Research Article Open Access
Predictive Eel Population Model Based on Fish Habitat Dependency and Fishing Pressure on Pisodonophis boro (Hamilton) in the Godavari Delta, India
Babu DE*, Ratna MR, Lakshmikantamma K and Tarun KDepartment of Zoology, Andhra University, Visakhapatnam, Andhra Pradesh, India
- *Corresponding Author:
- Babu DE
Department of Zoology, Andhra University
Visakhapatnam-530 003, Andhra Pradesh, India
Tel: +91-9849100756
E-mail: babucrabd@yahoo.com
Received Date: November 18, 2016; Accepted Date: December 30, 2016; Published Date: January 27, 2017
Citation: Babu DE, Ratna MR, Lakshmikantamma K, Tarun K (2017) Predictive Eel Population Model Based on Fish Habitat Dependency and Fishing Pressure on Pisodonophis boro (Hamilton) in the Godavari Delta, India. J Fisheries Livest Prod 5: 224 doi: 10.4172/2332-2608.1000224
Copyright: © 2017 Babu DE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The habitat dependency of the rice-paddy eels, Pisodonophis boro is described in this paper with emphasis on natural production. The eels use mangrove mudflats as homing ground for feeding and breeding; perform anadromous migration during breeding season; total mangrove dependency of the eels has been confirmed from field observations on eel fishery and mangrove degradation during the period 1981-2012. Total loss of mangrove habitat resulted in migration or mortality of the species and recruitment of the fish populations preceded rejuvenation and ramification of mangrove flora and associated fauna. Pisodonophis boro needs perennial freshwater inflow for successful reproduction; thus, the annual breeding cycle is triggered and regulated by the freshwater release from the river dam, and the artisanal fishery is correlated to these events. Sexes are separate and females with bilobed ovaries grow larger. The fish is being caught by trap nets and hooks as these have a sediment burrowing behavior and carnivorous food habits. A novel method of estimating eel population by applying Mini Tab. 16 software and the unique SARIMA, 1.1.1 model is highlighted. Critical factors influencing/triggering migration of eels from the Godavari estuary to the freshwater canals and paddy fields were seasonality, temperature and salinity. Smoked eels are delicacies of the entire central delta with a total population of 12,00,438; the proximate composition of the fish was analysed and health problems if any in smoked fish consumers was assessed by documenting health symptoms.
Keywords
Rice-Paddy eel; Biology; Forecasting model; Population ecology; Nutritive value
Introduction
Species diversity demands a suitable habitat that supports nursing and nourishing ground as evidenced in the rice-paddy eel, Pisodonophis boro Hamilton Buchanan 1822, the species exclusively habituates the shallow mangrove mudflats of Godavari delta adjoining paddy fields. There were only meager reports on the distribution of the burrowing ricepaddy eels or biology, when Jayaram [1] reported them from Sunderban mangroves in the vicinity of Bay of Bengal, east coast of India and Frantisek et al., [2] recorded them from the brackish water localities of Phan-Nga province of South-western and Phuket Island, Thailand.
In the present studies, emphasis was laid on the effect of environmental factors, especially season, temperature and salinity on the migratory and reproductive behaviour of the fish; this species was found as seasonal breeder, breeds during the southwest monsoon, commencing in the month of June and north-east monsoon commencing in October. One of the highlights of the study is developing an ecological model fishery with implicating environmental factors especially the influence of freshwater flow from the 3.599 km long Sir Arthur Cotton anicut (dam, constructed by the British Engineer, Sir Arthur Cotton during the years 1847-‘51on the river Godavari 16º56’0.8.38”N, 81 º45’08.77”E), on the diversity of the fish. This migratory eel described in this paper carries an important scientific message on the habitat preference of animal populations, demands shallow and submerged mangrove mud flats having perennial freshwater and seawater influx that regulate their reproductive behaviour. Another important scientific message is on specific habitat dependency of animals; mangrove habitat loss for aquaculture purpose in the western delta (16 º19’ 10.17” N 81 º 43’ 35.58” E to 16 º 22’ 04.77” N 81 º 44’ 58.26” E towards the East side of the river to 16 º 19’ 59.16” N 81 º 42’ 13.31” E to 16 º 22’ 22.25” N 81 º 40’ 31.14 E towards the West side of river) during the years 1981-1984 resulted in total loss of the rice-paddy eel populations from the area.
Since smoked fish are a preferential diet of a large population, biochemical analysis of the fish including fatty acid composition was evaluated to reveal the nutritive value of fresh and smoked fish, data were collected on fish consumers to establish any harmful effects of consuming the processed smoked fish.
Materials and Methods
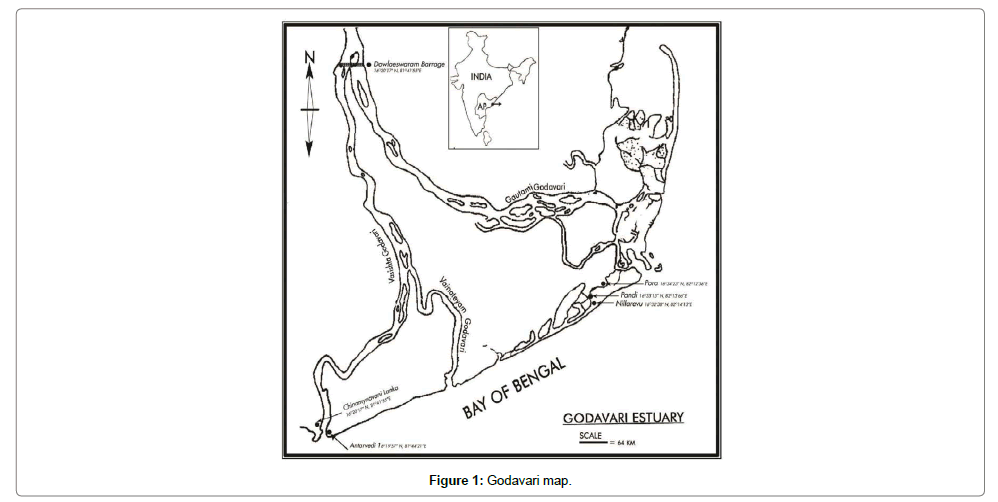
Rice-paddy eel landings were recorded from the estuarine and Backwaters of Bay of Bengal during the years 1980-2012 and results of frequency of distribution of the eels was given for the years 1999-2002 and compared to 2009; the frequency of distribution was more or less similar during all the years of study; data on numerical abundance and biomass estimation of eels were shown for the years 2008-2011. Studies pertaining to population dynamics including recruitment, growth, mortality and production of eels from the mangrove mudflats were estimated for the period 2010-2012. The common fishing methods followed for eel fishery were by removing them from their burrowed hideouts by using a shovel, hook or by trapping the eels through bag nets, laid across the estuarine creeks during low tides (Figure 1); the usual practice followed was that all the eels landed were smoked in dry mangrove wood after sun-cure for five to eight hours. Biological data on length – weight relationship, fecundity, gonado (ovary)-somatic index and proximate composition of the muscles along with calorific value was estimated and a comparison was made between smoked and fresh eels. Eels were subjected to salinity tolerance experiments in the laboratory.
