Research Article Open Access
The Influence of Pesticides on Hepatic and Renal Functions in Occupational Sprayers of Rural Malihabad, Lucknow (India)
Farrukh Jamal*, Quazi S Haque and Sangram Singh
Department of Biochemistry, Dr. Ram Manohar Lohia Avadh University, Faizabad-224001, India
- *Corresponding Author:
- Farrukh Jamal
Department of Biochemistry
Dr. Ram Manohar Lohia Avadh University
Faizabad-224001, India
Tel: 919415075554
E-mail: farrukhrmlau@gmail.com
Received date: October 05, 2015 Accepted date: January 21, 2016 Published date: February 01, 2016
Citation: Jamal F, Haque SQ, Singh S (2016) The Influence of Pesticides on Hepatic and Renal Functions in Occupational Sprayers of Rural Malihabad, Lucknow (India). Toxicol open access 1:106. doi:10.4172/2476-2067.1000106
Copyright: © 2016 Jamal F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
Pesticides affect several human organs, resulting in a wide variety of physiological changes. In context with the occupational pesticide sprayers it is important to understand the influence of toxic exposure on human health. The present study is a comparative analysis of hematologic parameters, hepatic and renal function in the occupationally exposed pesticide sprayers of mango plantation of rural Malihabad, Lucknow. This area is predominantly a mango cultivation belt in North India. The select group of study comprises of sixty (60) pesticide sprayers [study group] and the control group included thirty (30) pesticides-unexposed normal healthy persons engaged in normal usual agricultural work [age group 20 to 45 years corresponding to age group of select subject group] from rural Malihabad, Lucknow [India]. Blood samples were collected from both groups. Questionnaire, interview and observation were employed to obtain demographic, occupational, dietary and clinical data. The sprayers as compared with control participants showed significantly increased serum C reactive protein, liver function marker enzymes, serum bilirubin, creatinine, blood glucose, and blood urea; whereas the acetyl cholinesterase activity and the level of serum cholesterol declined. No significant alteration was observed in the serum total protein, globulin, and the albumin/globulin ratio; however, a slight downfall in the level of serum albumin was recorded. Compared with the control group, hematologic parameters significantly decreased in pesticide sprayers. It is suggested that a high degree of pesticide absorption in occupationally exposed pesticides sprayers is responsible for decrease in the level of serum acetyl cholinesterase and consequently there is impairment of liver and kidney functions and slightly altered hematologic parameters. The present study suggests the toxic effect of pesticides on the occupational sprayer and precautionary measures must be taken to ameliorate their health status. It also suggests that restrain must be imposed on the indiscriminate use of lethal pesticides as it affects the ecosystem.
Keywords
Pesticide sprayers; Hematologic; Hepatic; Renal functions
Background
Pesticides are extensively used worldwide in agricultural practises to control pests and increase crop yield. In recent years, their use has increased considerably. As an agricultural developing nation India too relies predominantly on chemical pesticides to sustain a large population. Pesticides have potential to kill a wide variety of insect pests and in doing so they harm the ecosystem in general and human health in particular. Being agriculture oriented nation the primary occupation of rural population depends on spraying indiscriminately a diverse group of agro-chemicals to save the crops. Among the most commonly used pesticides are organophosphorus [OPs] insecticides. These OPs are very powerful neurotoxins that manifest different degree of neurotoxicity in users when exposed to different levels of exposure [1]. India represents the largest manufacturer of basic pesticides in Asia and ranks 12th on the global spectrum. Of the total pesticide consumption in India insecticides account for ~75%, followed by fungicides [~ 12%] and herbicides [~ 10%] [2].
For controlling the different types of pests a wide variety of chemical pesticides are used. These are primarily organochlorines, organophosphorous, carbamates, pyrethroids compounds, and various inorganic compounds. Among the common organophosphorous and carbamates pesticides used in mango plantation are Dichlorovas 76% EC, Monocrotophos 36% EC, Dimethoate 30% EC, Phosphamidon 85% SL, Endosulfan 35% EC, Carbaryl, 25% EC, Monocrotophos 36% SL, Methomyl. It is generally through the skin, eyes, by inhalation, or by ingestion that pesticides reaches the target organs. Through intact skin, the fat-soluble pesticides, and to some extent, the water-soluble pesticides are absorbed. Sores and abrasions enhance the uptake of pesticides. The aerosol droplets with dimensions smaller than 5 μm in diameters are effectively absorbed through the lungs. The consumption of contaminated food or from using contaminated utensils may also provide ways to ingestion of these chemicals. The pesticide intake may also be an outcome of contaminated hands; like chewing pan, tobacco, smoking of bidi and while using pesticides for spraying, mixing, or handling [3]. For pesticide exposed workers, the dermal route of uptake is common.
