The Nandrolone Effect on Cardiac Muscle of Adult Male Albino Rat and the Possible Role of Nigella sativa: Light and Electron Microscopic Studies
Received: 25-Aug-2018 / Accepted Date: 04-Sep-2018 / Published Date: 11-Sep-2018
Keywords: Cardiac muscle; Intercalated disc; Nandrolone; N. sativa
Abbreviations
ND: Nandrolone Decanoate; NS: Negella Sativa; TQ: Thymoquinone; BS: Black Seed; H and E: Hematoxylin and Eosin; AAS: Anabolic Androgenic Steroids; DAB: Diamiobenzidine; GPx: Glutathione Peroxidase; GSH: Growth Stimulating Hormone; ROS: Reactive Oxygen Species, SOD: Superoxide Dismutase
Introduction
Nandrolone decanoate (ND) is a synthetic derivative of testosterone. It is anabolic androgenic steroids (AAS) administrated by athletes and adolescents and considered as one of the most commonly AAS. ND is supposed to promote muscle mass increase and improves physical appearance as well as sporting performance [1].
ND abuse is often associated with serious adverse effects, interfering with many body systems such as the musculoskeletal system, the endocrine system and the reproductive system [2]. Also, ND may suppress the hypothalamic pituitary gonadal axis resulting in diminished endogenous testosterone production [3].
vNandrolone can be administrated by many routs such as oral pills, injectable steroid, creams and gel. Nandrolone was taken as a supportive therapy to increase body weight in some pathological conditions as cachexia that associated with some chronic diseases. Also, it was administrated to treat anaemia. But now, it is widely used mostly by young men for non-medical purposes, to improve their body building [4,5].
Nandrolone has many adverse effects especially when used by healthy people. Many serious adverse effects can be resulted from longterm intake of excessive doses of anabolic steroids.
Hypercholestrolemia, hepatic dysfunction and hypertension are the most common adverse effects [6]. Cardiovascular diseases are the most important side effects, as they increased the incidence of young male body builder’s death.
Nandrolone decanoate also reported to increase in atherosclerosis, tachycardia, cardiac dysfunction, arrhythmia, premature acute ischaemic heart diseases, myocardial infarction and even sudden death. Other effects as depression, nervousness, testicular atrophy and liver damage were also reported [7,8].
The use of medicinal plants for the prevention of cardiovascular diseases has been increasing recently.N. sativa(NS), also known as black cumin or black seed, has been proved to possess blood pressurelowering effects in animals as well as humans [9]. NS has been used in medicinal purposes for centuries in the Middle East, India and Northern Africa [10]. Also, it has been widely used to treat nervous system diseases such as memory impairment, epilepsy, neurotoxicity and pain.
The N. sativa therapeutic properties are due to the presence of thymoquinone (TQ) and polyphenols which are the essential oil major bioactive components [11]. In traditional medicine, NS seeds are used in the treatment of different illnesses like obesity, hypertension, gastrointestinal problems, diarrhoea, back pain, cardiac diseases, bronchitis, asthma, sexual diseases, rheumatoid arthritis and skin disorders [12,13].
The aim of this work is to study the possible histological alterations that may occur in the cardiac muscle structure of adult male albino rats receiving nandrolone and the possible role of N. sativa supplementation.
Materials and Methods
Drugs and chemicals
Nandrolone decanoate (Deca-Durabolin): It was in the form of ampoules (25 mg/ ampoule). It was provided by Nile Company pharmaceuticals-Cairo.
N. sativa : Black seed (BS) was purchased from Kahira Pharm. and Chem. Ind. Co., Cairo, Egypt. Black seed is present as soft gelatin capsules (500 mg). The capsule was dissolved in 10 cm of vegetable oil and taken at dose of 50 mg/kg body weight via gastric gavage [14].
Animals
Thirty adult Wistar male albino rats weighing (200-250 gm.) were purchased from the Breading Animal House, Faculty of Medicine, Zagazig University, Zagazig, Egypt. They were housed at room temperature under standard laboratory conditions. They were maintained on standard laboratory food and water ad libitum during the period of the experiment.
All procedures of the experiment were approved and carried out according to the guidelines of the Institutional Animal Care and Use Committee accepted by Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Experimental design
The animals were classified into three equal Groups (each contains 10 rats):
Group I: control Group: Included 10 animals which subdivided into two equal subgroups:
Subgroup Ia: Received vegetable oil.
Subgroup Ib: (N. sativa treated): N. sativa was given at a dose of 50 mg/kg body weight per day via gastric gavage for four weeks [14].
