Research Article Open Access
Synthesis and Conversion of Gold Nanosphere into Nanoprism Platform using Laurencia papillosa: A Novel Natural Method
Younes AM1, Hegazi MM1, Beall GW2, Al-Sharkawey AE3, Dashti NH4 and Montasser MS4*
1Department of Marine Biology, Faculty of Science, Suez Canal University, Ismailia, Egypt
2Department of Chemistry and Biochemistry, Texas State University, San Marcos, Texas, USA
3Nanoscopy Science Center, Faculty of Science, University of Kuwait, Kuwait
4Department of Biological Sciences, Faculty of Science, University of Kuwait, Kuwait
- *Corresponding Author:
- Montasser MS
Department of Biological Sciences
Faculty of Science
University of Kuwait, Kuwait
Tel: +965 6991 9966
E-mail: Magdy.montasser@ku.edu.kw
Received Date: November 09, 2015; Accepted Date: January 11, 2016; Published Date: January 25, 2016
Citation: Younes AM, Hegazi MM, Beall GW, Al-Sharkawey AE, Dashti NH, et al. (2016) Synthesis and Conversion of Gold Nanosphere into Nanoprism Platform using Laurencia papillosa: A Novel Natural Method. J Fisheries Livest Prod 4:163. doi:10.4172/2332-2608.1000163
Copyright: © 2016 Younes AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
This is the first report on a rapid green synthesis of gold nanoparticles (GNPs) using red algae (Laurencia papillosa). The green synthesis of eco-friendly nanoparticles is of a great interest in Nanoscience for biomedical applications and specifically for clinical diagnostic applications. GNPs especially nanoprism represents a new advanced tool to study cell function and useful in optoelectronics, in developing a drug delivery system to control plant virus diseases and in nanomedicine. Conventional physical and chemical methods have been developed for the synthesis of metal nanoparticles, but these methods are expensive and require the use of toxic and aggressive chemicals. In this paper it is demonstrated that a rapid, low coast and ecofriendly method for synthesis of gold nanosphere and its conversion into gold nanoprism has been developed. The method involves using water solvent extract of Laurencia papillosa as a reducing agent. Nanoscopy analysis revealed that the nanoprism and other different morphologies were obtained just by varying the concentration molarity of tetrachloauric acid (HAuCl4), keeping the concentration of pure algal extract (PAE) constant. The best concentration of AuCl4 was 5 mM and best concentration of the red algae extract was at 0.05 g/ml. The functional groups responsible for conversion of nanospheres into nanoprism were NH and OH groups found in the contents of the red algae extract. The as-synthesized gold nanoprisms were characterized by several physicochemical techniques. The nanoprisms are single crystalline, whose basal plates surface are atomically flat "111" planes. We anticipate our results to be a starting point for more applications in medicine and industrial technology.
Keywords
Nanosphere; Nanoprism; Biomimetic; Tetrachloauric acid; Optoelectronics
Introduction
Currently, there is a growing need to develop environmentally benign nanoparticles synthesis process without using toxic chemicals in the synthesis protocols. The secrets gleaned from nature have led to the development of biomimetic approaches to the growth of advanced nano-materials. Gold nanoprism represents a new advanced tool to study cell function and useful in optoelectronics [1-3], in developing a drug delivery system using gene gun technology to control plant virus diseases and in nanomedicine [4]. The green synthesis of eco-friendly nanoparticles is of great interest in Nanoscience for biomedical applications [5]. This synthesis would be simpler, more economical and free of adsorbed chemicals on the surface of as-synthesized GNPs. Recently, several authors have been engaged in green synthesis of GNPs with different sizes and shapes [6-9]. The phytochemicals from the red algae served as an easy reducing and stabilizing agents for GNPs synthesis [10]. There are several important factors that affect the synthesis of nanoparticles, including pH of the solution, temperature, concentration of the extracts, size and shapes of GNPs [11]. Trace quantities of gold (Aureat, Au) and silver (Ag) nanoprism have been observed as by-products of methods that predominately produce spheres [12]. Gold nanoprism have been used to study cell function and catalysis [13]. No published data are available about using the red algae Laurencia papillosa and the phytochemicals derived from them as a strong reducing and stabilizing agent in conversion of gold nanospheres into gold nanoprisms. The main objective of this work was to study the feasibility of using laurencia papillosa assisted synthesis in production of gold nanosphere and conversion into gold nanoprisms.
Materials and Methods
Tetrachloauric acid (HAuCl4), (Sigma, Aldrich chemicals, USA) was used without further purification. Initially different concentrations (Table 1) of HAuCl4 solution (0.25, 0.5, 1, 2, 3, 4 and 5 mM) were prepared in sterile water according to Pal [14]. Samples of Laurencia papillosa were collected, then washed with fresh water repeatedly. Samples were air dried in the shade for 5 days, then ground into small pieces. Five grams of the dried samples were boiled in 100 ml sterile water for 5 min [15]. The boiled extract solution was centrifuged at 5000 rpm for 5 min at 5°C. The centrifuged pellet was discarded and the supernatant (PAE) was used for the synthesis of GNPs. The GNP were produced in a one-step reaction, where each reaction had a total final volume of 4 ml with different concentrations of HAuCl4 each as shown in Table 1. The PAE volume and concentration were kept constant, at room temperature.
| Exp No. | AuCl4 Conc.* (mM) |
Reaction vol/ ml | AuCl4 (10 mM Stock soln.) vol/ ml | Water vol/ ml | Total vol/ ml of AuCl4 Stock conc. |
|---|---|---|---|---|---|
| 1 | 0.25 | 4 | 1.25 | 48.75 | 50 |
| 2 | 0.5 | 4 | 2.5 | 47.5 | 50 |
| 3 | 1 | 4 | 5 | 45 | 50 |
| 4 | 2 | 4 | 10 | 40 | 50 |
| 5 | 3 | 4 | 15 | 35 | 50 |
| 6 | 4 | 4 | 20 | 30 | 50 |
| 7 | 5 | 4 | 25 | 25 | 50 |
Table 1: Reaction conditions of GNPs synthesis using 10 mM of AuCl4 at different concentrations.