A number of enzymes, physiological systems, and organs of mammalian systems, i.e., reproductive, nervous, immune, and endocrine systems; as well as blood coagulation and hematologic, cardiovascular, sensory organs, skin, and respiratory systems are influenced by pesticides. Other effects include metabolic disturbances, fluid and electrolyte imbalance, and carcinogenic and mutagenic potential. Among the several human organs that are affected by pesticides liver is most susceptible. An increased lipid peroxidation in several tissues, mainly brain, skeletal muscle, red blood cells [RBC], etc., is due to enhance generation of reactive oxygen and nitrogen species and depletion in the antioxidant status of population exposed to different pesticides [4,5]. The induction or inhibition of certain enzymes by pesticides results in altering the biochemical status in an individual. Inhibition of cholinesterase alters liver and kidney functions, decrease hemoglobin [Hb], impair oxidative stress and cause antioxidants imbalance. In pesticide-exposed workers, the adverse effects on drug metabolising enzymes in the liver have been reported [4,6]. Therefore, the present study is aimed at assessing the influence of pesticides exposure and their toxicity on hematologic parameters and on liver and kidney function tests in occupationally exposed pesticides sprayers of mango plantation in Malihabad, Lucknow [India].
Materials and Methods
Materials
This study group included 60 subjects who were occupational pesticide sprayers of mango plantation and 30 normal healthy individuals as control subjects who were not exposed to pesticides. The subjects were taken from Malihabad area of Lucknow, UP, India whose age ranges from 20 to 45 years. The control subjects were of similar age groups and were field workers from the same area. They neither performed any spraying activities nor have any kind of pesticide exposure. The pesticide sprayers were updated with the information about the aim of study and health hazards of exposure to pesticides and written consent was taken before collecting relevant data and biological material. A set of questionnaires and interview were used to collect demographic, occupational and clinical data.
All the subjects involved in the study belonged to agricultural families with almost similar socio-economic status. The subjects lacked any past history of major illness. The food habits including dietary intake of all subjects were comparable as observed from periodical checkups of their lunch boxes. Besides, this their routine breakfast and dinner were also verified. Of the study group subjects the educational status was: primary schooling [40%], passed high school [50%] and higher education [10%]. Control subjects were selected to provide a similar educational distribution. All subjects consuming drugs for minor illness were excluded. The individual males who were healthy and had attributes of non-smokers, non-alcoholism and occupationally sprayers of mango plantation with more than five and upto 15 years duration were considered for the study.
The study group subjects were routinely engaged in spraying pesticides continuously for four to five hours daily spreading from the month of October to January. The blood was collected at the end of this period as exposure to pesticides is maximal during this time of year. The experiments were conducted with the approval of the institutional ethical committee, and followed the guidelines of Helsinki declaration of 1964 [7].
Collection of blood samples
Blood samples were collected in heparin containing tubes by puncturing the anticubital vein.
Measurement of acetyl cholinesterase
Serum acetyl cholinesterase was measured using the Accucare kit method of Knedel et al. [8]. In the presence of potassium hexacyanoferrate [III] serum cholinesterase hydrolyses butyrylthiocoline to produce thiocoline. The cholinesterase activity of the sample is measured by the proportional decrease in the absorbance.
Measurement of serum C-reactive protein [CRP]
Serum CRP was measured according to Andersen et al. [9] and Lothar [10]. TURBILYTE- CRP™ is a turbidimetric immunoassay for determining CRP in human serum. It is based on the principal of AN agglutination reaction. The serum sample is reacted with activation buffer, TURBILYTECRP™ latex reagent, resulting in the formation of an insoluble complex which is monitored at 546 nm. The concentration of CRP in the serum specimen corresponds to the turbidity.
Measurement of SGOT and SGPT
Liver and kidney functions tests were measured using a fully automated biochemistry analyzer [Eurolyser] on the same day of sample collection. The transaminases SGOT [AST] and SGPT [ALT] were measured by the UV-kinetic method [11]. The generation of NAD+ in both transaminase reactions was measured at 340 nm.
Measurement of serum total protein, serum albumin and total bilirubin
Serum total protein was measured by the Biuret method [12]. Serum proteins react with cupric ion in alkaline pH to produce a colored complex, the intensity of the color complex was measured at 546 nm and directly proportional to the protein concentration in the specimen. Serum albumin was measured by the BCG method [13]. Serum albumin binds with 3.3',5.5'-tetra bromocresol sulfonapthalein [BCG] in acidic medium at pH 4.2, and the blue-green colored complex formed is measured at 600 nm. Serum globulin and the A/G ratio were calculated by using serum total proteins and albumin values.
Serum total bilirubin was estimated by the Jendrassik method [14]. Serum bilirubin reacts with diazotized sulfanilic acid to produce azobilirubin [pink color]. Dimethyl sulphoxide [DMSO] catalyzes the formation of azobilirubin from free bilirubin. The intensity of the pink color is proportional to the bilirubin concentration, measured at 546 nm.
Measurement of serum alkaline phosphatase and blood urea
Serum alkaline phosphatase was measured by the King Armstrong method using Span diagnostics kit [15]. Phenol is released by an enzymatic hydrolysis from disodium phenyl phosphate under defined conditions of time, temperature, and pH. The phenol reacts with 4- aminoantipyrine in the presence of alkaline oxidizing agent to produce a red compound measured at 520 nm against a reagent blank. Colour development is rapid and stable for at least one hour in bright light. Sodium hydroxide is added immediately after incubation to raise the pH and stop the reaction. Potassium ferricyanide is the oxidizing agent and sodium bicarbonate is added to provide an alkaline medium.
Blood urea was measured by glutamate dehydrogenase [GLDH] method [16]. Blood urea is decomposed by urease to form ammonia and carbon dioxide. Ammonia combines with 2-oxoglutarate in presence of glutamate dehydrogenase and NADH to form L-glutamate and NAD. The rate of NAD formation was measured at 340 nm and was directly proportional to blood urea. Each molecule of urea hydrolyzed liberates two molecules of NAD+.