For studies on population recruitment, growth, mortality and production of mangroves, a ‘Hook and Trap’ method was developed basing on the sedentary burrowing behaviour of the adult eels and swimming and snaky behaviour of the juveniles, for this two types of traps were designed, the first one was baited hooks of different sizes and the second one was basket net trap made of double layered brown nylon mesh cloth of 500 μ containing minced fish and crab meat, laid serially in vertical rows, submerged in the waters at regular intervals of two to three meters. The hooks and the basket traps were used on three successive days at dusk and dawn time, each trial of two hours duration, a total of six trials were attempted in shallow mudflats during the years 2010-2012. To prevent immigration of eels, a polythene sheet with supporting bamboo poles was fixed surrounding 50 × 20 meter (length × width) mangrove mudflat area. Since, all the eels were removed during the fishing trials, the fishing ground was shifted to the adjacent site for the next trial.
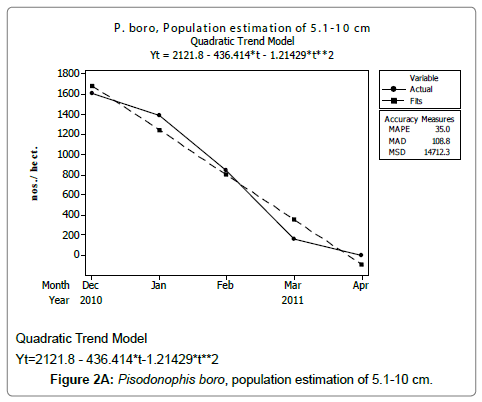
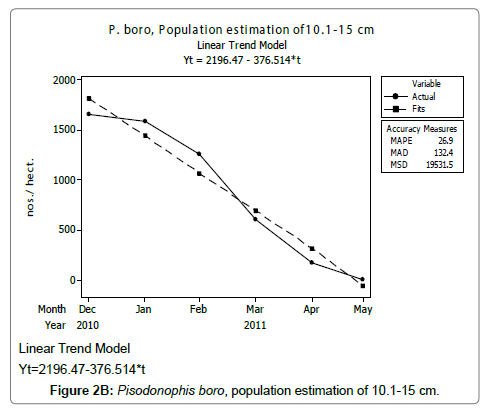
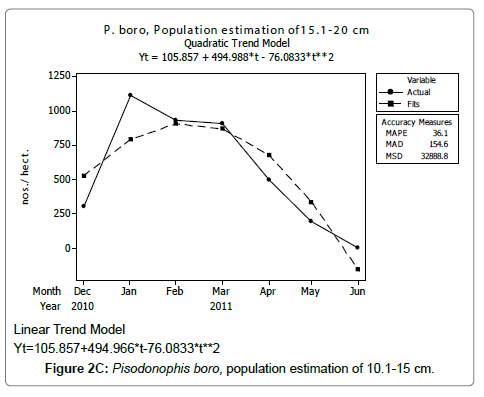
