Microgravity Effects on the Growth, Cell Cytology Properties and DNA Alterations of Three Iraqi Local Plants
Received: 13-Feb-2019 / Accepted Date: 27-Mar-2019 / Published Date: 05-Apr-2019
Abstract
In the current study, zero-gravity instruments circulated by the UNOOSA due to the proposals of the UN/Malaysia Expert Meeting on Human Space Technology in November 2011 were used to test gravity impacts and diverse media types (ddH2O and agar) on three plant species in particular, rice-Anber, rice-Jasmine. Microgravity impacts on cell division and root tips progression were assessed at 90 rpm, 90° clockwise at various gravity times relying upon the utilized plant species that was tested. The primary results of microgravity on plants were portrayed by huge declines in crisp and dry weight, less root stretching and numbering. Comet parameters demonstrated that controls (Comet length (px) 31.96 ± 1.92, Comet zone (px) 614.12 ± 57.78 mean ± SE in rice Anber ) were superior compared to the microgravity treatment (Comet length (px) 40.72 ± 1.08, Comet zone (px) 942.91 ± 36.79 mean ± SE in rice Anber). In the same wein, the effects of the agar (Comet length (px) 34.20 ± 2.53, Comet range (px) 644.30 ± 72.34 mean ± SE in rice Anber ) were better to those acquired from the cotton medium under zero gravity conditions (Comet length (px) 38.13 ± 1.39, Comet zone (px) 890.36 ± 48.42 mean ± SE in rice Anber). The clinorotated tests demonstrated fundamentally extraordinary qualities of cell cytology contrasted with those of the 1 g control. Local Iraqi plants, Oryza sativa (rice Jasmine and rice Anber) presented to microgravity had weaker development properties as the 1 g control bunches demonstrated a superior development rate achieving ~25% of the roots than the clinorotated gatherings. Moreover, an expansion in the mitotic record has been influenced by the aggregation of cells in a specific period of division primarily when the agar medium was utilized. In addition, chromosome variations, for example, stickiness in the metaphase and telophase, scaffolds and slacking chromosomes were recorded. The Comet test was further performed to decide how Single-Strand Breaks (SSBs) as the underlying harm added to DNA relocation. In general, the Comet examination senses a strategy for perceiving SSBs, and it had been demonstrated that a low level of SSB was detected as the concealed harm can't be seen by the Comet measure in light of the way that these SSBs vanish following a repair occasion and that SSBs as at an opportune time DNA insidiousness can be particularly perceived in the cellular Comet test (cellular test). Tests performed in microgravity have enormously added to the comprehension of how plants sense the gravity heading and react to it. In any case, the entire flag transduction process isn't yet comprehended in details between the two gatherings, although the research is potentially useful in the definition of cultivars to be used in spaceflight.
Keywords: Zero gravity; One-axis clinostat; Rice; Comet assay
Introduction
Up-to-date, a few studies on cell cytology properties have been carried out under a microgravity environment, since it is difficult to produce true microgravity conditions for enough time on earth and the opportunity for space experiments is still limited. Microgravity conditions can be produced on earth by centrifugation [1]. Acceleration due to centrifugation has been used to analyse the mechanism of gravity perception and gravicurvature. When plants are grown under hypergravity conditions, some changes in chromosome abnormalities, DNA damage, spindle structure and development of higher plants are observed [2]. However, changes in the physical properties of plants placed under hypergravity conditions have not yet been determined. Ionizing radiation and microgravity are considered the fundamental factors of space environment that can induce mutations or cause various biological effects on a wide range of organisms [3]. Morphogenesis and cellular changes have been observed during and right after space flights or post ground simulated experiments. Gene and protein expression changes have also been detected in a study carried out by Wang et al. [4] who investigated the proteomic changes of three heritable rice mutants induced by the space environment. Proteins were extracted from leaf tissues during tiller development and systematically analyzed by 2-D PAGE coupled with MS. Random positioning machines have also been used in substitution studies to simulate the effects of microgravity on several biological processes, such as plant growth, development and gene expression in ground-based researches [5]. Many genotoxicity assays have been developed to identify DNA damage. The Comet assay or the Single Cell Gel Electrophoresis (SCGE) assay is a gel electrophoresis-based method that can be used to measure DNA damage in an individual eukaryotic cell [6]. Due to its sensitivity, simplicity, and ability to directly measures DNA damage in individual cells, it has become an invaluable tool and gained rapidly importance for clinical applications, human bio-monitoring, genotoxicity, genetic ecotoxicology and carcinogenesis [7,8].