Measurement of serum creatinine, and cholesterol
Serum creatinine was estimated by Jaffes method [17]. Serum creatinine in alkaline medium reacts with picrate to produce an orange colour that absorbs light at 492 nm. The rate of increase in absorbance is directly proportional to the concentration of creatinine in specimen. Blood glucose was measured by employing enzymatic method [18].
Serum cholesterol was measured [19]. In the former, glucose oxidase converts glucose to gluconic acid, yielding hydrogen peroxide [H2O2]. Cholesterol esterase hydrolyses cholesterol esters into free cholesterol and fatty acids, and cholesterol oxidase then converts the cholesterol to H2O2 and cholest-4-en-3 one. H2O2 in the presence of peroxidase oxidatively couples with 4-aminoantipyrine and phenol to produce a red quinoneimine dye, having an absorbance maximum at 510 nm. The intensity of the red colour is proportional to the amount glucose or total cholesterol in the specimen, respectively. Hematologic parameters were measured by using a fully automated Hematology analyzer Sysmax K-4500 [20].
Statistical Analysis
Statistical analyses between the study and control groups were done using the unpaired student's T-test.
Results
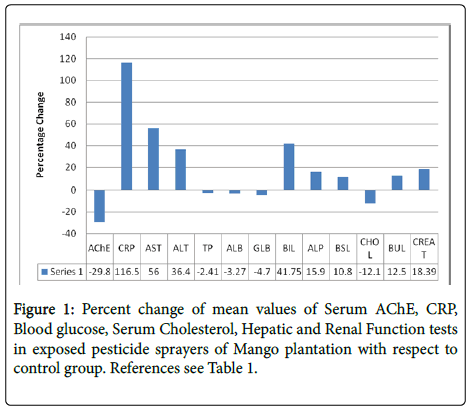
Compared with control participants, sprayers showed the followingsignificantly increased serum C reactive protein 116.5%, liver function marker enzymes-serum aspartate transaminase [AST] 56%, alanine transaminase [ALT] 36.4%, alkaline phosphatise [ALP] 15.9%, serum bilirubin [BIL] 41.8%, creatinine [CREAT] 18.4%, blood glucose [BSL] 10.8%, and blood urea [BUL] 12.5%; and decreased acetyl cholinesterase activity [AChE] 29.8% and serum cholesterol [CHOL] 12.12% as shown in Table 1 and Figure 1.
| Parameters | Study Group[N=60] | Control Group[N=30] | |
|---|---|---|---|
| Acetyl Cholinesterase [AChE] [U/L] | 4829 ±1375*** [876-7093] |
6993±1054 [4825-8524] |
|
| C Reactive Protein [CRP] [mg/dL] | 0.157 ±0.191** [0.043-0.892] |
0.072 ±0.019 [0.039-0.152] |
|
| Liver Function Tests: | |||
| Aspartate Transaminase [AST] [U/L] | 27.73±12.54*** [14-78] |
17.56 ±4.32 [10-28] |
|
| Alanine Transaminase [ALT] [U/L] | 32 50 ±14.66** [14-88] |
23.66 ± 4.96 [15-35] |
|
| Total Protein [gm/dL] | 7.22 ±0.383* [6.4-7.9] |
7.40 ±0.322 [6.4-8.0] |
|
| Albumin [gm/dL] | 4.14 ± 0.198* [3.8-4.6] |
4.28 ± 0.250 [3.60-4.70] |
|
| Globulin [gm/dL] | 3.13 ± 0250* [23-3.6] |
3.26 ± 0 478 [2.5-4.9] |
|
| Albumin/Globulin [A/G] Ratio | 1.330 ±0.1 23* [1.05-1.78] |
1.337 ±0.235 [0.79-1.76] |
|
| Alkaline phosphatase [ALP] [U/L] | 148 ± 21.29** [89-185] |
127 ±25.16 [68-179] |
|
| Bilirubin [mg/dL] | 0 954 ± 0.506* [0.40-3.84] |
0.673 ±0.252 [0.23-1.30] |
|
| Blood glucose [mg/dL] | 104±13.66**[79-145] | 94 ±13.34 [69-125] | |
| Serum cholesterol [mg/dL] | 157±30.57&**[92-218] | 179±23.44[150-250] | |
| Renal Functions Tests: | |||
| Blood Urea [mg/dL] | 28 ± 4.21* [19 -36] | 25 ± 4.56[18-36] | |
| Serum Creatinine [mg/dL] | 086 ±0.07*** [0.76-1] | 0.73 ± 0.13[0.45-1.0] | |
*P <0.05; **P <0.01; ***P <0.001
* Not significant compared with control.
Table-1: Biochemical parameters in exposed pesticide sprayers of Mango Plantation and healthy participants
AChE: Serum Acetyl cholinesterase; CRP: C-reactive Protein; AST: Aspartate Transaminase; ALT: Alanine Transaminase; TP: Total Protein; ALB: Albumin; GLB: Globulin; BIL: Bilirubin; ALP: Alkaline Phosphatase; BSL: Blood Glucose; CHOL: Serum Cholesterol; BUL: Blood Urea; CREAT: Creatinine.