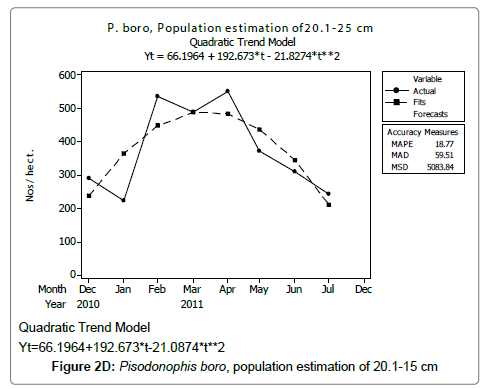
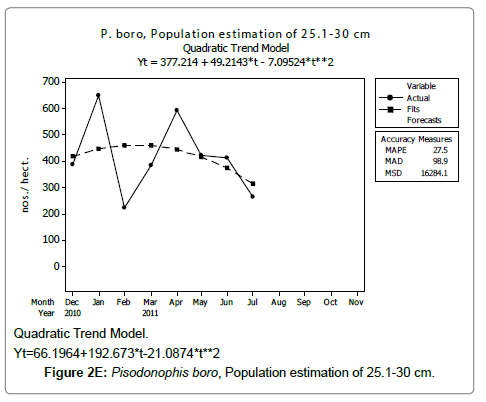
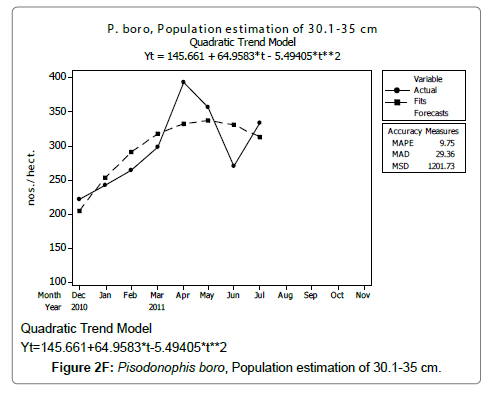
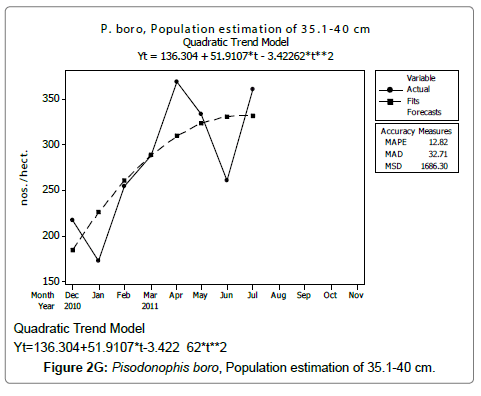
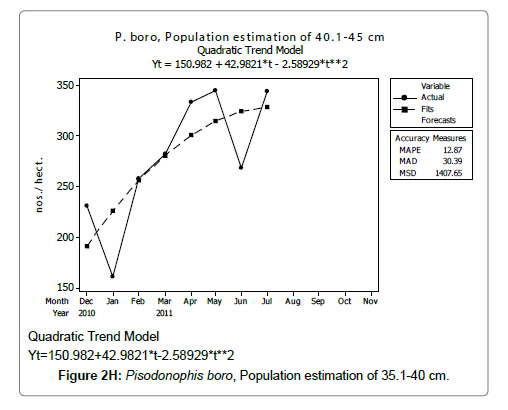
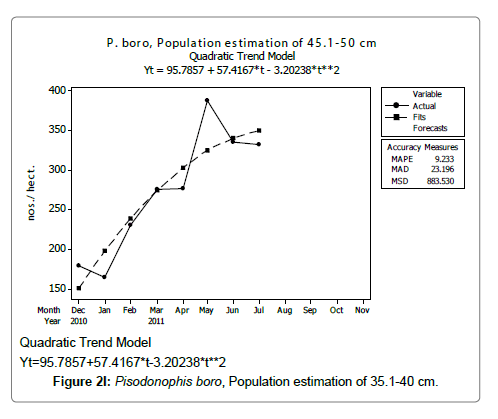
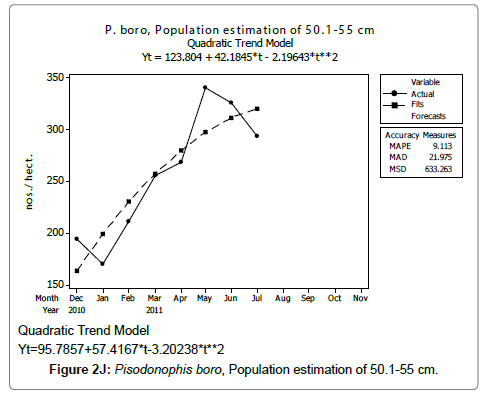
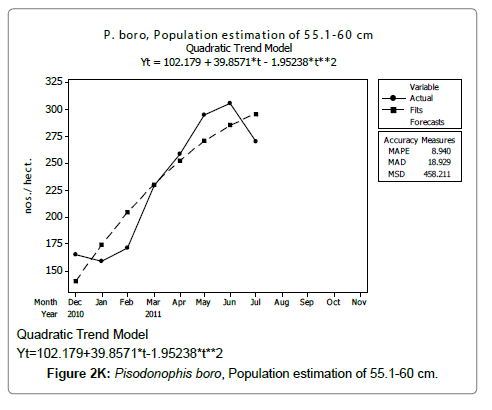
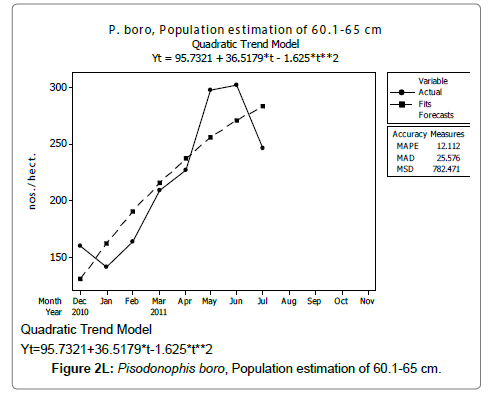
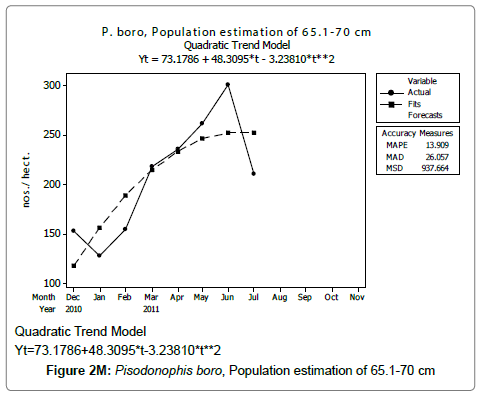
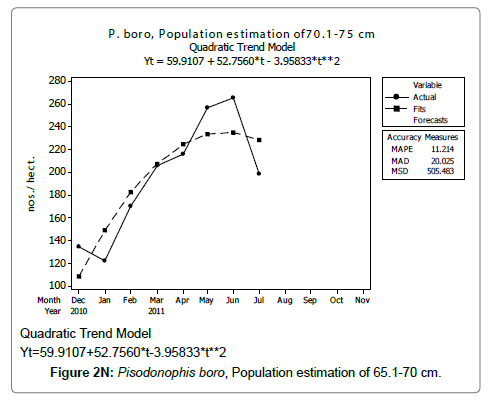
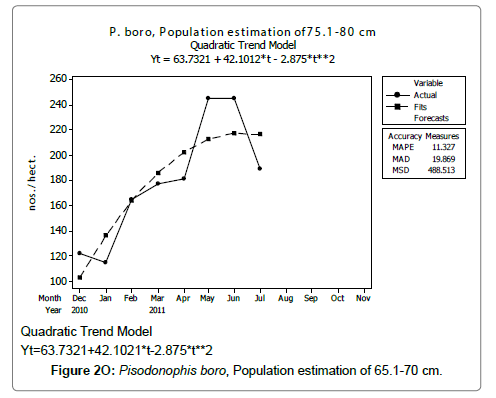
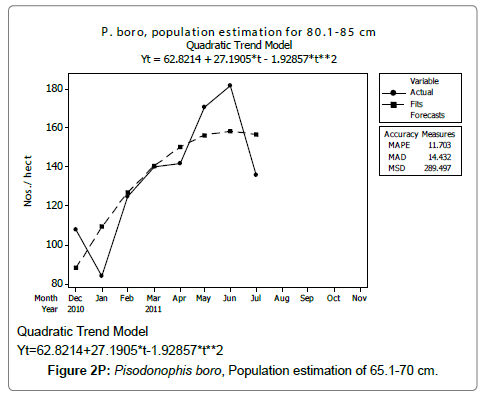
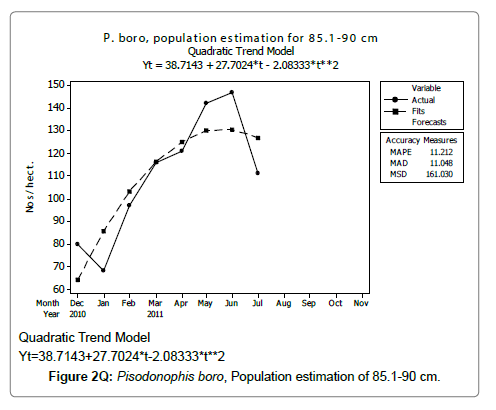
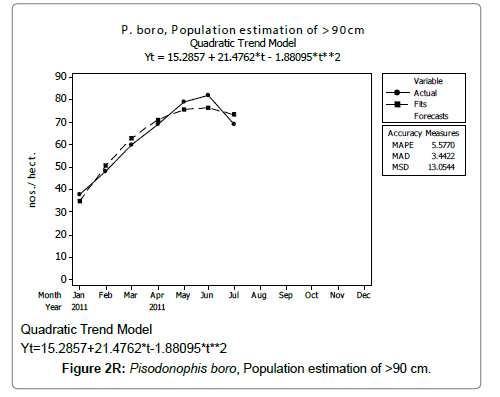
Linear and quadratic trend analysis was applied for population estimation by using ‘Mini Tab 16’ software, thus it was possible to estimate the total populations of eels. The best curves for each size were fitted applying either linear or quadratic models and using mean absolute deviation as a criterion with the projected values of different size populations (Figure 2A-2R). ‘SARIMA (1, 1, 1)’ model was fitted to estimate the eel population for the succeeding two years. The average fish size of each month with their standard deviation is also computed.