Kordyum [9] studied the effects of real and simulated microgravity on statolith positioning, mitochondria, tubulin and the endoplasmic reticulum, providing a significant progress in identifying stimulus-responsive elements. Iida et al. [10] worked on mechano-sensitive channels in a putative role in gravity sensing Arabidopsis. Grolig et al. [11] reported on the role of auxin transport, which is an essential component of the signalling pathway of root and shoot gravitropism. Progress made in this field which results from the contributions of three different topics: plant physiology, plant molecular biology and the gravitropic response pathway, is highlighted in the paper by Geisler et al. [12], describing approaches used for the analysis of auxin redistribution and quantification. Additionally, typical Alaska pea and the agravitropic pea mutant, ageotropum, less than 1 g conditions and on the 3-D clinostat uncovered that the apical snare of the epicotyl frames by advancement of the bend molded plumule of the developing life existing in the non-sprouted seed. The procedure of arrangement comprises of two phases: improvement and halfway opening and controlled by some natural property of the plumule. Roughly when the epicotyl rises up out of the seed, the snare is set up in both pea assortments. In Alaska the built up snare is maintained or upgraded by gravity, bringing about a deferral of snare opening contrasted and on a clinostat, which may give an off base thought that gravity causes snare development. Use of auxin polar transport inhibitors stifled the ebb and flow of snare in Alaska and in addition in ageotropum, proposing that the development of the snare includes auxin polar transport freely of gravity activity [13].
Consequently, a rotating clinostat was used in the current study to simulate weightlessness and to observe the effects of microgravity on growth properties and cell cytology characteristics of three Iraqi local plants placed under different types of culturing medium.
Materials and Methods
Plant germination
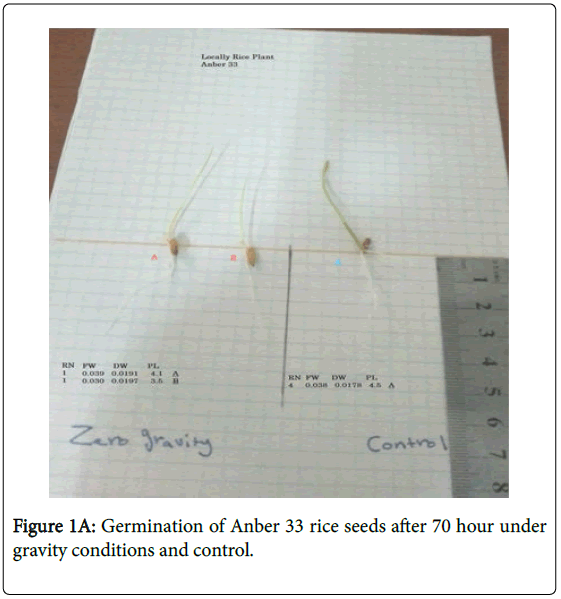
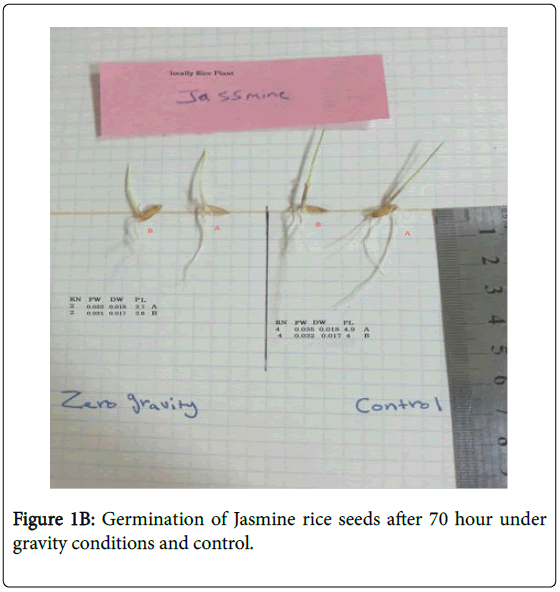
Two genotypes of rice (Oryx saliva L.) were hydroponically grown in two types of medium (15 g/L plant agar or cotton only) to test for cytological changes. Seed surface sterilization was carried out with 1.75% NaOCl for 5 min followed by three washes with sterile distilled water (ddH2O). Seeds were then placed in petri dishes each containing either 10 mL of ddH2O solidified with 15 g/L of plant agar or a sterilized wet cotton layer (Figures 1A and B). Cultured media were maintained at 24 ± 2ºC in a growth chamber in the dark for 3 days before they were divided and half was transferred to a clinostat under zero gravity conditions where one-axis clinostat was selected for distribution due to the easiness and simplicity of use. The one-axis clinostat used in the current research has one rotational axis, the direction of which can vary from 0 degree (parallel to the ground).