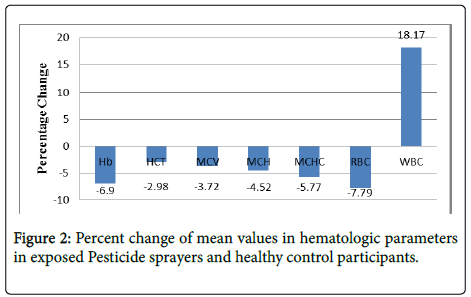
Serum total protein [TP], Globulin [GLB], and the Albumin/ Globulin A/G] ratio were not significantly altered; however, serum albumin [ALB] decreased slightly 3.3%, [P<0.05]. Compared with the control group, hematologic parameters significantly decreased in pesticide sprayers- hemoglobin [Hb] 6.9%, hematocrit [HCT] 2.98%, mean corpuscular volume [MCV] 3.72%, mean corpuscular hemoglobin [MCH] 4.5%, mean corpuscular hemoglobin concentration [MCHC] 5.8%, and red blood cell count [RBC] 7.7%, whereas the white blood cell count [WBC] increased 18.2% as shown in Table 2 and Figure 2.
| Hematologic Parameters | Study GroupN=[60] | Control GroupN=[30] |
|---|---|---|
| Hemoglobin Hb [gm/dL] | 13.83 ±1.47* [9.6-174] |
14.86 ±080 [1290-1620] |
| Hematocrit [HCT%] | 44.02 ± 2.97* [3290-51.70] |
45.37 ± 2.50 [39.80-5020] |
| MCV [fl] | 85.55 ± 4.55* [66.30-96] |
88.95 ±4.08 [76.90-96.40] |
| MCH [pg] | 28.70 ± 2.73** [19.40-33.70] |
30.06 ± 1.61 [25-33.90] |
| MCHC [gm/dL] | 31.80 ± 2.13** [23.90-35.90] |
33.75 ±1.83 [28.50-36] |
| RBC count [Million/µL] | 5.04 ±0.42*** [413-5.87] |
5.46 ±0.64 [390-65] |
| WBC count [mm3] | 7.74 ± 1.58** [5-12.20] |
6.55 ±1.18 [4.50-10] |
Hemoglobin; MCHC - Mean Corpuscular Hemoglobin Concentration;
RBC - Red Blood Cells; WBC - White Blood Cells; Figures indicate
Mean ± SD values range in parenthesis].
*Ρ <0.05; **Ρ <0.01; ***Ρ <0.001
Table-2: Comparison of hematologic parameters of Pesticide Sprayers and healthy control participants.
Hb: Hemoglobin, HCT: Hematocrit; MCV: Mean Corpuscle Volume; MCH: Mean Corpuscle Hemoglobin; MCHC: Mean Corpuscle Hemoglobin Concentration; RBC: Red Blood Cell Count; WBC: White Blood Cell
The biochemical markers of occupational exposure to pesticides include serum urea, creatinine, bilirubin, aspartate amino transferase and alanine amino transferase. The significantly increased ALT, AST, and ALP found in pesticide sprayers of mango plantations as compared with the control group indicate liver tissue damage following high exposure to pesticides. A decrease in serum acetyl cholinesterase activity in occupationally exposed pesticides sprayers indicated a high degree of pesticides absorption. The fall out of decreased acetyl cholinesterase activity is reflected in the impairment of both liver and kidney functions with slightly altered hematologic parameters. The increased serum AST, ALT, and ALP in our study could be due to increased malondialdehyde formation. The decreased serum cholesterol level seen in pesticide sprayers of mango plantation might be due to regular farm activity. The decrease is total cholesterol and favorable effects on serum lipids and lipoproteins may be due to regular exercise. The finding of increased serum creatinine and blood urea levels in pesticide sprayers of mango plantation agrees with the results of prior studies reporting alterations in nephrotoxicity of occupational pesticide sprayers. Compared with the control group, all hematologic parameters studied here in sprayers of mango plantations study group] decreased, except for the WBC, which increased.
Discussion
Mango growers use pesticides for the control of various insects and pests on mango plants. We collected blood samples at the end of the month of January because all sprayers were exposed daily for three months to all kinds of pesticides. During blood sample collection, most sprayers complained of fatigue, headache, loss of appetite with nausea, dizziness, excessive sweating and salivation, blurred vision with excessive watering, muscle weakness, anemia, all signs and symptoms of mild OP and carbamate exposure. We conducted a follow-up study in few cases whose serum cholinesterase was below 2000 U/L and observed that after almost two months, the altered biochemical parameters returned to normal level and the clinical symptoms were also reduced. The health of pesticide sprayers may be further worsened, but during a gap of four months they remain unexposed to any pesticides.
Although all pesticide sprayers know about the toxicity of pesticides, they do not take any precautions while spraying the pesticides on mango plantations because practically such precautions are not possible. For example, the sprayers are advised to use goggles or a helmet while spraying, but due to wind flow, fog develops on the protective device and the sprayer visibility is comprised. Hence, the exposure to various pesticides is higher for sprayers of mango plantation than for other types of exposure.