For experiments on biochemical composition, the smoked fish were cut into 2” pieces and after a thorough wash, boiled in a stainless steel container for physical observations on odour and for testing the palatability of the fish. Proximate composition of the muscle tissue was analyzed by taking samples from the anterior, middle and posterior regions and averages are presented. Dry weight determinations were made at 95-105ºC for 24-48 h and the dried tissue was taken for proximate analysis. Total lipid was estimated by extracting the fat in methanol-chloroform mixture as a solvent in a Soxhlet extraction apparatus [3,4] and total protein was estimated by colorimetric determinations of Lowry et al. [5] in a spectrophotometer at 560 nm and the Calorific value of fish was determined in a Bomb calorimeter. The acid value of the fat was determined by neutralizing the fatty acid with 0.1 M KOH, [6]. The degree of un-saturation of the fatty acids was determined as iodine value that the halogen iodine adds across the double bonds of the un-saturated fatty acids [6-8]. Muscle tissues of smoked eel were incubated in cornmeal, Littman oxgall agar, potato dextrose, rice grain medium, Sabouraud dextrose agar, Sabouraud broth and nutrient agar media for identification of any fungal moulds [9,10]. 1000 smoked fish consumers were used for sample studies so as to evaluate the probable harmful effects of processed and smoked fish due to histamine; symptoms of diarrhea, skin and respiratory allergies, nausea and headache were taken as the main criteria. Data on smoked mullet (Mugil cephalus) consumers were simultaneously collected and compared to smoked eel consumers.
Results
The Godavari estuary was identified potential habitat for mangroves that support rich flora and fauna, as per the earlier revenue records, it was observed that mangroves were distributed in about 404.64 km2 in eastern delta, 380.06 km2 in central delta and 975.6 km2 in western delta of river Godavari; currently there are 32,222 hectares of mangroves in the Eastern and central delta distributed in 322.22 km2 and <1000 hectares of isolated rejuvenating mangrove patches in the western delta. Pisodonophis boro, commonly referred to as ricepaddy eels were recorded from the mangrove mudflats adjacent to the backwater zones of central and western deltas of Godavari estuary, India (16Ë?33’12.36”N, 82Ë? 12’ 13.46” E and 16Ë?19’10.17”N, 81Ë? 43’ 35.58’E); the eels habituate the burrows made in the shallow mangrove mudflats, which were subjected to perennial freshwater and seawater influx. A precarious situation observed was that the fish were totally migrated from the western delta and parts of the central delta (Figure 1) after 1982 when thousands of hectares of mangrove habitats were cut and recently recruitment of few numbers of eels was observed in some of the rejuvenating mangrove mudflats in abandoned aquaculture areas from the year 2010 onwards; retrieving of mangroves started from the year 2003 onwards. On site observations made during very low tides prevailing in the months of January and February revealed mangrove faunal associates such as polychaetes, nematodes, crustaceans, mollusks and gobiid fish at densities (excluding nematodes) 1.72-8.35/m2 in the mangroves of central delta and 0.76-2.88/ m2 in the retrieving mangrove mud flats of western delta, east coast of India. It was interesting to notice mangrove crabs belonging to families Ocypodidae and Grapsidae as initial invaders of the retrieving mangroves. Subsequently, other associated fauna also took shelter in the area, the mangroves, took 6-8 years to reach a height of 3-31/2’ and eel re-habitation took place only after the resilience of flora and associated fauna.
Biology
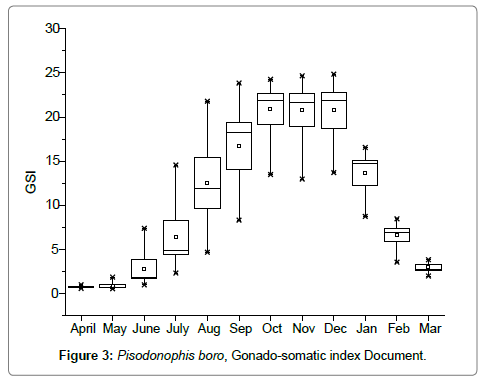
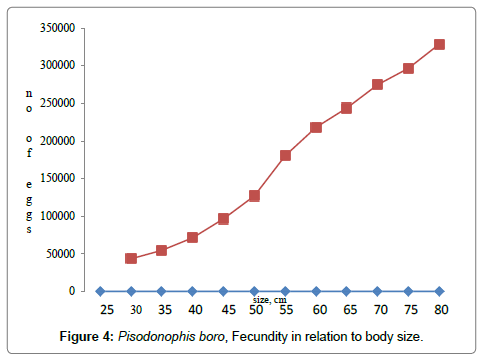
Salinity tolerance tests conducted in the laboratory have resulted to understand that the eels were euryhaline, survived well in pure freshwater to salinities up to 45 PSU without any mortality. Gut content analysis of the eels have revealed their carnivorous behaviour depending on sedentary gobiid fish, mangrove crabs, shrimps, hermit crabs, polychaetes, nematodes, molluscs etc. Sexes are separate, males matured at 24-26 cm body length while the females matured at 28-30 cm, the females have bilobed ovaries, originating below the liver lobe in the anterior region, the ovaries extended till the rounded gonopore; in fully mature stages the ovarian lobes were found extending 2-3 cm below the gonopore. The left lobe of the ovary originates 5-10 mm anterior to the right lobe. In males, the testes appeared bilobed white tubular structures, the vasdeferens extended and opened into the gonopore; before opening, the vasdeferens was found enlarged as a receptacle to store spermatophore. The onset of reproductive activity as evidenced by the growth of the ovary with a gradual increase in the ovariansomatic index was associated with freshwater flow into the mangrove mud flats in the month of June every year (Figure 3). Peak GSI values of were observed in the months August-November, gradually declined in subsequent months reaching minimum in the months March-May; the anadromous eel migrates from the estuarine mangrove mudflats to the nearby rice fields and freshwater canals. Most of the eel migrations were observed in the months of July through December in accordance with increased freshwater flow from the irrigation canals and also due to monsoon showers, eel migrations were totally stopped by March every year when the freshwater flow from the irrigation canals was stopped from 1st April of every year. In mature condition, the oocyte diameter ranged 750-1200 μm and the fecundity values ranged 43,372–3, 28,858, for the sizes 30-80 cm (Figure 4). Maximum size of the eel recorded in December 2001 measured 122 cm with a body weight of 520 g, such large sized eels of > 108 cm were not traced after the year 2002.