Cytological changes were tested for seeds germinated under clinostat construction (40 rpm) and plants grown for 70 h at 90º rotational axis angle and clockwise rotation direction. Seeds were germinated as mentioned above and once the roots reached a length of about 2 cm to 3 cm, root tips were removed and fixed according to the method described by McCurdy and Gunning [14]. Immunofluorescence labelling was carried out with an anti-α-tubulin (Sigma T-9026) (1:500) and an anti-mouse-FITC (Sigma F-02571) (1:16). All incubations were performed at 37°C for 40 min, followed by three washes each for 10 min with Phosphate Buffer Saline (PBS). Controls using only the second antibody showed no labelling. Confocal laser scanning microscopy was carried out using a Bio-Rad MRC-600 confocal microscope with BHS filters. Images were contrast enhanced before recorded on Kodak TMAX-100 films [14].
Comet assay
Preparations of solutions and stains for the comet assay were prepared according to Dhawan et al. [15]. Nuclei isolation was conducted according to Gichner [16] and Gichner et al. [17]. Plant roots were excised and placed on ice in a 60 mm petri dish containing 300 μL of cold 400 mM Tris-HCl buffer, pH 7.5. Using a razor blade, the root was gently sliced into a "fringe" to release nuclei. The petri dish was kept tilted in the ice so the isolated nuclei will be collected in the buffer.
Microscope slides for comet assay were prepared according to Gichner [16] and Gichner et al. [17]. Cleaned slides were dipped into melted 1% Normal Melting Agarose (NMA) and left to be solidified, a second layer of 100:l mixture of 1% of Low Melting Agarose (LMA) and freshly prepared nuclei suspension that was previously placed on coated slides with NMA, covered with a cover slip, placed on ice until solidified. The slip was then removed and the slides were dipped again in a 1% low melting agarose to form the third layer, placed on ice until solidified and finally the cover slip was removed and DNA was ready for the DNA unwinding step. The alkaline version of the Comet technique with DNA unwinding and electrophoresis at pH >13 was performed. Thus, the slides were placed in the alkaline buffer for 15 min before electrophoresis to allow DNA unwinding.
All operations were conducted under a dark field. Electrophoresis was done on 24 V, 250 mA for 30 min at 4°C and adjusts the current to 250 mA by raising or lowering the buffer level.
Ethidium bromide as a flurochromes was used in the comet assay for DNA staining. The staining was followed by the examination of comet cells under a fluorescent microscope using software, linked to a digital camera mounted on the microscope that automatically analyses individual Comet images.
Amino acid analysis
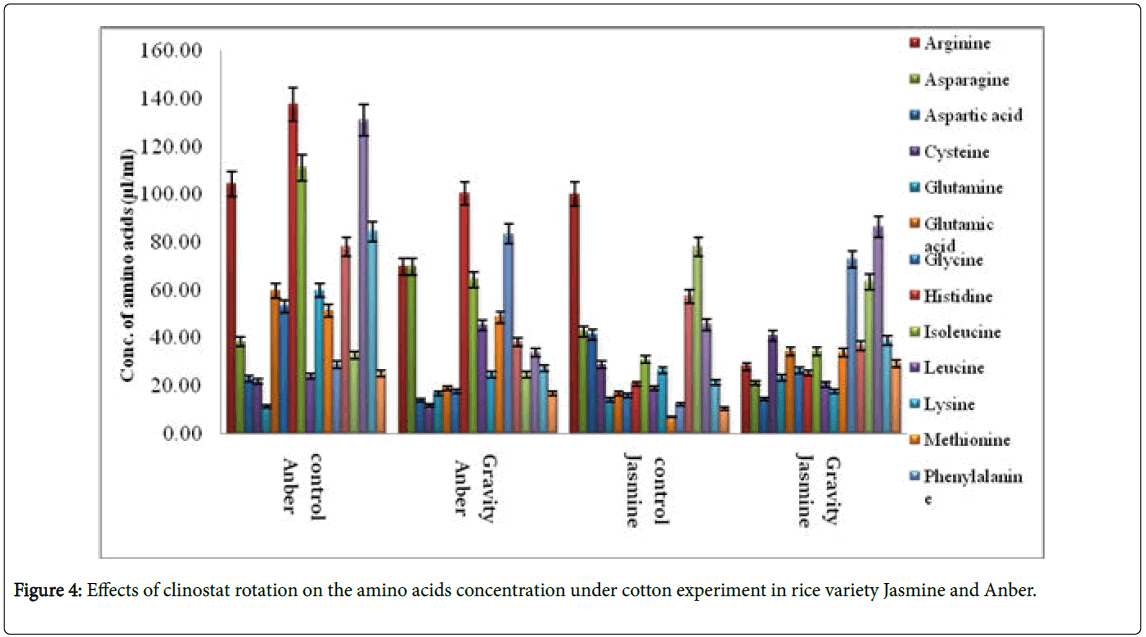
Amino acids in seedlings of rice were estimated in triplicate following reported method elsewhere [18]. Briefly, 3 mL of HCl (6 N) was added in 0.1 g of sample in a test tube. The test tube placed in the oven at 110°C for 1 h. Then, volume was increased to 50 mL by adding distilled water and mixture was filtered. The samples were dried in rotary evaporator at 40°C and solid samples were melted in 2 mL of HCl (0.01 N) and transferred to tightly closed test tube and stored at -4°C until analysis. Amino acids analyzer (Shimadzu 20AT LC System, Shimadzu, Tokyo, Japan) [19] was used for amino acid analysis. The Statistical Analysis System-SAS (2012) program was utilized and the Least Significant Difference (LSD) test was used to compare between means.