The decrease in serum AChE in pesticide sprayers in the present study was mainly due to organophosphorus and carbamate pesticides, confirming reports that OP and carbamate compounds inhibit AChE activity [21,22]. The primary target of OP and carbamate pesticides is acetyl cholinesterase, mechanistically involving carbamoylation of the active serine residue of the enzyme, resulting in cholinergic hyperactivity [23]. The activity of AChE is inhibited after both acute and chronic pesticides exposure. The reversible initial symptoms of burning eyes, mouth, and throat result from the direct contact with the pesticide and its carrier solvent, whereas subsequent neurological features that arise after sufficient absorption leads to the inhibition of AChE and the accumulation of excess amounts of ACh at muscarinic and nicotinic sites, including the neuromuscular junction. An acronym MUDDLES Miosis, Urination, Diarrhea, Diaphoresis, Lacrimation, Excitation of CNS, and Salivation] has been proposed to characterize the principal effects of AChE inhibition, whether caused by carbamates or OP pesticides compounds [24]. AChE can be synthesized by a variety of cells, including neurons, muscle cells, and erythrocytes. The enzyme is found in the pcrikaryon, dendritcs, and axon of cholinergic neurons, in the cholinergic synapses, and at the surface and infoldings of the post-junctional motor end plates.
A separate gene encodes for butyrylcholinesterase [BuChE], also known as plasma cholinesterase or pseudocholinesterase. Butyrylcholinesterase has been detected in the liver, plasma, and glial cells of the nervous system [25]. Although ACh is hydrolyzed faster by AChE than by [BuChE], OP compounds inhibit both enzymes. The function of BuChE not fully understood, but the enzyme appears to act as a scavenger of such AChE inhibitors as OP compounds and carbamates. The cholinesterase activity in plasma can be almost completely inhibited whereas erythrocyte AChE still retains 50% activity. This relative inhibition varies between compounds and with the route of absorption. It also depends on the duration of exposure if it has been chronic, acute or acute-on-chronic. Further, the rate of reversal of cholinesterase inhibition is depends on the type of the pesticides either carbamate or [OP]. For all practical purposes, plasma cholinesterase inhibition is a useful indicator of exposure to OP or carbamates, whereas a normal plasma cholinesterase activity effectively rules out acute poisoning by these compounds. It is difficult to decide if the low activity is indeed due to poisoning or to some other cause that may be physiologic, pharmacologic, or genetic. Cholinesterase activity also decreases in liver diseases, malnutrition, and pregnancy. Erythrocyte AChE is membrane-bound enzyme and the apparent measured activity is dependent on the methods used in purifying it from residual plasma cholinesterase. There is a lack of standard procedure in assessing the membrane bound Ache activity and delineating it from soluble state. The activity of AChE in erythrocyte also depends on the rate of erythropoiesis. The newly generated erythrocytes have a high activity, which decreases with the progress in time. Thus the erythrocyte AChE activity is not only a function of the number but also depends on the age of the cell population. However, the low activities of both plasma and erythrocyte AChE are strongly indicative of poisoning with pesticide that is either OP or carbamates.
C-reactive protein is a serum protein that is synthesized in the liver [26]. An acute injury or the onset of inflammation enhances the rate of synthesis and secretion of CRP within hours which may reach to as high as 20 time normal levels. In this study, the increase in CRP levels could possibly be an outcome of acute injury or due the onset of inflammation in the liver. An elevated serum concentration of CRP is an evidence of an active tissue damage process. Apart from indicating inflammatory disorders, CRP levels helps in differential diagnosis, and in the management of neonatal septicemia and meningitis for which standard microbiological investigations are difficult to perform. The CRP levels invariably rise after major surgery but fall to normal within 7-10 days. The absence of such a fall indicates septic or inflammatory postoperative complications. There exists an excellent correlation between peak levels of CRP and creatinine phosphokinase which is useful information for diagnosing patients with myocardial infarction [26]. An epidemiologic survey of rural agricultural workers by Allazov [27] reported a high incidence of infectious inflammatory lesions of the liver among those workers compared with a control population.
The biochemical markers of occupational exposure to pesticides include serum urea, creatinine, bilirubin, aspartate amino transferase and alanine amino transferase. The significantly increased ALT, AST, and ALP found in pesticide sprayers of mango plantation of Malihabad as compared with the control group indicate liver tissue damage following high exposure to pesticides. Increased levels of transaminase enzymes and ALP have been reported in several OP and carbamate pesticides exposed populations [28,29]. These findings are supported by studies carried out in rats rats fed with OP pesticides and endrin [30-32]. In vitro studies have shown that AST, ALT, lactate dehydrogenase and AChE are inhibited by glyphosate and paraquat [33]. Mostafa et al. [33] have reported that mice treated with carbofuran have elevated levels of serum transaminases and blood urea nitrogen, indicating toxicity to hepatic and renal structures.
The increased serum AST, ALT, and ALP in our study could be due to increased malondialdehyde MDA] formation. MDA formed by hepatic lipid peroxidation is toxic, mutagenic, and inactivates enzymes by altering lysine residues. Increased MDA and decreased activities of CAT, SOD, and GSH levels were found in rats following a deltamethrin oral dose LD50150 mg/kg] for 30 days. The pathogenesis may be through free radical [O2-] formation as deltamethrin undergoes metabolism in the liver via hydrolytic ester cleavage and oxidative pathways [34]. The metabolism of various drugs, chemicals, and pesticides primarily takes place in the liver [35]. Several studies have shown that a high intake of drugs, chemicals, or higher pesticides exposure is responsible for onset of biochemical and histopathological changes in liver involving cytoplasminolysis, nuclear pyknosis, and necrosis leading to complete exhaustion and disintegration of hepatocytes [36]. Several studies have reported that pesticides exposure decreases protein biosynthesis in the liver [37,38]. In the present study, however, serum total protein, globulin, and the A/G ratio were not significantly altered and serum albumin was only slightly decreased P<0.05] as compared with control participants. The lack of a significant alteration in protein levels in this study might be due to a high intake of a non-vegetarian diet.