UV-Visible spectroscopy
The bioreduction and optical properties of the freshly prepared GNPs were investigated according to Priya et al. [16] by measuring the UV-vis spectrum between 200-800 nm in a 10- mm -path-length quartz cuvette with a 1 nm resolution (Agilent Cary UV-Vis NIR Spectrometer).
Transmission electron microscopy (TEM)
TEM samples were prepared via drop casting on carbon-coated grid [17]. TEM measurements were carried out on a JEOL model 1200 EX instrument operated at an accelerating voltage 120 KV.
Atomic force microscopy (AFM)
GNPs were prepared by solution-casting onto highly oriented graphite substrate and analyzed by AFM in the contact mode on a VEECO digital instruments multimode [18].
Field emission scanning electron microscopy (FESEM)
Morphology of the as-synthesized gold nanoparticles was studied using JSM-6763 LA instrument [19].
X-ray Diffraction(XRD)
XRD measurements of films of the as-synthesized gold nanoparticles solvent cast onto glass slides were done on a Bruker AXS D8 Advance X-Ray Powder Diffractometer operating from 20 to 80° two theta at a voltage of 40 KV to determine the crystalline structure of GNPs [20].
Fourier transformed infrared (FTIR) spectroscopy
In order to determine the possible functional groups of the phytochemical present in PAE that help in the reduction of HAuCl4 to gold nanoparticles and its stabilization [21], FTIR (RX spectrophotometer, Perkin Elmer, Waltham, MA analysis was carried out. After the removal of the free biomolecules that were not adsorbed by the nanoparticles after repeated centrifugation and re dispersion in water, the nanoparticles were subjected to FTIR analysis.
X-ray photoelectron spectroscopy (XPS)
XPS measurements were obtained on a KRATOS-AXIS 165 instrument equipped with dual aluminum-magnesium anodes using MgK radiation, operating at 5 KV and 15 mA with pass energy of 80 eV and an increment of 0.1 eV. The samples were degassed for several hours in the XPS chamber to minimize air contamination on the surface [19].
Results and Discussion
UV-visible spectroscopy
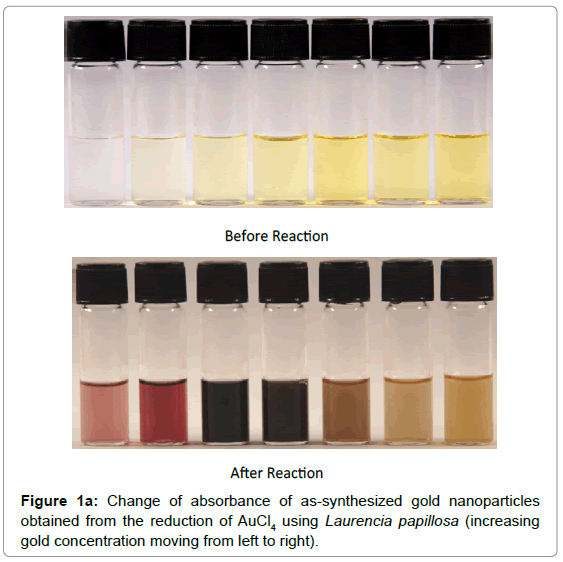
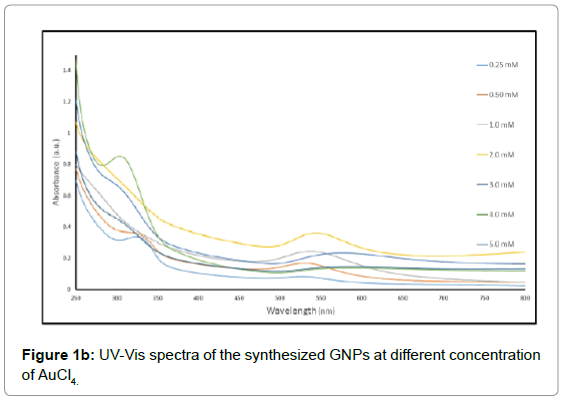
The color of solutions listed in Table 1 changed from yellow to pale violet and finally ruby red, indicating formation of gold nanoparticles (Figure 1a). There is an abrupt change in color between 2 mM and 3 mM. UV-vis spectra recorded as a function of HAuCl4 concentration at room temperature showed an increase of a Surface Plasmon Resonance (SPR) band from 526 to 586 nm, clearly indicating the formation of GNPs [21-23] with different peak intensity as shown in Figure 1b. The absorption value at 526-586 nm was due to longitudinal excitation of the SPR vibration as resulted from the light scattering and absorption which is determined by the size of the GNPs, as the Plasmon band of GNPs can range from 510 to 560 nm (26). It can be seen that there is a steady shift in the UV-vis absorbance peak at 525 nm for 0.25 mM to 545 nm for 2 mM. The peak then shifts abruptly to around 570 nm and becomes broader at concentrations above 3 mM. The UV-vis spectral trends confirmed the color changes observed visually.