Population ecology
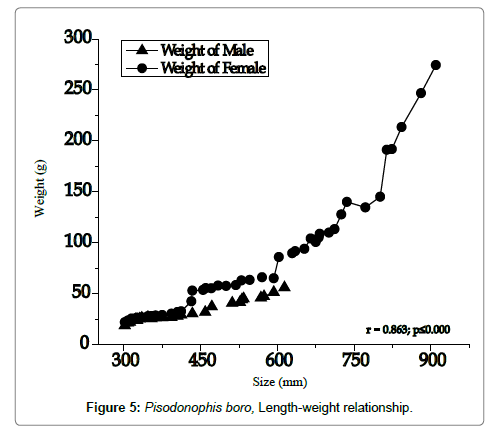
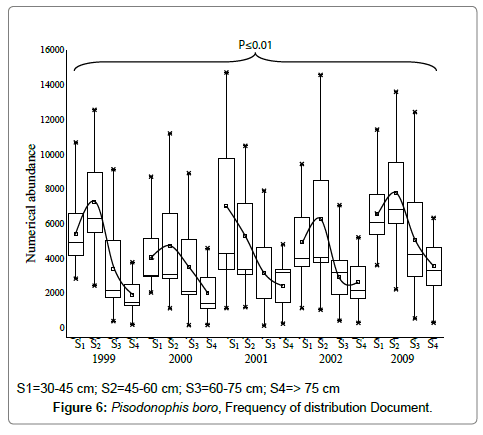
Rice-paddy eel landings of central delta were found dominant in the months of July–January that coincides with the south-west and north-east monsoon seasons, vis-à-vis the breeding season, the eels mainly were caught in the freshwater canals during their backward migration to the estuary. The length-weight relationship has clearly indicated that females grow to larger sizes and males grow only up to 62 cm; the weight increase observed was gradual up to a body size of ~60 cm. afterwards a steep increase was evident (Figure 5). Maximum landing of the eels was observed in size ranges 45-60 cm of both the sexes (Figure 6), in all the years of study, the size groups 45-60 cm dominated, but during the year 2001, 30-45 cm sizes outnumbered all other sizes. During the peak breeding season, most of the eels landed were in the size range 55-75 cm, indicating abundance of reproductive females, most of the males fell in the size range 30-45 cm. Usually, few large size females, >80 cm were observed in the creeks during the months October-December. During the breeding season, more than 70% of the eels landed were females with fully ripened or ripening ovaries in advanced stages of vitellogenesis. Such eels were observed to ascend the freshwater canals and were usually trapped in the bag nets laid across the estuary, mostly at night times. The few numbers of the eels landed during the months April and May represent those recovered from their burrowed hideouts.
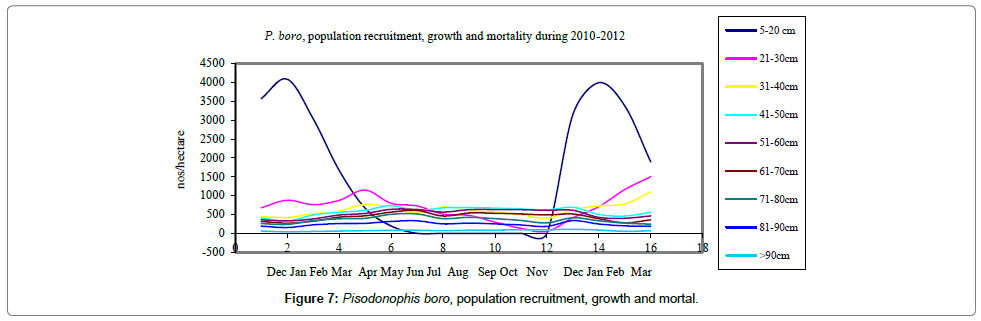
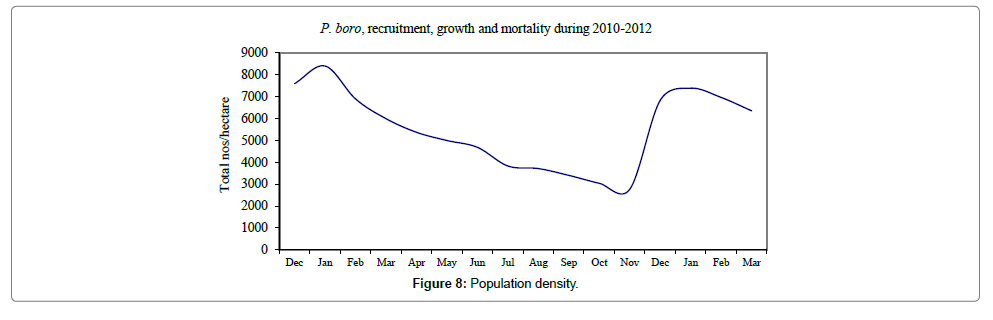
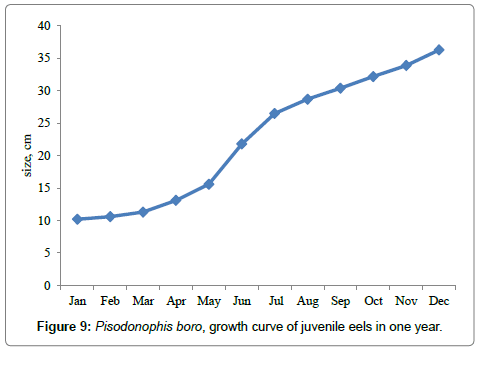
Size at recruitment was found to be 8-11 cm when the elvers were settled on the humous mangrove mudflats, indicated by their peak numbers in December and January 2010 and 2011 (Figures 7 and 8). It was observed that the entire juvenile population has reached the maturity stage of 26-28 cm by July (Figure 9) when they were sexually matured and commenced migration to freshwater canals in the month of July or August. Repeated observations made on the fish landings have revealed all spent female or male populations towards the middle and end of January that the migration of reproductive females stopped by then. Natural mortality of juvenile population was mainly due to predation by the adult eels, carnivorous fish and the crabs.