Group II: Nandrolone treated Group: Included 10 rats received nandrolone by intra-muscular injection at a dose of 10 mg/kg/week for four weeks [15].
Group III: Nandrolone treated and N. sativa supplemented Group: Composed of 10 rats, each received nandrolone and N. sativa simultaneously at a dose similar to the previous Groups. 24 hour after the last injection, all the Groups were sacrificed.
At the end of the experiment, rats were anaesthetized by intraperitoneal injection of pentobarbitone sodium 60 mg/kg body weight. A midline incision was done on the anterior aspect of the chest, sternocostal junctions were cut.
Samples from the heart fixed in 10% neutral buffered formalin and processed for preparation of paraffin sections for histological: (Hematoxylin and Eosin) [16], van Geison stain [17]. For ultrastructural study, specimens were immediately fixed in 2.5% phosphate-buffered glutaraldehyde (pH 7.4). Thereafter, they were postfixed in 1% osmium tetroxide in the same buffer at 4°C, dehydrated, and embedded in epoxy resin [18].
Methods
Histological study
Paraffin sections (5 μm thick) stained with (H and E) for examination of overall morphology and Van Geison for examination of collagen fibers.
Immunohistochemical study: Immunohistochemical reaction for Caspase 3 protein was done by using streptavidin-biotin complex immunoperoxidase system. Serial sections of paraffin-embedded specimens were deparaffinized on charged slides. The sections were incubated in 0.1% hydrogen peroxide for 30 min to block the endogenous peroxidase, then incubated with the primary antibody. The primary antibody used for caspase-3 was ready-to-use rabbit polyclonal antibody (CAT-No: RB-3425-R2). The slides were incubated with the secondary anti-rabbit antibody versal kits (Zymed laboratories), diluted 1: 200 for 30 minutes, staining was completed by incubation with chromogen, called diamiobenzidine (DAB). Mayer’s hematoxylin was used as a counterstain [19].
Ultrastructural study: Semithin sections 1 μm thick were stained with 1% toluidine blue for light microscopic examination. Ultrathin sections were stained with uranyl acetate and lead citrate [18], examined and photographed using (JEOL JEM -2100) Transmission Electron Microscope (Jeol Ltd, Tokyo, Japan) in Electron Microscope Research Unit, Faculty of Agriculture, Mansoura University, Egypt.
Histomorphometric analysis
The image analyzer computer system Leica Qwin 500 (Leica Ltd, Cambridge, UK) at the Image Analyzing Unit of Pathology Department, Faculty of Dentistry, Cairo University, Egypt, was used to measure the area percent of collagen fibers and caspase 3 immunoreaction. The area percent was measured using the interactive measure menu. The measuring frame of a standard area equal to 118476.6 mm² was chosen so that the brown positive immune reaction could be seen and masked by blue binary colour to be measured. Examination of ten readings from five non-overlapping sections from each rat of all Groups was done.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 13.00 (Chicago, Illinois, USA). Statistical significance was determined by one-way analysis of variance for differences between the means of different Groups. Further analysis was carried out using the post-hoc test to compare the parameters between the different Groups with each other. Probability of P less than 0.05 was considered statistically significant.