Results and Discussion
Plant growth
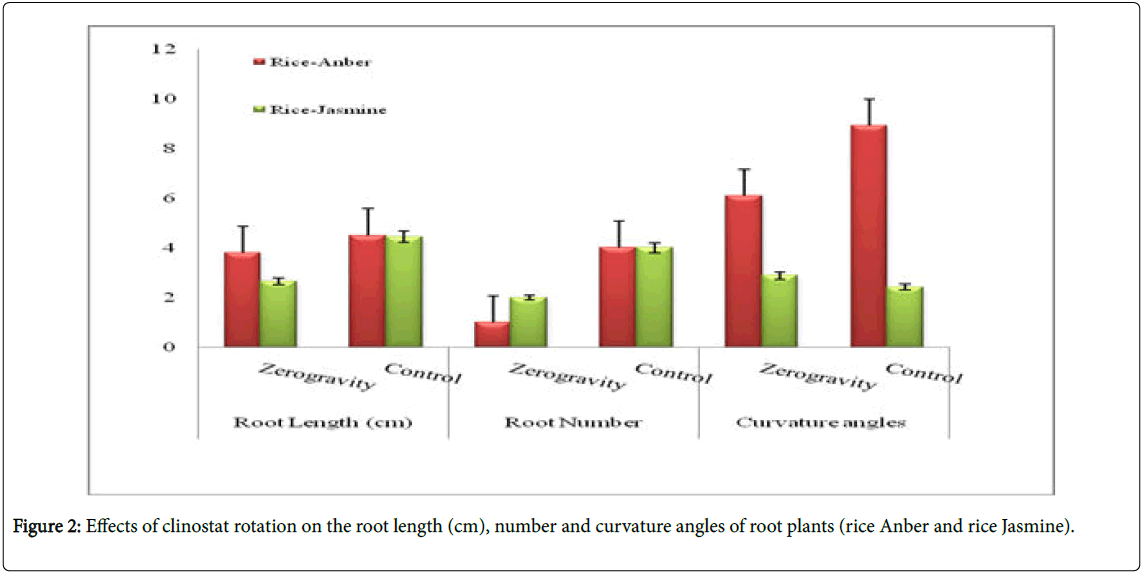
Root: Universally, plants grown off the clinostat showed more roots in contrast with their respective controls (plants developed under zero gravity conditions). Root number of plants, for example, rice-Anber and rice-Jasmine expanded incredibly when grown off the clinostat. Essentially, rice-Anber and rice-Jasmine grown off the clinostat demonstrated a higher root length (Figure 2). The clinostat impact was basically extraordinary in upgrading root length in some plant species but not in grain plants.
In addition, plants, grain, rice-Anber wheat placed off the clinostat demonstrated more root arch edges (Figure 1), with the exception of rice Jasmine. A minimal measure of root ebb and flow was gotten when grown off the clinostat. Root parameters are a restraint of development (regularly around 25%), recognizable after incitement with recuperation following 2 or 3 days of germination. Root length development, root number, and bend points are likewise repressed in rice Jasmine more than half due to the clinostat treatment. Bend points an expansion in seedling spiral augmentation in zero gravity due to ethylene because of the mechanical burdens, thus it was proposed that the clinostat epinasty was just a reaction to mechanical anxieties.
Save ingestion in the root game-plan of the clinorotation seedlings as the level clinostat influenced the difference in the root structure, it was fundamental to pick if the substance of metabolites in these organs on the even clinostat was remarkable in association with that of the controls. The levels of lipids, starch, sucrose, dissolvable sugars and proteins (new weight present) of the root framework did not vary between both culturing conditions [1].