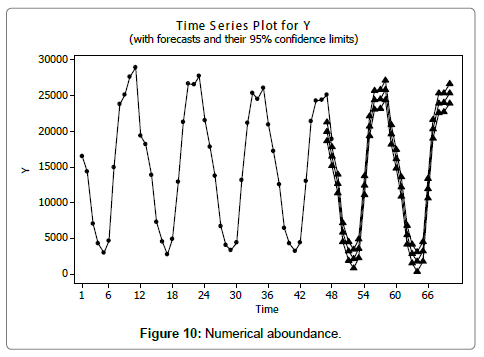
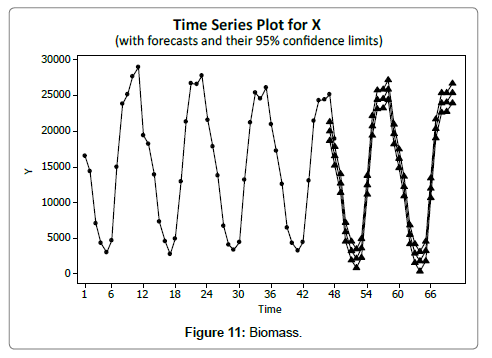
Eel Population estimation and mangrove production. The total population of rice-paddy eels were estimated for one hectare area by employing ‘Hook and Trap’ method, maximum numbers were recorded in the month of January coincided with active juvenile recruitment and minimum numbers recorded in the month of November; the maximum and minimum numbers followed the reproductive behavior, recruitment pattern and fishing mortality. The distribution of eel populations changed from left skewed to symmetric during December to May and became right skewed from June (Figure 2A–2R). The juvenile population was found high during December- April and thereafter declined mostly due to their conversion to next sizes and fishing mortality; active eel migrations started from July onwards resulted in mortality due to fishing. A gradual increase in the sub-adult and adult populations was evident from April onwards that reached minimum in November when recruitment commenced during the last quarter of the month (Figures 7 and 8). It was estimated that the average production of the eels was 0.84/m² during January and 0.27/ m² in the month of November. The ‘SARIMA’ forecast model shown in Figures 10 and 11 and Table 1 is based on numerical abundance and biomass of eels landed during the period January 2008 to December 20012, the model predicts future eel fishery for the next 24 months. The model exhibits a cyclical and seasonal pattern with peak landings during August to December and minimal landings in the months of April and May, this was true in all the four years of study; the model forecasted a similar pattern for the next 48 months. The other pertinent point observed was about sustainability of eel fisheries and the quantities landed; with minor fluctuations, fish landings were stable throughout. The landings were based on the limited fishing ground and the eel migratory behavior that active migrations commence in July and continued till January, initial landings in the months June-August were mostly due to small sized eels and larger sizes mostly were observed in the months September onwards, reflected on the biomass.
| Numerical abundance, Values of Y 95% limits | Biomass, Values of X, 95% limits | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Forecast | Lower | Upper | Actual | Forecast | Lower | Upper | Actual | |
| 48* | 19975.5 | 18639 | 21311.9 | 18958 | 1314.7 | 1227.64 | 1401.76 | 1258.8 | |
| 49 | 16532 | 15195.6 | 17868.5 | 690.3 | 603.24 | 777.36 | |||
| 50 | 12685.9 | 11349.5 | 14022.4 | 524.47 | 437.41 | 611.53 | |||
| 51 | 5863 | 4526.5 | 7199.4 | 205.67 | 118.61 | 292.73 | |||
| 52 | 3228.5 | 1892.1 | 4565 | 66.21 | -20.86 | 153.27 | |||
| 53 | 2099.8 | 763.3 | 3436.2 | 41.49 | -45.57 | 128.56 | |||
| 54 | 3554.4 | 2217.9 | 4890.9 | 143.51 | 56.45 | 230.57 | |||
| 55 | 12395.6 | 11059.1 | 13732 | 576.83 | 489.77 | 663.9 | |||
| 56 | 20743.1 | 19406.7 | 22079.6 | 995.56 | 908.5 | 1082.62 | |||
| 57 | 24405.3 | 23068.9 | 25741.8 | 1383.93 | 1296.87 | 1470.99 | |||
| 58 | 24509.9 | 23173.5 | 25846.4 | 1507.79 | 1420.73 | 1594.85 | |||
| 59 | 25798 | 24461.5 | 27134.4 | 1710.34 | 1623.28 | 1797.4 | |||
| 60 | 19587.5 | 18233.4 | 20941.7 | 1290.18 | 1199.87 | 1380.49 | |||
| 61 | 16129.8 | 14775.6 | 17483.9 | 666.5 | 576.18 | 756.81 | |||
| 62 | 12249.8 | 10895.6 | 13603.9 | 500.84 | 410.53 | 591.16 | |||
| 63 | 5459.8 | 4105.7 | 6814 | 181.58 | 91.27 | 271.89 | |||
| 64 | 2846 | 1491.8 | 4200.2 | 42.03 | -48.28 | 132.35 | |||
| 65 | 1720.2 | 366 | 3074.4 | 17.39 | -72.92 | 107.7 | |||
| 66 | 3161.3 | 1807.2 | 4515.5 | 119.3 | 28.99 | 209.62 | |||
| 67 | 11991.3 | 10637.1 | 13345.5 | 552.75 | 462.44 | 643.07 | |||
| 68 | 20344.5 | 18990.3 | 21698.7 | 971.49 | 881.18 | 1061.8 | |||
| 69 | 23968 | 22613.8 | 25322.2 | 1359.37 | 1269.06 | 1449.68 | |||
| 70 | 24072.8 | 22718.6 | 25426.9 | 1484.5 | 1394.19 | 1574.81 | |||
| 71 | 25337.9 | 23983.7 | 26692.1 | 1687.53 | 1597.22 | 1777.84 | |||
*Forecasting commenced from 48th month.