In the present study, almost 60% of pesticide sprayers were receiving alternate day non-veg and protein-rich food in their diet. Most sprayers were also the owners of mango orchards of more than five hectors. Therefore, they are economically very strong and mainly due to social customs are taking non-vegetarian food in their diet. The serum bilirubin was markedly increased in pesticide sprayers of mango plantation. Mild increase of bilirubin in the blood may be caused by hemolysis or the increased breakdown of erythrocytes.
The peroxidation of lipids in biological membranes is initiated by chemical agents, ionizing radiation, and pesticides which in turn generates MDA [39,40]. Erythrocytes serves as excellent models for taking up studies related with the interaction of xenobiotics with biomembranes as even the slightest damage can cause hemolysis and leakage of cellular constituents [41]. Increased hemolysis due to cellular injury by many xenobiotics has been associated with the lipid peroxidation of erythrocytes [40-42]. Moreover, in severe OP insecticide poisoning transient hyperglycemia and glycosuria have often been noticed. The significantly increased blood glucose level found in sprayers could be due to increased catecholamine levels. Pesticides affect the adrenal gland and stimulate the secretion of epinephrine, which in turn stimulates glycogenolysis and increases blood glucose while depleting glycogen in the liver [43]. Diazinon is a widely used OP compound that is associated with toxicity in humans, rats and other animals [44-48]. These compounds induce hyperglycemia and inhibit cholinesterase [49,50]. In diazinon-treated 10 mg/kg, 20 mg/kg, and 40 mg/kg, [i.p.] hyperglycemic animals, the glycogen content of the brain depleted the activities of glycogen phosphorylase [51]. Enzymes, phosphoglucomutase, and hexokinase were significantly increased whereas the activity of glucose-6- phosphatase remained unchanged. The treatment with diazinon increased lactate dehydrogenase activity. The induced changes may compensate for the energy requirement of the stimulatory effects caused by diazinon [51].
The decreased serum cholesterol level seen in pesticide sprayers of Malihabad, Lucknow might be due to regular farm activity. Regular physical exercise has favorable effects on serum lipids and lipoproteins level especially in reducing total cholesterol [52]. The finding of increased serum creatinine and blood urea levels in these pesticide sprayers of mango plantations agrees with the results of prior studies reporting subtle nephro-toxic changes in workers occupationally exposed to pesticides [53]. Acute renal insufficiency has reported in malathion-exposed sprayers [54]. Rhabdomyolysis, a well-known complication of severe poisonings, appears to be relatively frequent in severe OP pesticide intoxication, including diazinon. If not treated correctly, it may lead to acute renal failure and paresis in later stages [55]. Due to the contraction of the smooth muscle of the bladder symptoms may include strangury and frequent involuntary urination. When exposed to a mixture of pesticides monocrotophos, hexachlorocyclohexane, and endosulfan] at varying intervals, histopathologic changes were observed in the liver, kidney, and muscles of normal, protein-malnourished, diabetic as well as both protein-malnourished and diabetic albino rats. The examination in the pesticides-exposed rats revealed hepatotoxic, nephrotoxic, and muscular necrotic effects. In case of protein-malnourished and diabetic animals or in animals with both these disorders the toxicity was aggravated and severe [55,56].
Therefore, based on past reports, the present study indicates that the slight nephro-toxicity in pesticide sprayers of Malihabad, mango plantations due to the use of various pesticides used. Compared with the control group, all hematologic parameters studied here in pesticide sprayers study group] decreased, except for the WBC, which increased. Several studies have reported decreased Hb levels in humans exposed to OP pesticides [60-61]. Due to the disruptive effect of the pesticides on the bone marrow, which produces red and white blood cells there was a decreased Hb and RBC. The hematology assay provides information on bone marrow activity and on the status of other organs governing the synthesis, function, and destruction of components of the circulatory system. Λ disturbance in erythrocytes parameters often reflects an imbalance between the production and loss of RBC [58].
In the present study, the decrease in erythrocytes, PCV%, and the Hb concentration may be due to the injury to hematopoietic stem cells by various pesticides used in Malihabad, mango plantations. For example, the adverse effect of parathion is that it alters the cloning potential of bone marrow precursor stem cells [59]. Therefore, a detailed study is required to know at which level the pesticides studied here affected and altered the hematologic parameters. Pesticides and their residues can inhibit many steps of heme biosynthesis. The anemia and shortening of the life span of circulating erythrocytes reported in pesticides-exposed workers might be due to the interference of Hb biosynthesis [60]. Hb, hematocrit, and RBC were slightly reduced in mild and high dose males guinea pigs, whereas in high dose females it was moderately [37,61]. The hematologic parameters were not affected severely, except for the WBC. A recent study from China reported that despite normal blood cholinesterase activities, acute hemolysis, jaundice, renal function impairment, and leukocytosis occurred from incidental exposure to trichlorfon, an OP pesticide . Therefore, the markedly increased WBC count found in the present study is likely due to the pesticides and/or their residues.