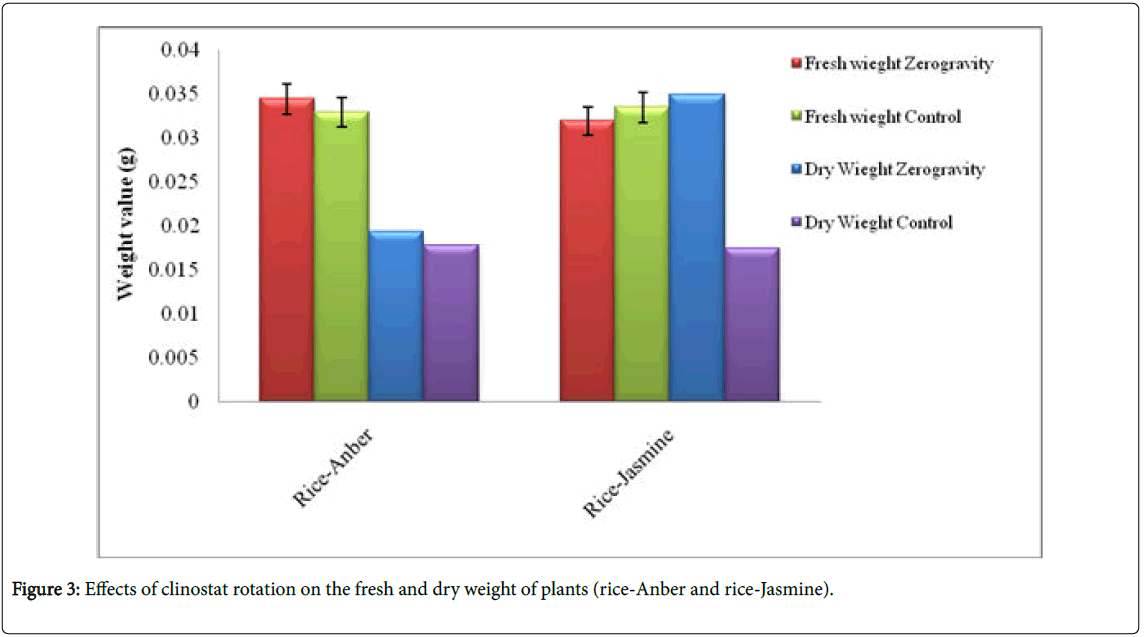
Seedlings dry and fresh weight: The dry weight of rice Jasmine developed under zero gravity is higher than that of the control grown under normal conditions (Figure 3). Subsequent to being put under recreated microgravity for 70 hours, dry weight increments by over 4%, which implies there is a great degree of contrast (t-test, p<0.01) between untreated control and reproduced microgravity. In the wake of drying the seedlings under re-enacted microgravity for 70 hours, early apoptosis builds, which implies a critical contrast (t-test, p<0.05) between untreated control and under recreated microgravity. Fresh weight of rice-Anber and Jasmine are moderately equivalent under zero gravity (Figure 3).
Cell cytology
Clinostat is wildly used for cell culturing, tissue engineering and space biology research. In order to investigate the effects of simulated microgravity on cell cytology, cells are cultured under simulated microgravity to establish the cell growth curves in comparison to those grown under normal conditions (controls). The inhibition of cell growth is observed under simulated microgravity for all cell lines used in this research. As shown in Table 1, cells grow into the logarithmic phase before the 70 h under simulated microgravity, and the numbers of cells under untreated control exhibit significant differences (t-test, p=0.05).
| Species | Medium | Statement | No. of cells divided | No. of cells, abnormal divided | |
|---|---|---|---|---|---|
| Metaphase I | Anaphase I and Telophase I | ||||
| Rice-Anber | Agar | Control | 117 | 5 | 6 |
| Zero gravity | 188.2 | 2 | 5 | ||
| Cotton | Control | 91.43 | 7 | 6 | |
| Zero gravity | 126 | 5 | 7 | ||
| Rice-Jasmine | Agar | Control | 61.25 | 6 | 4 |
| Zero gravity | 93.6 | 4 | 4 | ||
| Cotton | Control | 84.375 | 7 | 6 | |
| Zero gravity | 124.25 | 5 | 6 | ||
Table 1: Meiotic configurations and chromosome behaviour of plants.
There is an enormous qualification between control assembling and re-enacted microgravity gathering. While ensuing to being put under re-enacted microgravity for 70 hours, Mitotic Index (MI) shows critical changes in agar and cotton media. MI shows an incredible degree of important difference (test, p<0.001). These results indicate that MI increased ends up being more basic with the development of treatment time. The explanation behind the development in MI can be deciphered using animated cell duplication or collection of cells in the midst of mitotic (metaphase catch). It can be seen from the telephone advancement twists that the extension in MI in the midst of the examination can be added to the social affair of cells in metaphase, which infers a metaphase blockage. Furthermore, plants cell growth had increases in cell formation under simulated microgravity compared with the controls and the effect increased along with the extension of treatment medium (agar, cotton), which is consistent with previous research results [20].
In order to see whether cellular reproduction level is affected by simulated microgravity, MI is analyzed where the total number of cells, the number of mitotic cells are counted and the MI is calculated using the following formula: MI (%)=(the number of mitotic cells/the total number of cells) 100%.