Transmission electron microscopy (TEM)
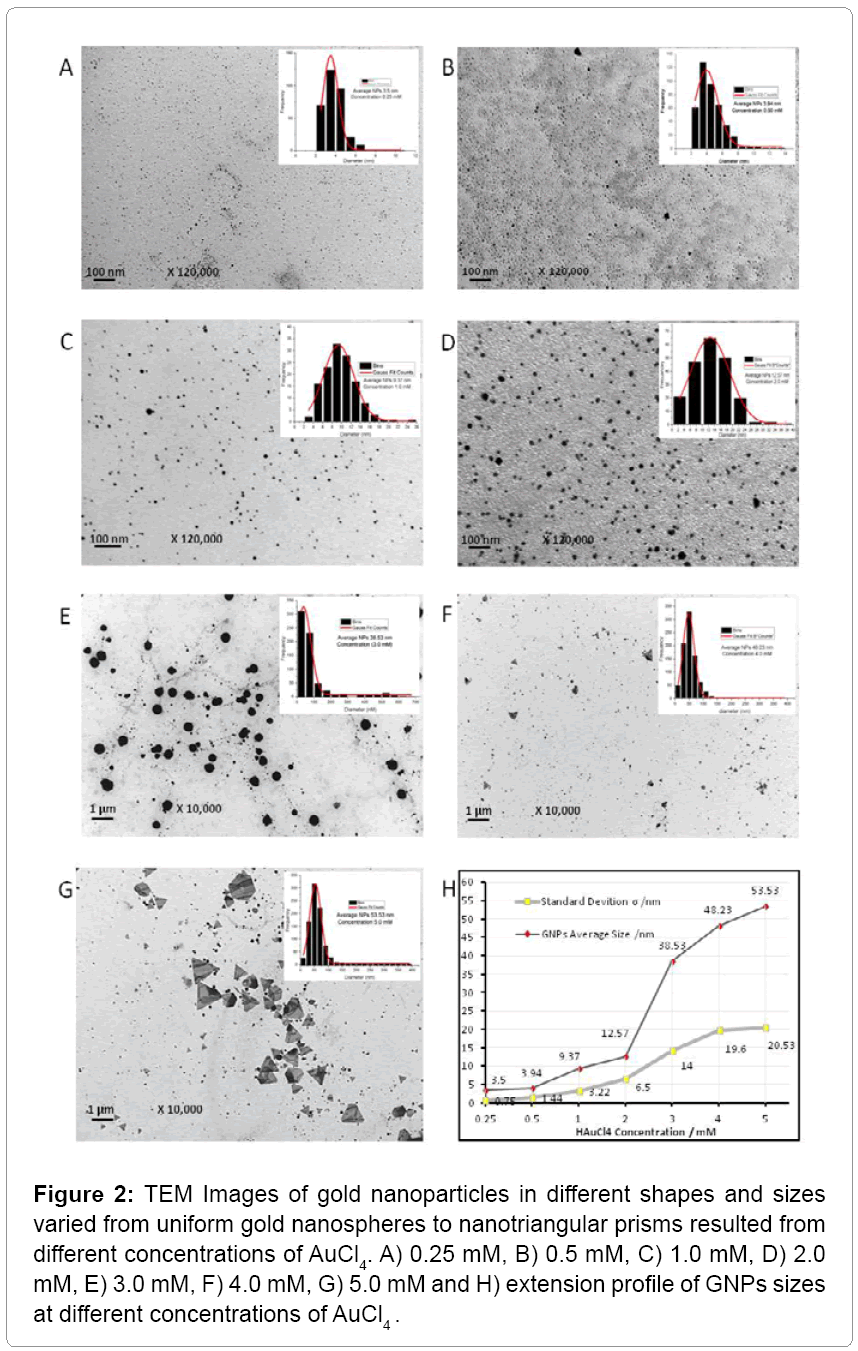
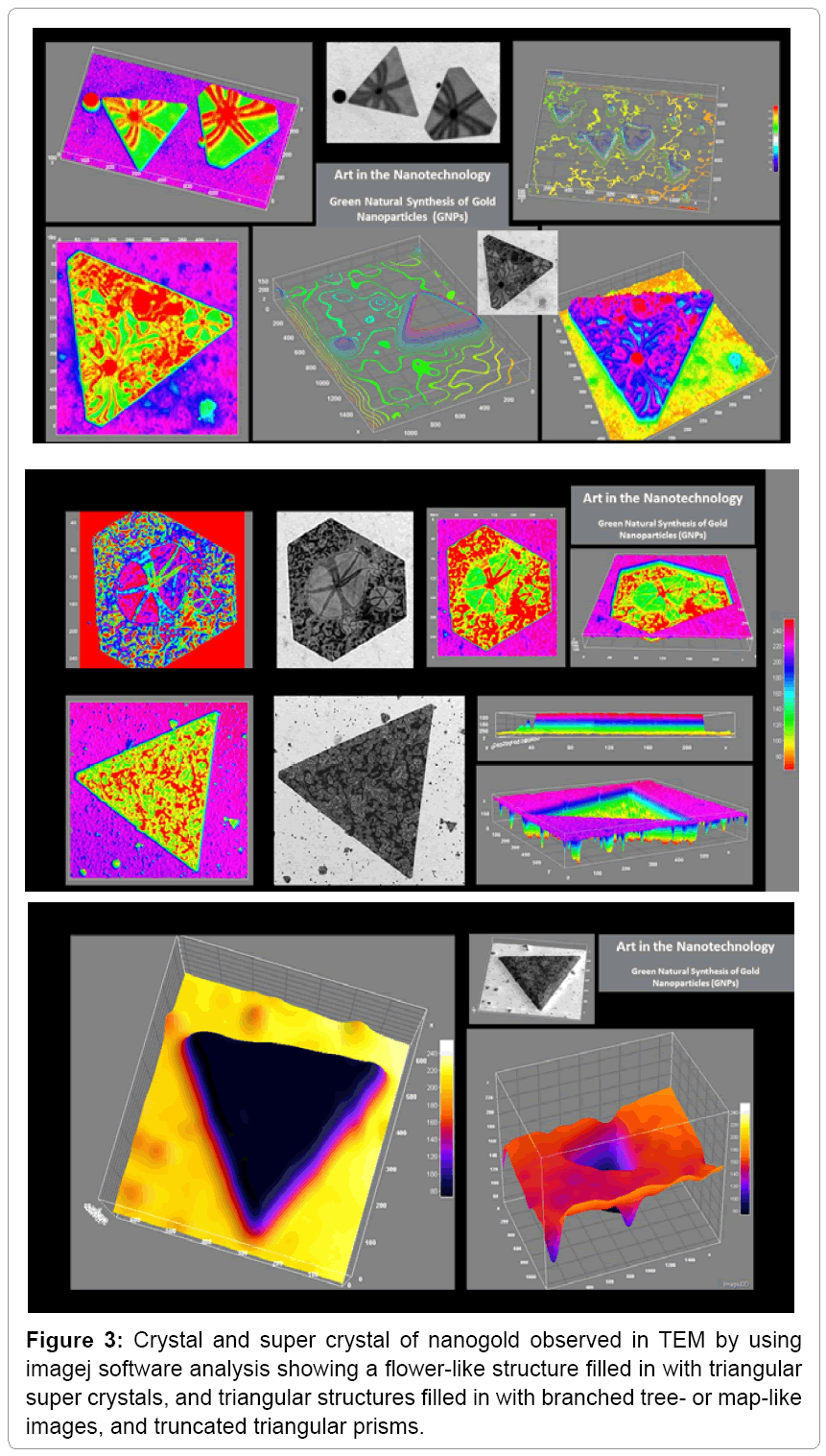
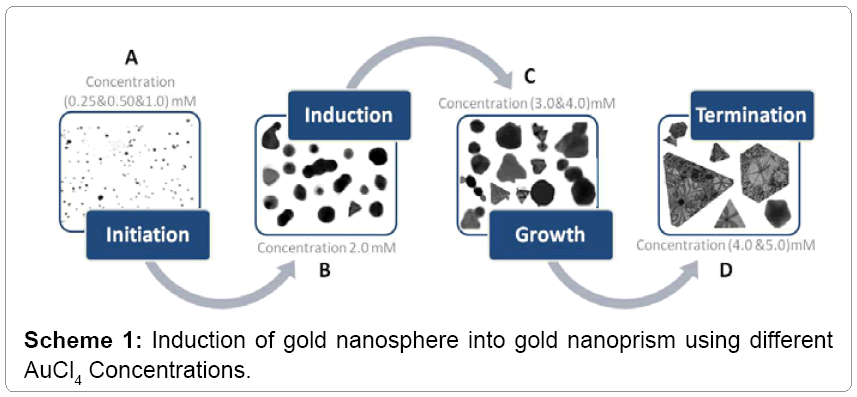
Different concentrations of AuCl4-PAE resulted in various GNPs sizes of 3.5 nm up to 53 nm and various shapes of spherical, hexagonal, octaganol, triangular and truncated nanoprism (Figure 2). The results suggested that the biosynthesis of triangular and truncated nanoprism were formed in four steps: initiation, induction, growth and termination as shown in Scheme 1. In the initiation step the spherical nanoparticles were seeded in different sizes. In the induction step the spherical nanoparticles were aggregated into small triangular nanoprism mixed with other crystal shapes. As the concentration of AuCl4 increased, the triangular and truncated nanoprism grows into super crystals of nanoprism (Figure 2). These results correlated with the fact that weak reducing agents can generally form bigger nanoparticles (nanoprism) with different shapes. We observed a flower-like structure filled in with triangular crystals, as well as triangular structures filled in with artistically branched tree or map-like images (Figure 3). Also it was noticed that biequilateral triangular nanoprism and truncated nanoprism were resulted from the conversion of nanosphere using various concentrations of AuCl4 as shown in Scheme 1. The computational analysis of TEM images showed two types of nanoprism: one flat plate and the other type was hopper growth triangular nanoprism (Figure 4). In general, nanoprism shapes represented ~ 90% of the total number of particles observed. This considered a significantly higher number than what was reported earlier [24]. The resulted GNPs ranged from 3.5 nm to 12 nm for concentrations less than 2 mM. However, triangular nanoprism ranged from 12 to 53 nm that often showed truncated nanoprism rather than what was reported previously [24]. At concentrations greater than 3 mM a bimodal distribution of particles appeared where there were two classes of particles. There was one class in the 30 to 50 nm range but there were also very large crystals that were hundreds of nm in size. The micrographs of the 5 mM best illustrate these phenomena. This change in particle size distribution again correlates with color changes and the UV-vis spectra.