Table 1: Pisodonophis boro, Numerical abundance and Biomass, forecast values of X & Y for the years 2012-2014.
Nutritive value
Biochemical estimations of fresh and smoked fish have revealed marked changes in the protein and lipid profile, a decline in the total protein and fat content was evident in smoked fish; 5.95% of protein decline and 14.4% lipid degradation was observed after smoking. It was also observed that the protein and lipid decrease was associated with an increase in the water soluble fraction of protein and fatty acid content (Table 2). An increase in the degree of un-saturation of fatty acids was noticed in the smoked fish muscle samples. When the fish muscle was kept in water, a layer of fat was observed floating on the surface, thorough homogenization of the dry fish meat made the water more turbid. No fungal moulds or hyphae were noticed on the skin or muscles of the dry fish even after storage for 60 days in the laboratory kept at temperatures 26-35ºC. Observations made by sampling on smoked eel consumers of different age groups have indicated no ill health reactions of histamine or other microbes. It was an interesting observation made that symptoms of nausea, vomiting and chronic diarrhea were observed in 28-31% of persons after consumption of smoked mullets, the symptoms were prevalent in people consuming the smoked mullets after 4 or 5 days after smoking; prevalent in children <12 years of age.
| Component | Fresh eel | Smoked eel |
|---|---|---|
| Water (%) | 67.9 ± 2.3 | 19.23 ± 1.48 |
| Total protein (% dry weight) | 66.32 ± 2.28 | 62.37 ± 2.51 |
| Water soluble protein (%) | 5.63 ± 0.95 | 9.4 ± 1.08 |
| Total lipid (% dry weight) | 17.42 ± 2.32 | 14.91 ± 1.27 |
| Fatty acid (mg/100mg) | 35.02 ± 2.29 | 40.81 ± 3.41 |
| Iodine value (mg/100 mg) | 53.1 ± 2.12 | 61.31 ± 4.91 |
| Ash (%) | 8.04 ± 0.96 | 8.31 ± 0.76 |
| Calorific value (K. cal) | 142.3 ± 12.7 | 128.6 ± 9.1 |
Table 2: Pisodonophis boro, proximate composition of fresh and smoked fish.
Discussion
The rice-paddy eels form minor but consistent fishery of the central delta, that are caught in the vicinity of mangroves, which are subjected to both freshwater and seawater influx; multiple branches of the freshwater irrigation canals flow over the shallow mangrove mudflats surrounding Pandi and Pallam villages. Peak fishery of the eels coincided with the monsoon and post-monsoon seasons, when there was sufficient inflow of freshwaters into this zone. Absence of the eels from other parts of the mangroves reflects on the topography and its support to eel fishery. The rice-paddy eels are limited to the shallow estuarine area having clayey and sandy pervious bottom with mangrove tree cover that the substratum supports the eels in making deep burrows having two openings, the eels hide themselves in the burrows made amongst the projecting respiratory roots of the mangrove flora. The eels interestingly were not recorded from any other locations of the 32,222 hectares of Godavari mangroves. De Shepper et al. [11] have emphasized the detailed burrowing behavior of the rice–paddy eel. Sumptuous quantity of juveniles of the estuarine crabs, shrimps, hermit crabs, bivalve and gastropod molluscans, polychaetes, juvenile gobiids, etc., provide an ideal feeding ground to the adults and breeding ground in the vicinity. Triggering of reproductive activity of the eels followed the periodic inflow of freshwater to the estuary from the irrigation canals in the month of June every year when the salinity of the estuary falls. Steady flow of water further promotes breeding and the eel migrations were associated with an increase in turbidity due to active south-west monsoon. During the months April and May, the mangrove mudflats receive only tidal water with salinity 35 PSU and >35 PSU, which drops to <25 PSU when the freshwater overflows the entire area; this sudden drop in salinity was primarily responsible for initiation of gonadal growth; the cycle persists every year. Even in case of a delay in setting up of south-west monsoon or in conditions of weak monsoon with scanty rainfall, eel fishery sustains because of a steady inflow of freshwater from the agricultural fields, except for the fact that the landings were low due to decreased migrations; good landings were recorded during the years with good rain fall salinity and temperature appears to be the limiting factors; the eels migrate and breed during the south-west and north-east monsoon, (June-November) when the surface water temperatures of the estuary falls <25ºC and the turbidity of the water increases due to suspended silt and clay particles due to incessant rains. These fish appeared to be permanent residents of the sub-merged mangrove mudflats, exhibiting hiding behaviour in the burrows made amongst the mangrove shrubs and trees.
The pertinent point of discussion is about the status of eel fishery earlier to 1851 before the construction of the anicut (dam) across the river Godavari and when there was no regularized water flow, thanks to Sir Arthur Cotton, the British Engineer and architect of Godavari anicut that the controlled water release to agriculture (paddy) helped in the development of eel fishery. The requirement of specific habitat on species diversity is well established in this context that total extinction of the eel population from the estuarine areas of western delta directly reflected on mangrove degradation during 1980s primarily was due to habitat loss in turn reflecting on the loss of feeding ground. Continued efforts to understand the habitat dependency of eels were successful when re-habitation of few numbers of eels started from 2010 onwards that was associated with re-juvenation of mangrove, restoration of freshwater flow and invasion of associated fauna, mangrove leaf fall and its decay makes the soil slushy, a habitat requirement. It is here the mangrove crabs of the genus Metapograpsus, Sesarma and Neoepisesarma contribute in making the soil pervious because of their feeding behaviour of climbing and plucking the young shoots and keeping the vegetation in their burrows, the genus Uca, Ocypoda, Metapograpsus and Metaplex were found involved in making burrows and leaf degradation. Absence of flora and fauna makes the mudflat relatively impervious; moreover the crabs served as a direct food to the eels. The estuarine condition supported by nearby freshwater inflow from the rice fields is a primary requirement for rice-paddy eels to complete their life cycle, the more the density of mangroves the greater the eel populations. Because of their specific habitat preference, the rice-paddy eels are very much limited in their distribution to a tropical estuarine climate; diversity of these species demands an estuarine environment for hiding and feeding, flooding freshwaters for breeding, fishery development demands a relatively larger mangrove ecosystem with a surrounding habitat as described above.