As shown in Table 1, MI changes follow the same tendency for plants cultured in both media, agar and cotton. There is a significant difference between rotation control group and simulated microgravity group. MI shows significant changes under simulated microgravity for rice plants, but no significant difference between the two types of media. This increase in MI index could be the result of stimulating cellular proliferation or accumulation of cells during mitotic phase (metaphase arrest). It can be suggested from the cell growth curves that the increase in MI during the experiment can be contributed to the accumulation of cells in metaphase, which means a metaphase blockage.
Chromosome behaviour: Earlier cytogenetic study revealed that chromosome pairing between cultivated rice and barely meiotic behaviour was regular in all the interspecific plants at metaphase (Figure 3). A significant difference was observed in zero gravity fragment compared with control. Stickiness of chromosomes in zero gravity statement was higher than the control for both rice plants. Cmetaphase was the highest under zero gravity treatment compared with the control, but ring chromosomes showed no significant variation [21].
At anaphase and telophase, all types of anomalies were not recorded within a single parent however, more than two abnormalities were commonly observed. Frequent abnormalities as bridges, ring chromosomes, laggards and micronuclei are found in homozygous plant varieties. Few bridges and fragments observed under zero gravity conditions compared with the control. High frequency of bridges formation was observed in various rice species at the telophase [22].
Therefore, it could be concluded that simulated microgravity can alter the structure of spindle microtubules, and stimulate the formation of multipolar spindles together with multicentrosomes. This causes the overexpression of proteins to block abnormal cells in the metaphase, thereby inhibiting cell proliferation [23].
Comet assay: The Comet assay is selected to evaluate the genetic assessment of DNA damage in rice-Anber and Jasmine under various Comet parameters (Comet length, Comet area and percentage of DNA in the tail).
Results regarding the Comet length and area for rice-Anber plants (Tables 2 and 3) show striking differences between treatments and controls, 40.72 px ± 1.08 and 31.96 px ± 1.92 respectively. The greater the length of Comet parameters indicates more DNA damage and vice versa. In the same vein, there was a huge distinction between media sorts, agar or cotton, and this divergence was significantly higher when plants were put under zero gravity but lower than microgravity medications (40.72 ± 1.08 and 942.91 ± 36.79). Whereas the agar analyze (Mean ± SE) (34.20 ± 2.53 and 644.30 ± 72.34 individually) was lower than the cotton one (Mean ± SE) (38.13 ± 1.39 and 890.36 ± 48.42 separately). The best interaction value (the lower length of Comet parameters means less DNA damage) with a significant difference was in the control treatment of the agar experiment (it was 29.00 ± 3.64). While, the percentage of DNA in the tail of Comet parameters of Anber did not show any significant difference between treatments, experiments and interaction (Tables 4 and 5).
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar Experiment | Cotton Experiment | ||
| Control | 29.00 ± 3.64 | 34.44 ± 1.43 | 31.96 ± 1.92 |
| Microgravity | 39.40 ± 1.46 | 41.83 ± 1.54 | 40.72 ± 1.08 |
| Mean | 34.20 ± 2.53 | 38.13 ± 1.39 | - |
Table 2: Effect of treatment and experimental in Comet length (px) of rice Anber.
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar Experiment | Cotton Experiment | ||
| Control | 432.60 ± 9.66 | 765.38 ± 45.96 | 614.12 ± 57.78 |
| Microgravity | 856.00 ± 32.34 | 1015.33 ± 44.24 | 942.91 ± 36.79 |
| Mean | 644.30 ± 72.34 | 890.36 ± 48.42 | - |
Table 3: Effect of treatment and experiment in Comet area (px) of rice Anber.
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar Experiment | Cotton Experiment | ||
| Control | 33.20 ± 1.80 | 40.83 ± 2.62 | 37.36 ± 1.97 |
| Microgravity | 39.60 ± 2.37 | 40.50 ± 1.66 | 40.09 ± 1.34 |
| Mean | 36.40 ± 1.76 | 40.67 ± 1.48 | - |
Table 4: Effect of treatment and experiment in Comet length (px) of rice Jasmine.
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar Experiment | Cotton Experiment | ||
| Control | 0.00121 ± 0.00 | 0.01957 ± 0.018 | 0.01122 ± 0.010 |
| Microgravity | 0.00086 ± 0.00 | 0.00905 ± 0.008 | 0.00533 ± 0.004 |
| Mean | 0.00103 ± 0.00 | 0.01431 ± 0.009 | - |
Table 5: Effect of treatment and experiment in % DNA in the tail of rice Anber.