Histological Results
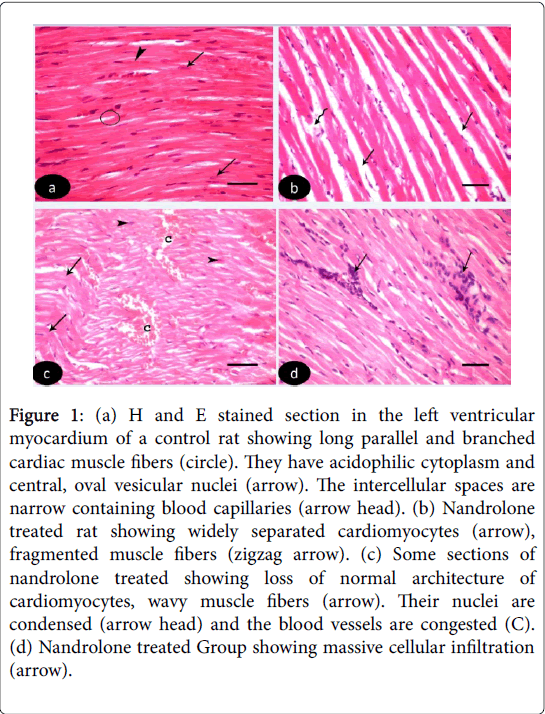
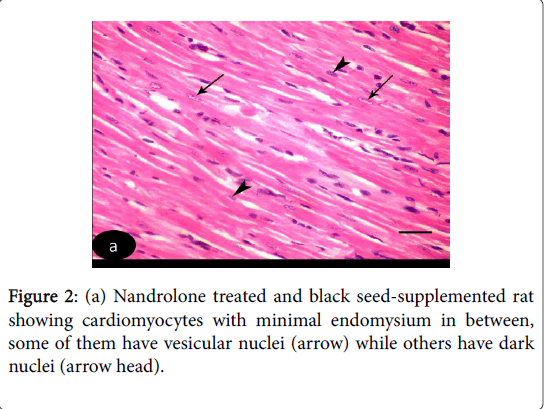
H and E stained sections of left ventricular myocardium of both control Groups Ia and Ib revealed nearly similar results. H and E stained sections of left ventricle of control Groups revealed cardiomyocytes with acidophilic sarcoplasm and central oval vesicular nuclei with clear perinuclear regions. The intercellular spaces are narrow containing blood capillaries (Figure 1 (a-d)). Nandrolone- Treated Group, sections revealed focal variable changes in the cardiac muscle. Some of them showed separated and degenerated muscle fibers with widening of the endomysium. The cytoplasm of the cardiomyocytes appeared deeply acidophilic with condensation and flattening of some nuclei. Others affected areas revealed wavy muscle fibers with loss of their normal architecture and congested blood vessels. Massive cellular infiltration was noticed between the muscle fiber (Figure 1 (b-d)). Nandrolone treated and black seed supplemented Group, sections of the left ventricular myocardium revealed minimal changes in their histological features. Little intercellular spaces were observed in between muscle fibers. Some cardiomyocytes nuclei were vesicular and others appeared dark (Figure 2).
Figure 1: (a) H and E stained section in the left ventricular myocardium of a control rat showing long parallel and branched cardiac muscle fibers (circle). They have acidophilic cytoplasm and central, oval vesicular nuclei (arrow). The intercellular spaces are narrow containing blood capillaries (arrow head). (b) Nandrolone treated rat showing widely separated cardiomyocytes (arrow), fragmented muscle fibers (zigzag arrow). (c) Some sections of nandrolone treated showing loss of normal architecture of cardiomyocytes, wavy muscle fibers (arrow). Their nuclei are condensed (arrow head) and the blood vessels are congested (C). (d) Nandrolone treated Group showing massive cellular infiltration (arrow).
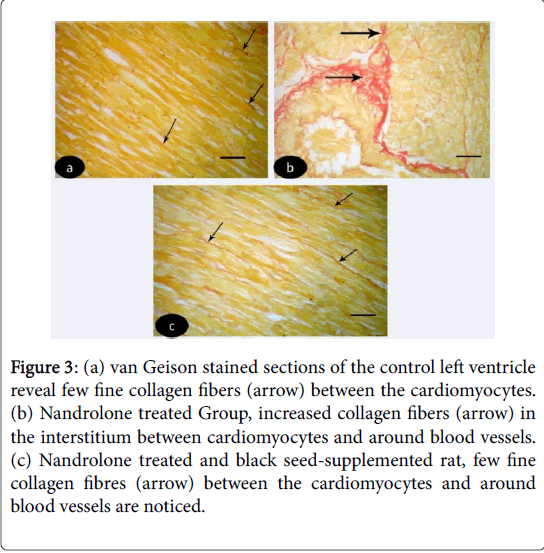
Van Geison stained sections of the control left ventricle revealed few fine collagen fibers between the cardiomyocytes (Figure 3 (a-c)). While in nandrolone treated rats, increased collagen fibers were noticed in the interstitium between cardiomyocytes and around blood vessels (Figure 3). In nandrolone treated and black seed supplemented rats, few fine collagen fibers between the cardiomyocytes and around blood vessels were noticed (Figure 3).
Figure 3: (a) van Geison stained sections of the control left ventricle reveal few fine collagen fibers (arrow) between the cardiomyocytes. (b) Nandrolone treated Group, increased collagen fibers (arrow) in the interstitium between cardiomyocytes and around blood vessels. (c) Nandrolone treated and black seed-supplemented rat, few fine collagen fibres (arrow) between the cardiomyocytes and around blood vessels are noticed.