Such an aquatic ecological niche of the shallow mangrove mudflats subjected to perennial freshwater (rice fields) and seawater inflow (backwaters) spreading over few km horizontally provide suitable hiding, feeding and breeding grounds in close vicinity is considered the limiting factor in the distribution of the rice-paddy eel, the other mangrove areas do not have such a topography, with typical backwater zone and nearby perennial freshwater inflow; in other mangrove zones, the freshwater and tidal water usually flows in deeper canals and creeks but do not spread on to the mangrove mud flats, even if the mudflats are inundated, it is only during the high tide and flood times.
The ‘Hook and trap’ method supported by ‘Time series” linear and quadratic trend analysis for population estimation is based on the principle that the eel population is closed, composite, representing different ages, their uniform distribution in the area, carnivorous feeding habit with burrowing and hiding behavior and fishing at regular intervals results in a declining trend in population. The larvae are pelagic and only the elvers are recruited into the mangroves, estimation of eel production of mangroves has clearly indicated that the % survival of juveniles is very low, which are subjected to predation by fellow eels, other fish, crabs etc., and likely chance of these being drifted away during cyclonic storms and flood waters, which are common in the months August-November. Symmetry in population growth was observed in the months of May and June when mortality due to fishing was minimal. Population growth from the month of August is limited due to active fishing when the eels are harvested during their reproductive migration to the freshwater canals and paddy fields. The total eel population and biomass is related to the extent of suitable mangrove habitat; the production was high during 1980s and early 1990s when there was no mangrove degradation in the central delta but decreased after 1996 when part of the potential mangrove cover was removed and converted to aquaculture ponds.
The predictive model of eel population is based on the trend in eel landings for the preceding four years and the succeeding two years, the habitat dependant model predicts biomass and numerical abundance, the predictive values are more or less equal to the actual values; this is true that the eel fishery is seasonal and quantities landed depend up on extent of mangroves and annual rainfall. This particular model is suitable for such of those fish that live in isolated areas with specific habitat requirements. This model can be applied for estimating fish populations of small estuaries and mangroves or for compound populations of lakes and reservoirs with specific food and reproductive habits of a particular species. Linear trend analysis can be applied to diminishing populations in time series of catches in different size groups.
Talwar and Kacker [12] reported occurrence of these eels from the Hoogly estuary from West Bengal, India, but no significance was attributed to their fishery possibly due to their scanty availability, similarly Frantisek et al. [2] reported the occurrence of eels from the estuarine regions of Phuket island with provision for freshwater inflow. Earlier reports FAO [13] and Day [14] indicated the distribution of these eels from East Africa, Southern India, Sri Lanka, East Indian coast throughout Indonesia to Polynesia./p>
The increasing demand for eels indicates their nutritional value and palatability, dried and smoked eels are preferential fish of many and when a comparison was made on the proximate composition of the fresh and smoked fish, major differences were noticed in the protein and lipid composition, that lipid and protein degradation was evident after smoking, this mainly reflects on the processing for smoking, sun curing of the fish for few hours before smoking appears the major causative factor for protein degradation due to autolysis. Fat loss observed mainly reflects on the oxidation of fats during heat smoking. The water content is always maintained <20% that the possibility of fungal contamination is reduced. High fatty acid content of the fish is a good indication of their nutritional value. The palatability of the eel depends on the unique aroma of these as also observed in the migratory fish Tenulosa ilisha, (unpublished) both have great demand throughout.
Acknowledgements
Part of this work on eels was carried under INCO-DC Project of the EU and the UGC, New Delhi and the first author express their gratitude for financial assistance. Thanks are also due to Prof. J. L. Narasimham, and, Professor K. Srinivasa Rao, dept. of Statistics and Dr. C. Annapurna, and Dr. M. Srinivasa Rao, dept of Zoology, Andhra University for their help in statistical analysis of the data. Special thanks are due to Mr. G. L. Narayana Rao, Asst. Executive Engineer (retd), Godavari barrage for his help in giving details of Godavari anicut, and Mr. P. Satyam and his associates from Pallam village for his valuable help in population estimation studies. The authors greatly acknowledge the help rendered by Prof. T. J. Pandian, Emeritus professor, Madurai Kamaraj University, India and Dr. Max Troell, Beijer Institute, Stockhom, Sweden for their advises and for editing the manuscript.
References
- Jayaram KC (1999) The freshwater fishes of Indian region. Narendra Publishing House, Delhi.
- Frantisek M, Horst T, Thairungroj AM, Maipanich W, Laoprasert T (2007) Heliconema longissimum (Ortlepp, 1923) (Nematoda: Physalopteridae) from Pisodonophis boro (Teleostei: Ophichthidae) in Thailand, with remarks on the taxonomy of the Proleptinae Schulz, 1927. Systematic Parasitol66: 73-80.
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification.Can J Biochem Physiol 33: 911-917.
- Buchanan FH (1822) An account of the fishes found in the river Ganges and its branches.Edinburgh & London.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Follin Phenol reagent. J Biol Chem 193: 365-375.
- Plummer DT (1988) An introduction to practical biochemistry, 3rd ed. Tata Mc Graw Hill Publishing Company ltd, New Delhi.
- Love RM (1980) The chemical biology of fishes, Advances 1968 -1977. Academic Press, London 2: 943. 7.
- Anon(1988) Fish smoking and drying, in the effect of smoking and drying on the nutritional properties of fish. Burt JR(ed) Elsevier Applied Science, London and New York 166.8.
- Pelczar MJ, Chan ECS, Kreig NR (1995) Microbiology, fifth ed., Tata Mc Graw- Hill Publishing Company limited, New Delhi.
- Godkar PB, Godkar DP (2005) Text book of medical laboratory technology, 2nd ed., Bhalani Publishing house, Mumbai.
- De ShepperN, De KegelB, AdriaensD (2007) Pisodonophis boro (Ophichthide: Angui-lliformes): specialization for head- first and tail- first burrowing. JMorphol268: 112-126.
- TalwarPK, KackerRK (1984) Commercial sea fishes of India. Ed. by the Director, Zoological Survey of India: 997.
- FAO (1984) FAO species identification sheets for fishery purposes. Fischer W, Bianchi G (eds), FAO Fisheries Department Rome, Italy.
- Day F (1994) The fishes of India, being a natural history of the fishes known to inhabit the seas and freshwaters of India, Burma and Ceylon, vol I & II Fourth Indian report.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 4131
- [From(publication date):
June-2017 - Sep 04, 2025] - Breakdown by view type
- HTML page views : 3156
- PDF downloads : 975