The results of comet length of Jasmine (Table 6) show that there was no significant difference between treatments and between experiments, but there was a significant difference in the value of interaction where the best result was (33.20 ± 1.80) in the control treatment of agar experiment. Whilst the results of Comet area (px) for Jasmine rice indicated that there were significant differences between treatments and between experiments where the best results were in control treatment and agar experiment (Mean ± SE) (782.36 ± 49.96 and 793.40 ± 57.58 respectively). Additionally, the best value of interaction with a significant difference was in the control treatment of the agar experiment (645.80 ± 8.03) (Table 5). A significant difference between treatments, experiment and interaction in the percentage of DNA in the tail of Jasmine rice was also observed (Table 7).
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar experiment | Cotton Experiment | ||
| Control | 645.80 ± 8.03 | 896.17 ± 58.39 | 782.36 ± 49.96 |
| Microgravity | 941.00 ± 62.95 | 991.67 ± 33.29 | 968.63 ± 32.96 |
| Mean | 793.40 ± 57.58 | 943.91 ± 35.13 | - |
Table 6: Effect of treatment and experiment in Comet area (px) of rice Jasmine.
| Treatment | Experiment | Mean ± SE | |
|---|---|---|---|
| Agar Experiment | Cotton Experiment | ||
| Control | 0.00097 ± 0.00 | 0.00903 ± 0.008 | 0.00536 ± 0.004 |
| Microgravity | 0.00082 ± 0.00 | 0.00645 ± 0.005 | 0.00389 ± 0.003 |
| Mean | 0.00089 ± 0.00 | 0.00774 ± 0.004 | - |
Table 7: Effect of treatment and experiment in % DNA in tail of rice Jasmine.
Cytological changes were tested for seeds germinated under clinostat construction (40 rpm) and plants grown for 70 h at 90º rotational axis angle and clockwise rotation direction. Seeds were germinated as mentioned above and once the roots reached a length of about 2 cm to 3 cm, root tips were removed and fixed according to the method described by McCurdy and Gunning [14]. Immunofluorescence labelling was carried out with an anti-α-tubulin (Sigma T-9026) (1:500) and an anti-mouse-FITC (Sigma F-02571) (1:16). All incubations were performed at 37°C for 40 min, followed by three washes each for 10 min with Phosphate Buffer Saline (PBS). Controls using only the second antibody showed no labelling. Confocal laser scanning microscopy was carried out using a Bio-Rad MRC-600 confocal microscope with BHS filters. Images were contrast enhanced before recorded on Kodak TMAX-100 films [14].
Few researchers have been studied the effects of gravity on DNA plant and Amino acids profile. Aubry-Hivet et al. [24] showed that changes in gene and protein expression in Arabidopsis thaliana cells were significant and fundamental changes in metabolic pathways were also revealed by these researchers. Also, a comprehensive analysis of gene expression of floral buds revealed that hyper gravity substantially changes expression of genes involved in the biosynthesis of phytohormones such as abscisic acid and auxin [25]. Nasir et al. [26] demonstrated the changes in expression of stress related genes under microgravity in Euglena gracilis.
Conclusion
The effects of microgravity on plant characteristics, including Iraqi cultivated rice were studied. The Clinostat has been used in the biologist's study on how organisms might adapt to the microgravity environment and what affects the force of gravity has on plant development, production and cytology behaviour [27]. It can therefore be concluded that simulated microgravity can alter the structure of the spindle microtubules, and stimulate the formation of multipolar spindles together with multicentrosomes, which causes the over expression of proteins to block the abnormal cells in metaphase, thereby inhibiting cell proliferation. The results of the Comet assay parameters show that the control treatments were in general better than those grown microgravity also the agar as a culturing medium was superior to cotton. It was hypothesized that microgravity can alter the growth, DNA and amino acid profile during germination. Therefore, present study was aimed to appraise the effect of microgravity (generated by clinostat) on rice, under normal, cotton experiment and agar supplementation in the definition of cultivars to be used in spaceflight.
References
- Mouhamad RS, Munawar Iqbal (2016) Effect of artificial gravistimulation on amino acid profile of Pea, Rice, Corn, Wheat during early growth stages. Information Processing in Agriculture 18: 45-51.
- Mosesso P, Schuber M, Seibt D, Schmitz C, Fiore M, et al. (2001) X-ray-induced chromosome aberrations in human lymphocytes in vitro are potentiated under simulated microgravity conditions (Clinostat).Phys Med 17: 264-266.
- Horneck G (1999) Impact of microgravity on radiobiological processes and efficiency of DNA repair. Mutat res 430: 221-228.
- Wang YC, Zhang S, Du TY, Wang B, Sun XQ (2009) Clinorotation upregulates inducible nitric oxide synthase by inhibiting AP-1 activation in human umbilical vein endothelial cells. J Cell Biochem 107: 357-363.