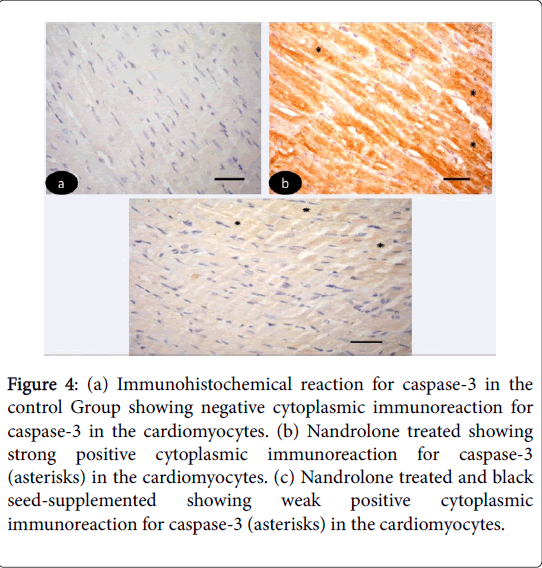
Immunohistochemical results: Immunohistochemical-stained sections of the control left ventricular myocardium revealed negative cytoplasmic reaction for caspase-3 in the cardiomyocytes (Figure 4 (ac)). While, in nandrolone treated strong positive cytoplasmic reaction for caspase-3 in the cardiomyocytes (Figure 4). On the other hand in nandrolone treated and black seed-supplemented subgroup revealed weak positive cytoplasmic immunoreaction for caspase-3 in the cardiomyocyte (Figure 4).
Figure 4: (a) Immunohistochemical reaction for caspase-3 in the control Group showing negative cytoplasmic immunoreaction for caspase-3 in the cardiomyocytes. (b) Nandrolone treated showing strong positive cytoplasmic immunoreaction for caspase-3 (asterisks) in the cardiomyocytes. (c) Nandrolone treated and black seed-supplemented showing weak positive cytoplasmic immunoreaction for caspase-3 (asterisks) in the cardiomyocytes.
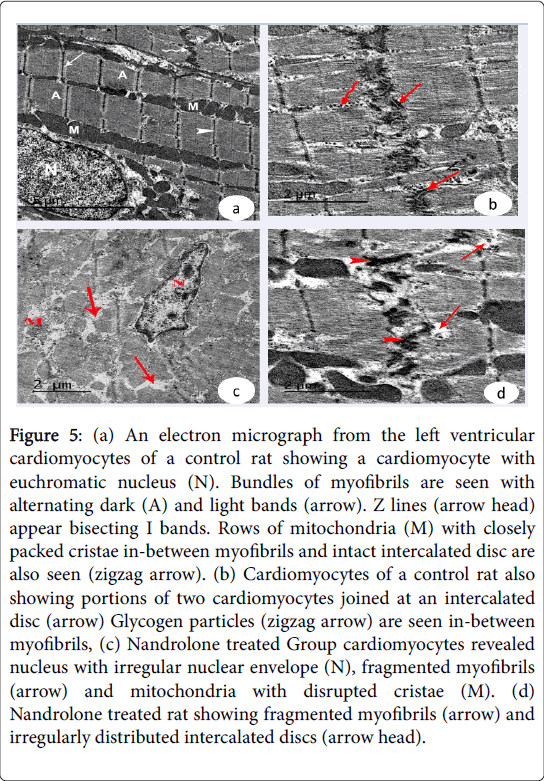
Ultrastructural results: Examination of the ultrathin sections of the left ventricular cardiomyocytes of the control Groups revealed euchromatic nuclei with dispersed euchromatin. The cardiomyocytes joined by intact intercalated discs with their transverse and lateral portion. The sarcoplasm showed bundles of myofibrils with rows of mitochondria with closely packed cristae in between them. Their myofibrils had alternating dark A and light I bands. Z lines appeared bisecting I band. Glycogen granules could be detected in between myofibrils (Figure 5 (a and b)). In nandrolone treated Group.
Figure 5: (a) An electron micrograph from the left ventricular cardiomyocytes of a control rat showing a cardiomyocyte with euchromatic nucleus (N). Bundles of myofibrils are seen with alternating dark (A) and light bands (arrow). Z lines (arrow head) appear bisecting I bands. Rows of mitochondria (M) with closely packed cristae in-between myofibrils and intact intercalated disc are also seen (zigzag arrow). (b) Cardiomyocytes of a control rat also showing portions of two cardiomyocytes joined at an intercalated disc (arrow) Glycogen particles (zigzag arrow) are seen in-between myofibrils, (c) Nandrolone treated Group cardiomyocytes revealed nucleus with irregular nuclear envelope (N), fragmented myofibrils (arrow) and mitochondria with disrupted cristae (M). (d) Nandrolone treated rat showing fragmented myofibrils (arrow) and irregularly distributed intercalated discs (arrow head).