Figure 2: TEM Images of gold nanoparticles in different shapes and sizes varied from uniform gold nanospheres to nanotriangular prisms resulted from different concentrations of AuCl4. A) 0.25 mM, B) 0.5 mM, C) 1.0 mM, D) 2.0 mM, E) 3.0 mM, F) 4.0 mM, G) 5.0 mM and H) extension profile of GNPs sizes at different concentrations of AuCl4 .
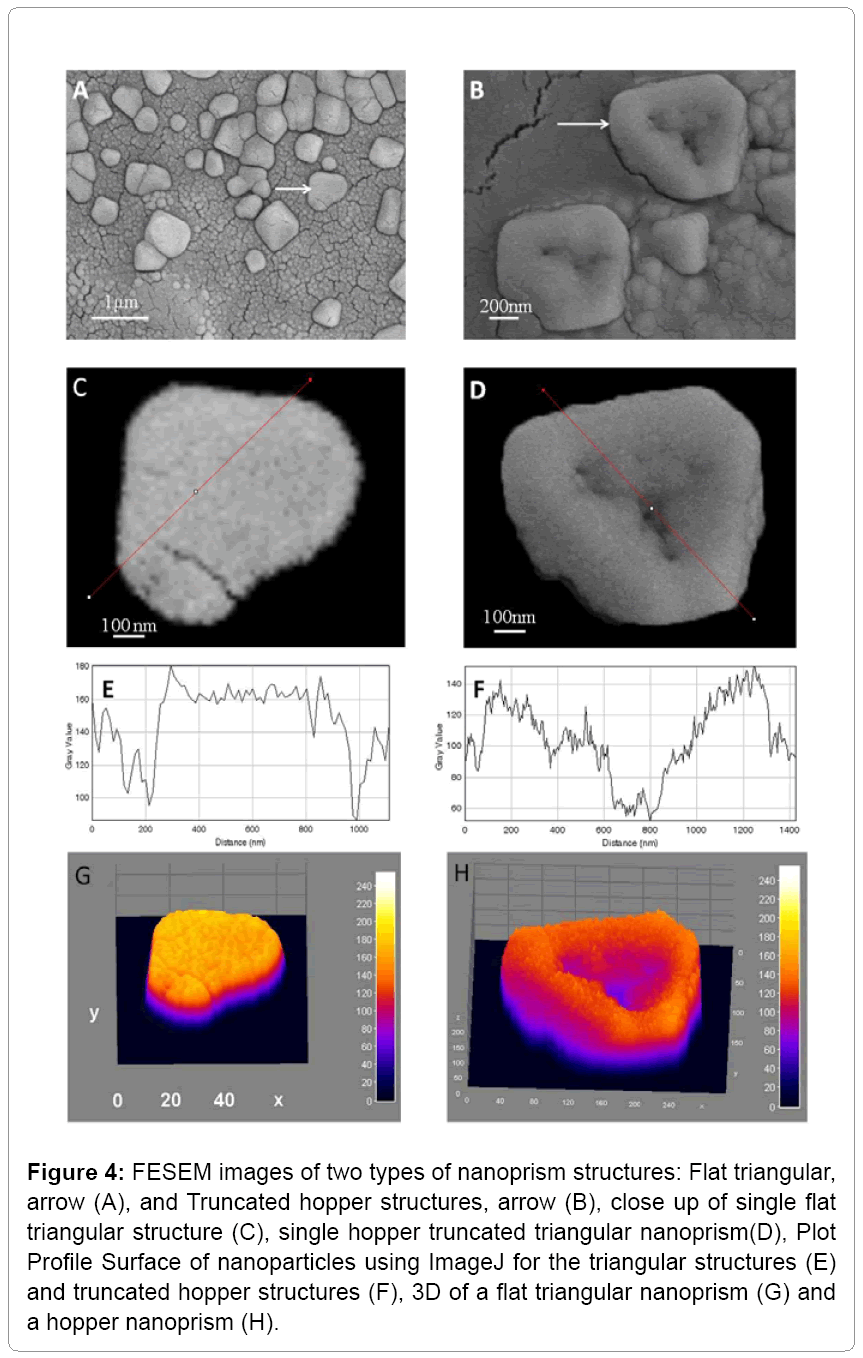
Figure 4: FESEM images of two types of nanoprism structures: Flat triangular, arrow (A), and Truncated hopper structures, arrow (B), close up of single flat triangular structure (C), single hopper truncated triangular nanoprism(D), Plot Profile Surface of nanoparticles using ImageJ for the triangular structures (E) and truncated hopper structures (F), 3D of a flat triangular nanoprism (G) and a hopper nanoprism (H).
Field emission scanning electron microscopy (FESEM)
The resulted GNPs showed two types of triangular structures one was flat and the other was of a hopper growth type as shown in Figure 4. The morphology of hopper crystals arise when crystal growth at the edges is more rapid than the center growth. The hopper structure is anticipated to be used as a starting point for more applications in industrial technology and in medicine, especially in drug delivery systems since it represents a higher surface area per mass.