Acknowledgement
We express our gratitude to Indian Institute of Toxicology Research IITR, [Lucknow] for collaboration, assistance and invaluable support in this study.
Conflict of Interest
The authors declare that there is no conflict of interest regarding this manuscript.
References
- Quazi S, Jamal F, Rastogi SK (2012) Effect of organophosphorus on biochemical parameters on agricultural workers. Asian J Biochem 7: 37-45.
- Indira DP, Bellamy R, Sunder SP (2007) Facing hazards at work-Agricultural workers and pesticide exposure in Kuttanad, Kerala. South Asian Network for Development and Environmental Economics 19: 1-4.
- World Health Organization (1990) Public Health Impact of Pesticides used in Agriculture, Geneva.
- World Health Organization (1992) Environmental Health criteria. No. 130, Endrin. Geneva.
- World Health Organization (1993) Environmental Health criteria. No. 145, Methyl Parathion. Geneva.
- Patil JA, Patil AJ, Govindwar SP (2003) Biochemical effects of various pesticides on sprayers of grape gardens. Indian J ClinBiochem 18: 16-22.
- Helsinki Declaration (1996) Amended by World Medical Assembly, Venice, Italy, 1983. Br Med J 313: 1448-49.
- Knedel B, Boettger R, Klin W (1967) Arbeitsgruppe enzyme der DeutschonGesellschall fur, KlinischeChemie 1989 Mitt DtschGesKlinChemi PS 20: 123-124.
- Anderson Hc, Mccarty M (1950) Determination of C-reactive protein in the blood as a measure of the activity of the disease process in acute rheumatic fever. Am J Med 8: 445-455.
- Lothar T (1998) Clinical Laboratory Diagnostics: Use and Assessment of Clinical Laboratory Results Frankfurt/Main: TH-Books Verlagsgeselschaft1: 1727.
- (1974) The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. Scand J Clin Lab Invest 33: 291.
- Henry RJ, Cannon DC, Winkelman JW (1974) Method for increasing shelf-life of a serum conjugated bilirubin reference composition and composition produced thereby. Clinical Chemistry, Principles and Techniques, Second Ed., New York, NY, USA. Harper Row Publishers 1038-1070.
- Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. ClinChimActa 31: 87-96.
- Varely H (1980) Practical clinical Biochemistry 1: 1012.
- Varley H (1975) Practical Clinical Biochemistry1: 453.
- Kassirer JP (1971) Clinical evaluation of kidney function--glomerular function. N Engl J Med 285: 385-389.
- Larsen K (1972) Creatinine assay by a reaction-kinetic principle. ClinChimActa 41: 209-217.
- Young DS, Pestaner LC, Gibberman V (1975) Effects of drugs on clinical laboratory tests. ClinChem 21: 1D-432D.
- Tarbutton PN, Gunter CR (1974) Enzymatic determination of total cholesterol in serum. ClinChem 20: 724.
- Schmidt-Kehl L (1927) Blood changes in chronic lead poisoning. ArchivFuer Hygiene 98: 1-22.
- Costa LG (1988) Interactions of neurotoxicants with neurotransmitter systems. Toxicology 49: 359-366.
- Avashia BH (1987) Is carbaryl as safe as its reputation? Am J Med 83: 1168-1169.
- Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87: 245-254.
- O'Malley M (1997) Clinical evaluation of pesticide exposure and poisonings. Lancet 349: 1161-1166.
- Lockridge O, Bartels CF, Vaughan TA, Wong CK, Norton SE, et al. (1987) Complete amino acid sequence of human serum cholinesterase. J BiolChem 262: 549-557.
- Anderson HC, Mccarty M (1950) Determination of C-reactive protein in the blood as a measure of the activity of the disease in acute rheumatic fever. Am J Med 8:445-455.
- Allazov S (1994) The role of pesticides in the occurrence of pathological changes in the kidneys. UrolNefrol (Mosk) : 42-44.
- [No authors listed] (1991) Occupational exposures in insecticide application, and some pesticides. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 16-23 October 1990. IARC MonogrEvalCarcinog Risks Hum 53: 5-586.
- Michalek JE, Ketchum NS, Longnecker MP (2001) Serum dioxin and hepatic abnormalities in veterans of Operation Ranch Hand. Ann Epidemiol 11: 304-311.
- Bushnell PJ, Padilla SS, Ward T, Pope CN, Olszyk VB (1991) Behavioral and neurochemical changes in rats dosed repeatedly with diisopropylfluorophosphate. J PharmacolExpTher 256: 741-750.
- Kagan J (1971) Topical questions about the toxicology of phosphorganic insecticides. Ernahrungsforschung 14: 503-504.
- Luckens MM, Phelps KI (1969) Serum enzyme patterns in acute poisoning with organochlorine insecticides. J Pharm Sci 58: 569-572.
- Mostafa IY, Zayed SM, Farghaly M, Mahdy F (1992) Bioavailability to rats and toxicity in mice of carbofuran residues bound to faba beans. J Environ Sci Health B 27: 399-405.