- Soh H, Auh C, Soh WY, Han K, Kim D, et al. (2011) Gene expression changes in Arabidopsis seedlings during short to long-term exposure to 3-D clinorotation. Planta 234: 255-270.
- Olive PL, Banáth JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1: 23-29.
- Choucroun P, Gillet D, Dorange G, Sawicki B, Dewitte JD (2001) Comet assay and early apoptosis. Mut Res 478: 89-96.
- Ventura L1, Giovannini A, Savio M, Donà M, Macovei A, Buttafava A, et al. (2013) Single cell gel electrophoresis (Comet) assay with plants: Research on DNA repair and ecogenotoxicity testing. Chemosphere 92: 1-9.
- Kordyum EL (2014) Plant cell gravisensitivity and adaptation to microgravity. Plant Biol 16: 79-90.
- Iida H, Furuichi T, Nakano M, Toyota M, Sokabe M, et al. (2014) New candidates for mechano- sensititve channels potentially involved in gravity sensing in Arabidopsis thaliana. Plant Biol 16: 39-42.
- Grolig F, Moch J, Schneider A, Galland P (2014) Actin cytoskeleton and organelle movement in the sporangiophore of the zygomycete Phycomyces blakesleeanus. Plant Biol 16: 167-178.
- Geisler M, Wang B, Zhu J (2014) Auxin transport during root gravitropism: transporters and techniques. Plant Biol 16: 50-57.
- Ueda J, Miyamoto K, Uheda E, Oka M, Yano S, et al. (2014) Close relationships between polar auxin transport and graviresponse in plants. Plant Biol 16: 43-49.
- McCurdy DW, Gunning BES (1990) Reorganization of cortical actin microfilaments and microtubules at preprophase and mitosis in wheat root- tip cells: a double label immunofluorescence study. Cell Motil Cytoskeleton 15: 76-87.
- Dhawan A, Bajpayee M, Pandey A, Parmar D (2003) Protocol for the Single Cell Gel Electrophoreses/ Comet Assay for Rapid Genotoxicity Assessment. Developmental Toxicology Division Industrial Toxicology Research Centre, India.
- Gichner T (2003) Differential genotoxicity of ethyl methanesulphonate, Nethyl-N-nitrosourea and maleic hydrazide in tobacco seedlings based on data of the Comet assay and two recombination assays. Mutat Res 538: 171-179.
- Gichner T, Patkov´a Z, Sz´akov´a J, Demnerov´a K (2004) Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombination in tobacco leaves. Mutat Res 559: 49-57.
- Cavanagh PR Licata AA, Rice AJ (2007) Exercise and pharmacological countermeasures for bone loss during long duration space flight Gravitat Space Res, 18.
- SAS (2012) Statistical Analysis System, User's Guide. Statistical. Version 9.1th ed. SAS. Inst Inc Cary N C. USA.
- Goyden J, Tawara K, Hedeen D, Willey JS, Oxford JT et al. (2015) The Effect of OSM on MC3T3-E1 Osteoblastic Cells in Simulated Microgravity with Radiation. PLoS ONE 10: e0127230.
- Wu C, Guo X, Wang F, Li X, Tian X, et al. (2011) Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing. PlosONE 7: 1-8.
- Yoshioka R, Soga K, Wakabayashi K, Takeba G, Hoson T (2003) Hypergravity-induced changes in gene expression in Arabidopsis hypocotyls. Adv Space Res 31: 2187-2193.
- Shen-Miller J, Gordon SA (1967) Gravitational compensation and the phototropic response of oat coleoptiles. Plant Physiol 42: 352-360.
- Aubry-Hivet D, Nziengui H, Rapp K, Oliveira O, Paponov IA, et al. (2014) Analysis of gene expression during parabolic flights reveals distinct early gravity responses in Arabidopsis roots. Plant Biol 16: 129-141.
- Tamaoki D, Karahara I, Nishiuchi T, Wakasugi T, Yamada K, et al. (2014) Effects of hypergravity stimulus on global gene expression during reproductive growth in Arabidopsis. Plant Biol 16: 179-186.
- Nasir A, Strauch SM, Becker I, Sperling A, Schuster M, et al. (2014) The influence of microgravity on Euglena gracilis as studied on Shenzhou 8. Plant Biol 16: 113-119.
- Zhang XY, Wang GZ, Ding B, Li YH, Tan YJ (2000) The effects of simulated weightlessness on cell cycle of osteoblast-like cells. Chin J Aerospace Med 11: 432-451.
Citation: Mouhamad RS, Shallal HS, Al-Daoude A (2019) Microgravity Effects on the Growth, Cell Cytology Properties and DNA Alterations of Two Iraqi Local Plants. J Rice Res 7: 207.
Copyright: © 2019 Mouhamad RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4878
- [From(publication date): 0-2019 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 3890
- PDF downloads: 988