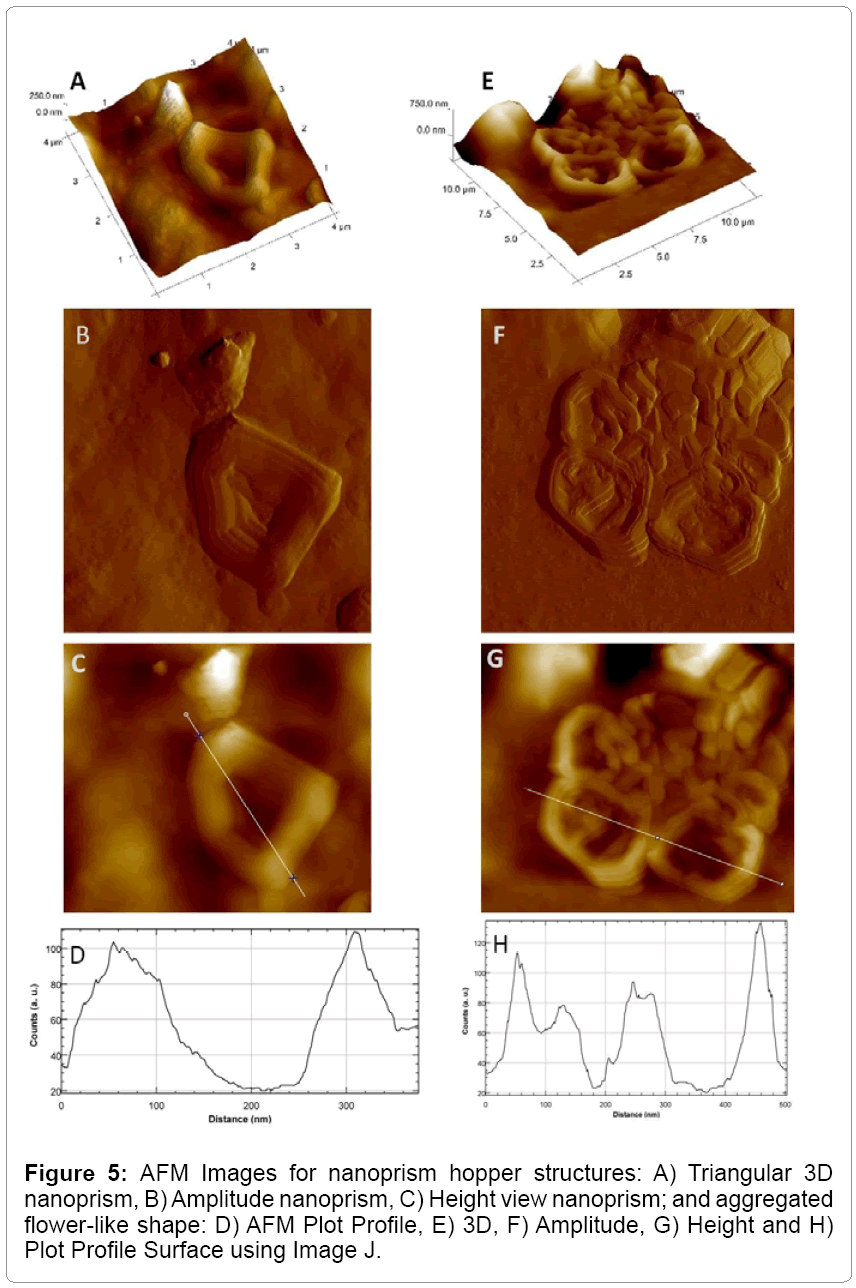
Atomic force microscopy (AFM)
AFM analysis showed 3D, Heights, Amplitude, and mixed images of GNPs including gold nanoprism. The resulted surface indicated by a dotted line through triangular axis was relatively smooth and flat for the purely triangular particles but in the hopper crystals, the scan shows a distinct valley between the edges as shown in Figure 5.
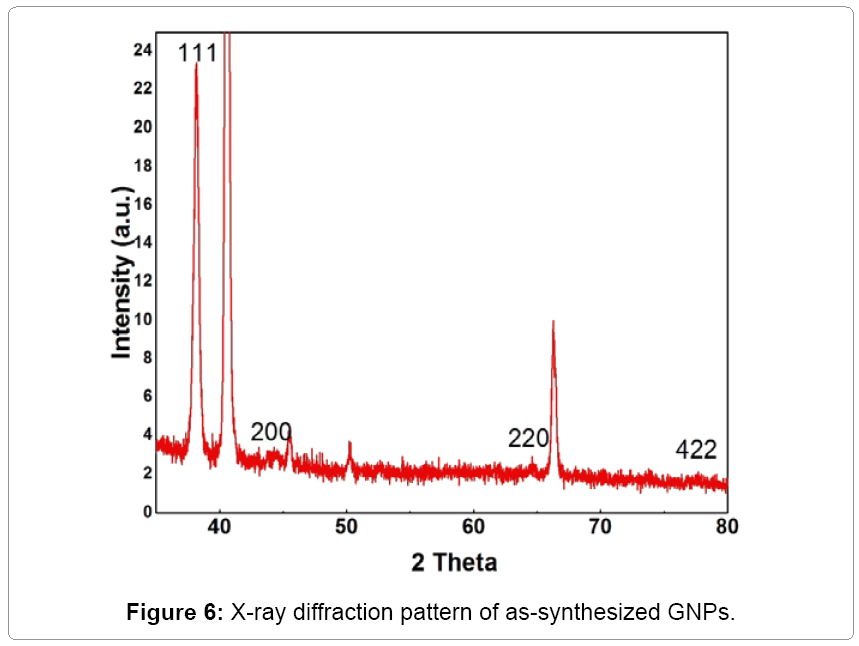
X-ray diffraction (XRD) spectroscopy
The crystal structures of the as-synthesized gold nanoprism obtained after the reduction of HAuCl4 using Laurencia papillosa extracts were identified using X-Ray Diffraction (XRD) spectroscopy analysis (Figure 6). The Bragg peaks equivalent to "111", "200", "220" and "422" demonstrate the formation of crystalline gold nanoprism [25]. The intensity of the "111" line would indicate that the face of the triangular particles is the (111) type which is consistent with the trigonal symmetry. All reflections are distinctly indexed to a face-centered cubic (fcc) phase of GNPs. All other diffraction lines arise from the extracted algae components, byproducts of the reaction or substrate. These results corroborate with earlier published literature [25]. The presence of these four intense peaks corresponding to the nanoparticles was in agreement with the Bragg's reflections of gold identified with the diffraction pattern [24]. Thus, the XRD pattern suggests that the gold nanoparticles were essentially crystalline in nature. Hence, the simulated solution exhibited tremendous performance on the synthesis of GNPs as that of Laurencia papillosa extract. Similar results of XRD for gold nanoparticles synthesized using brown algae S. marginatum were found previously [25].
