 3: 961-968.
- Evans W, Hales B, Strutton PG (2013) pCO2 distributions and air-water CO2 fluxes in the Columbia River estuary, Estuarine, Coastal and Shelf Sci117: 260-272.
- Fourqurean JW, Duarte CM, Kennedy H, Marba N, Holmer M, et al. (2012) Seagrass ecosystems as a globally significant carbon stock. Nature Geosci 5: 505-509.
- Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, et al. (2008) Global change and the ecology of cities. Sci319: 756-760.
- Hartnett HE, Keil RG, Hedges JI, Devol AH (1998) Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature 391: 572-574.
- Hendriks IE, Sintes T, Bouma T, Duarte CM (2007) Experimental assessment and modeling evaluation of the effects of seagrass (P. Oceanica) on flow and particle trapping. Mar EcolProgSer 356: 163-173.
- Raymond PA, Hartmann J, Lauerwald RS, McDonald C, Hoover M, et al. (2013) Global carbon dioxide emissions from inland waters. Nature 503: 355-359.
- Regnier PAG, Friedlingstein P, Mackenzie CFTN, Janssens GG, Laruelle RL, et al. (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nature Geosci 6: 597-607.
- Taylor PG, Townsend AR (2010) Stoichiometric control of organic carbon-nitrate relationships from soils to the sea. Nature 464: 1178-1181.
- Watanabe AT, Yamamoto K, Nadaoka Y, Maeda T, Miyajima YT, et al. (2013) Spatiotemporal variations in CO2 flux in a fringing reef simulated using a novel carbonate system dynamics model. Coral Reefs 2013 32: 239-254
- (1993) Matsunaga Chemistry and Chemical Industry 46: 763
- ZHi LZ (2009) Carbon and Nitrogen nutrient balance signling in plant. Plant Signaling and Behav4: 584-591.
- Coruzzi G, Bush D (2001) Nitrogen and Carbon nutrient and metabolite signaling in plant. Plant physiol125: 61-64.
- Davidson D (1998) Inhalated nitric oxide for the early treatment of persistent pulmonary hypertension. The 1-NO/PPHN Study Group. Pedriatrics 101: 325-334.
- Barry W, Debra LL, Diane lE, Jeffrey DL (2000) The Toxicity of inhaled Nitric oxide. ToxicolSci 59: 3-16.
- Ozaki S, Watanabe Y, Ogasawara T, Kondo Y, Shiotani N, et al. (1986) Total synthesis of optically active myo-inositol 1,4,5-tris(phosphate). Tetrahedron Letters 27: 3157-60.
- Ozaki S, Yoshihisa K, Naokazu S, Tomio O, Yutaka W (1992) Synthesis and some properties of D-myo-inositol 1,4,5-tris(dihydrogen phosphate).Jof the Chemical Society, Perkin Transactions 1: Org and Bio-Org Chem2: 729-737.
- Ozaki S, Yutaka W (1991) Synthesis of Inositol Polyphosphate and Their Derivatives. ACS Symposium Series 463 Inositolpolyphosphate and derivatives Edit Allen B. Reitz 41-64.
- Ozaki S, Watanabe Y, MaekawaT,HigakiY, Ogasawara T,et al. (1998) Synthesis of phosphatidyl-myo-inositol polyphosphates and derivatives. ACS Symposium Series 718 Phosphoinositides, Edit Karol Bruzik 212-221.
- Ozaki S (2015) Sulfo disaccharides co-working with Klotho. Studies on structure, structure activity relation and function.World J of Pharmacy and PharmaceutSci4: 152-175.
- Ozaki S (2016) Secret of Anti-aging: Anti-Aging Food Containing Glucosamine,Hyaluronic Acid and Chondroitin.Jacobs J of Physiology 2: 13.
- Ozaki S (2015) Glucosamine Derivatives. Sulfo disaccharides co-working with Klotho. Nutrand Food Sci 5: 416.
- Ozaki S (2015) Synthesis of Anti-Aging Reagent: Sulfo Disaccharide Co-working with Anti-Aging Gene. Arch of Med7: 17.
- Shoichiro O (2015)Nutrition for Good Health,Anti-aging and Long Life, Hyaluronic Acid. Glucosamine and Chondroitin. Maternal and PaediatricNutr J1:e102.
- Shoichiro O (2016) Food containing hyaluronic acid and chondroichin is essential for anti-aging. InternJ of aging and ClinRes1:101.
- Shoichiro O (2016) Toward Anti-Aging and Long Life. Jacobs J of Physiol2: 13-17.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 18547
- [From(publication date):
December-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 17438
- PDF downloads : 1109