- Manna S, Bhattacharyya D, Mandal TK, Das S (2005) Repeated dose toxicity of deltamethrin in rats. Indian J Pharmacol 37: 160-164.
- Piñeiro-Carrero VM, Piñeiro EO (2004) Liver. Pediatrics 113: 1097-1106.
- Ram RN, Singh SK (1988) Carbofuran-induced histopathological and biochemical changes in liver of the teleost fish, Channapunctatus (Bloch). Ecotoxicol Environ Saf 16: 194-201.
- Bomhard E, Loser E, Schilde B (1981) E605- Methyl Methyl parathion chronic toxicological studies in rats 2 year feeding trial Wuppartal -Elberfeld Bayer AG. Institute of toxicology. IPCS 145: 155.
- Subbotina SG, Belonozhko GA (1968) The effect of Sevin on the thermoresistance and fractionary protein content of blood serum. Gig PrimenToxicolPesticKlinOtrav 442-444.
- Zavodnik IB, Lapshina EA, Zavodnik LB, Soszyaski M, Bartosz G, et al. (2002) Hypochlorous acid-induced oxidative damage of human red blood cells: effects of tert-butyl hydroperoxide and nitrite on the HOCl reaction with erythrocytes. Bioelectrochemistry 58: 127-135.
- John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J NutrBiochem 12: 500-504.
- Kumar SS, Sika HC, Saxena J, Zweig G (1975) Membrane damage in human erythrocyte caused by captan and captafol. Pest BiochemPhysiol 5: 338-347.
- Altuntas I, Delibas N, Sutcu R (2002) The effects of organophosphate insecticide methidathion on lipid peroxidation and anti-oxidant enzymes in rat erythrocytes: role of vitamins E and C. Hum ExpToxicol 21: 681-685.
- Varshneya C, Singh T, Sharma LD, Bahga HS, Garg SK (1992) Immunotoxic responses of cypermethrin, a synthetic pyrethroid insecticide in rats. Indian J PhysiolPharmacol 36: 123-126.
- Hayes WJ Jr (1963) Clinical handbook on economic poisons. Emergency information for treating poisonings. U.S. Public Health Service Publication No. 476. Washington DC.
- Kessler H, Mracek JF (1973) Nonfatal accidental organophosphate pesticide intoxication in seven inmates of a correctional institution. J Med Assoc State Ala 42: 775-781.
- Kar PP, Matin MA (1971) Duration of diazinon induced changes in the brain acetycholine of rats. Pharmacol Res Commun 3: 351-354.
- Bruce RB, Howard JW, Elsea JR (1955) Pesticide toxicity, toxicity of O, Odiethyl O-2-Isopropyl-6-methyI-4-pyrimidyl] phosphorothioatediazinon]. J Agric Food Chem 3: 1017-1021.
- Holmstedt B (1959) Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev 11: 567-688.
- Dybing O, Sognen E (1958) Hyperglycaemia in rats after diazinon and other cholinesterase inhibitors. ActaPharmacolToxicol (Copenh) 14: 231-235.
- Weiss LR, Bryant J, Pitzhugh CG (1960) Blood sugar levels following acute poisoning with parathion and seven in three species. ToxicolApplPharmacol 6: 363-364.
- Matin MA, Husain K (1987) Changes in cerebral glycogenolysis and related enzymes in diazinon treated hyperglycaemic animals. J ApplToxicol 7: 131-134.
- Sutherland WH, Woodhouse SP (1980) Physical activity and plasma lipoprotein lipid concentrations in men. Atherosclerosis 37: 285-292.
- Al-Qarawi AA, Adam SE (2003) Effects of malathion plus superphosphate or urea on Najdi sheep. Vet Hum Toxicol 45: 3-6.
- Abend Y, Goland S, Evron E, Sthoeger ZM, Geltner D (1994) Acute renal failure complicating organophosphate intoxication. Ren Fail 16: 415-417.
- Benjamin N, Kushwah A, Sharma RK, Katiyar AK (2006) Histopathological changes in liver, kidney and muscles of pesticides exposed malnourished and diabetic rats. Indian J ExpBiol 44: 228-232.
- Bhatnagar VK, Sharma RP, Malviya AN (1980) Effects of pesticidal stress amongst pesticide factory workers in Agra, India. Public Health 94: 375-378.
- Merchant MA, Mod DN (2004) Acute and chronic effects of aspirin on hematological parameters and hepatic ferritin expression in mice. Indian J Pharmacol 36: 226-230.
- Gallicchio VS, Casale GP, Bartholomew PM, Watts TD (1987) Altered colony-forming activities of bone marrow hematopoietic stem cells in mice following short-term in vivo exposure to parathion. Int J Cell Cloning 5: 231-241.
- Ray PK, Prasad AK (1992) Pollution and health. New Delhi: Wiley Eastern Ltd.
- Daly IWA (1983) Evaluation report for USEPA of a two-year chronic feeding study of methyl parathion in rats. East Millstone, New Jersey, Biodynamics Inc. Unpublished report, submitted to W.H.O., by Bayer, AG, Leverkusen, Germany.
- Wu ML, Deng JF (2009) Acute hemolysis caused by incidental trichlorfon exposure. J Chin Med Assoc 72: 214-218.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 15976
- [From(publication date):
December-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 14700
- PDF downloads : 1276