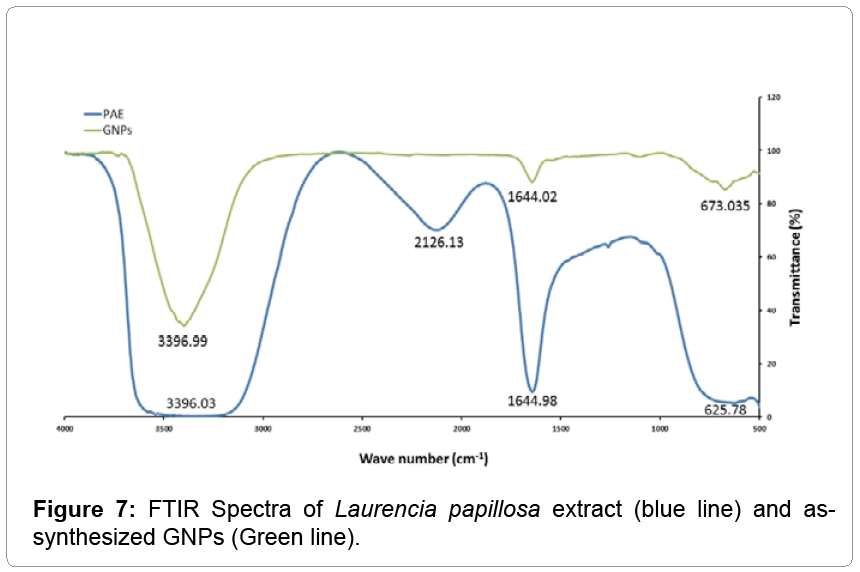
Fourier transformed infrared (FTIR) spectroscopy
In order to determine the possible functional groups of phytochemical or proteins in PAE that help in the reduction of HAuCl4 to gold nanoparticles and its stabilization, the FTIR spectroscopy analysis was carried out. The major stretching frequencies of Laurencia papillosa extract were observed at 3396.03, 2126.13, 1644.98 and 625.78 cm-1 (blue line in Figure 7) whereas the stretching frequencies of GNPs were observed at 3396.99, 1644.02 and 673.03 cm-1 (green line in Figure 7). The major IR spectrum, arises at 3396.03 cm-1 in blue line, due to the O–H stretching modes which are significantly reduced and become sharper upon coordination with gold nanoparticles as shown in Figure 7 green line [25], suggesting the role of phenolic groups in the reduction of HAuCl4 to GNPs. On the other hand, the IR spectrum at 1644.98 cm-1 (blue line) was due to the presence of amide in proteins of Laurencia papillosa [22]. The IR spectrum at 2126 cm-1 (blue line) due to the presence of C=O carbonyl group disappeared as indicated in green line due to the involvement of that protein in the reduction of HAuCl4 during the synthesis of GNPs.
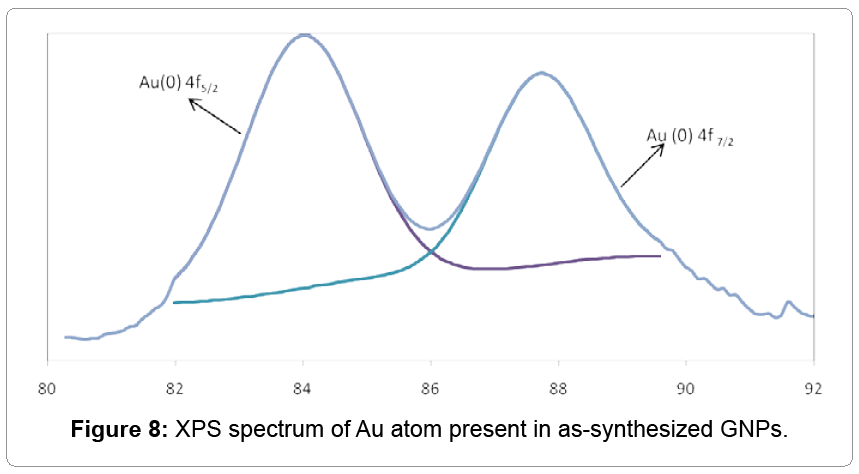
X-Ray photoelectron spectroscopy (XPS)
XPS data of gold nanoprism shown in Figure 8 indicating the binding energy (BE) of Au atoms. The BE was observed at both ~ 84, and 88 eV resulted in the presence of Au 4 f7/2 and Au 4 f5/2 respectively. The Au 4 f7/2 core attributed to Au (0) or metallic gold (Figure 8). The presence of Au0 on GNPs surface helps to stabilize the gold nanoparticles from aggregations [25].
Acknowledgements
We gratefully acknowledge funding support for this research work from the Research Administration, Projects #SL04/15 and #SL06/14, Kuwait University, Kuwait. We extend our appreciations to the Nanoscopy Science Center (NSC), Scientific Analytical Facilities (SAF), Faculty of Science, and Kuwait University, Kuwait.
End Note
This is the first report on green synthesis of Gold nanoparticles (GNPs) using red algae (Laurencia papillosa). The green synthesis of eco-friendly nanoparticles is of great interest in Nanoscience for biomedical applications and specifically for clinical diagnostics applications. GNPs especially nanoprism represents a new advanced tool to study cell function and useful in optoelectronics, in developing a drug delivery system to control plant virus diseases and in nanomedicine.
References
- Chandra M, Indi SS, Das PK (2007)Depolarized hyper-Rayleigh scattering from copper nanoparticles. JPhysChemC 111: 10652-10656.
- Segets D, Tomalino LM, Gradl J, Peukert W (2009) Real-time monitoring of the nucleation and growth of ZnO nanoparticles using an optical hyper-Rayleigh scattering method. J Phys Chem C 113: 11995-12001.
- Boyd RW (1992) Nonlinear Optics, Academic Press, San Diego.
- Singaravelu G, Arockiyamari JS, Ganesh KV, Govindaraju K (2007) Novel Extracellular Biosynthesis of Monodisperse Gold Nanoparticles Using Marine Algae, Sargassum Wightii Greville. Colloid Surf B: Biointerf57: 97-101.
- Kuppusamy V, Mahadevan A, Sathishkumar M, Pavagadhi S, Balasubramanian R (2011)Biosorptionand Subsequent Bioreduction of Trivalentaurum by a Brown Marine Alga Turbinaria Conoides. Chem Eng J167: 223-227.
- Baker S, Rakshith D, Kavitha KS,Santosh P, Kavitha HU, et al.(2013) Plants: Emerging as NanofactoriesTowards Facile Route in Synthesis of Nanoparticles. Bio Impacts 3: 111-117.
- Rajathi FAA, Parthiban C, Ganesh KV, Anantharaman P (2012) Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermummarginatum (kützing). SpectrochimActa A MolBiomol Spectrosc 99: 166-173.
- Nikoobakht B, Wang ZL, El-Sayed MA (2000)Self-Assembly of Gold Nanorods. J Phys Chem B104: 8635-8640.
- Rajeshkumar, Malarkodi C, Gnanajobitha G, Vanaja M, Paulkumar K, et al. (2013)Phytosynthesis of silver nanoparticles by Cissusquadrangularis: influence of physicochemical factors. Nanostructure in Chemistry 3: 1-6.
- Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, et al. (2004)Biological synthesis of triangular gold Nanoprisms. Nature materials 3: 482-488.
- Krishnamurthy P, Tan XF, Lim TK, Tit-Meng L, Prakash PK, et al.(2014) Proteomic Analysis Of Plasma Membrane And Tonoplast From The Leaves Of Mangrove Plant AvicenniaOfficinalis. Proteomics14: 2545-2557.
- Shankar SS, Ahmad A, Pasricha R, Sastry M (2003)Bioreduction of Chloroaurate ions by geranium leaves and its Endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13: 1822-1826.
- Ingale AG, Chaudhari AN (2013)Biogenic synthesis of nanoparticles and potential applications: an eco-friendly approach. Nanomedicine and Nanotechnology 4:12-19.
- PalA, Shah SS, Devi S (2007) Preparation of Silver, gold and Silver-gold bimetallic Nanoparticles in w/o Microemulsion Containing Triton X-100. Colloids SurfA: PhysicochemEngAspects302: 483-487.
- Mucalo MR, Bullen CR, Manley-Harris M, McIntire T (2002) Arabinogalactan from the Western larch tree: a new, purified and highly water-soluble polysaccharide-based protecting agent for maintaining precious metal nanoparticles in colloidal suspension. Journal of Materials Science37: 493-504.
- Priya MM, Selvia BK, Paul JAJ(2011) Green synthesis of silver nanoparticles from the leaf extracts of Euphorbia hirta and Neriumindicum. Digest Journal of Nanomaterials and Biostructures 6: 869-877.
- Gupta V, Gupta AR, Kant V (2013) Synthesis, characterization and biomedical Application of Nanoparticles. Science International1: 167-174.
- Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications.The Iranian Chemical Society7: 1-37.
- Shankar SS, Rai A, Ahmad A, Sastry M (2005) Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachtaindica) leaf broth. JColloid Interface Sci275: 496-502.
- Shukla R, Nune KS, Chanda N, Katti K, Mekapothula S, et al. (2008) Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 4: 1425-1436.
- Chandran PS, Chaudhary, M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold Nanotriangles and silver nanoparticles using Aloe Vera plant extract. BiotechnolProg 22: 577-83.
- El-Sayed MA, Huang X (2010) Gold nanoparticles: Optical properties and implementations in cancer diagnosis and Photothermal therapy. Advanced Research1: 13-28.
- Saxena A, Tripathi RM, Singh RP (2010) Biological Synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. DigestJ NanomaterBiostruct 5: 427-432.
- Krpetic Z, Scari G, Caneva C, Speranza G, Porta F (2009) Gold nanoparticles prepared using Cape Aloe active components. Langmuir 25: 7217-7221.
- Shankar SS, Rai A, Ahmad A, Sastry M (2005) Controlling the optical properties of Lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings.Chem Mater 17: 566-572.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 11238
- [From(publication date):
March-2016 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 10503
- PDF downloads : 735