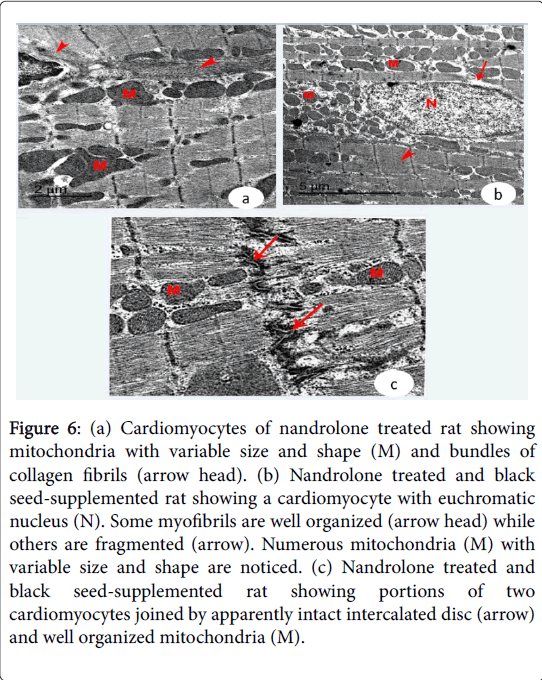
The cardiac muscle exhibited fragmentation and lysis of some myofibrils and pyknotic nuclei with irregular nuclear envelope. Increased collagen fibers were noticed in the endomysium. Irregularly distributed intercalated discs were demonstrated in-between the adjacent cardiomyocytes (Figure 5 (c and d) and Figure 6a). Nandrolone treated and black seed supplemented Group, cardiomyocytes contained large euchromatic nuclei with dispersed euchromatin. Some myofibrils were well organized while others were degenerated. Numerous mitochondria were demonstrated in-between the myofibrils. The cardiomyocytes were joined by intact intercalated discs with their transverse and lateral portion (Figure 6 (b and c)).
Figure 6: (a) Cardiomyocytes of nandrolone treated rat showing mitochondria with variable size and shape (M) and bundles of collagen fibrils (arrow head). (b) Nandrolone treated and black seed-supplemented rat showing a cardiomyocyte with euchromatic nucleus (N). Some myofibrils are well organized (arrow head) while others are fragmented (arrow). Numerous mitochondria (M) with variable size and shape are noticed. (c) Nandrolone treated and black seed-supplemented rat showing portions of two cardiomyocytes joined by apparently intact intercalated disc (arrow) and well organized mitochondria (M).
Histomorphometric and statistical results
Highly statistically significant increase in the mean area % of collagen fibres was revealed in Group II when compared with Group I and Group III. No statistical significant difference was detected between Group I, when compared with III (Table 1). Highly statistically significant increase in the mean area % of caspase 3 immunoreaction was noticed in Group II when compared with Group I, Group III. No statistical significant difference was detected between Group I, when compared with III (Table 2).
| Parameters | Mean ± SD | F | P-value |
|---|---|---|---|
| Group I | 12.31 ± 7. 3 | 90 | ≤ 0.0001** |
| Group II | 55.12 ± 10.5 | ||
| Group III | 14.81 ± 5.2 |
Table 1: Area % for collagen fibres in different studied Groups.
| Parameters | Mean ± SD | F | P-value |
|---|---|---|---|
| Group I | 17.03 ± 5.7 | 32 | ≤ 0.0001** |
| Group II | 45 ± 13.9 | ||
| Group III | 19.36 ± 0.31 |
Table 2: Area % of caspase 3 immunoreaction in different studied Groups.
Discussion
AAS; nandrolone; although forbidden in sports, are still widely used by professional and recreational athletes who intend to quickly gain muscle mass and improve cardiovascular system performance [20]. Their anabolic actions are mainly due to increase synthesis and reduce the degradation of the muscle proteins as mentioned by [21,22]. In the present work, nandrolone abuse induces many cardiac changes as regard structure, ventricular thickness, size, as well as heart connective tissue content. These results are in accordance with previous findings which stated that, chronic nandrolone abuse had great effect on many organs function and structure [23-28]. Others added that the effect of nandrolone is dose dependant [29]. In the current work, nandrolone treated Group (Group II) showed marked histological, ultrastructural and histochemical changes in cardiac muscle when compared with control Group (Group I). Minimal changes were noticed when nandrolone and N. sativa are co-administered (Group III).
In the present study, H and E-stained sections of the left ventricular myocardium revealed focal variable changes in the cardiac muscle. The less apparently affected areas showed separated and degenerated muscle fibers with widening of the endomysium. Other focal changes revealed hypertrophied muscle fibers. The cytoplasm of the cardiomyocytes appeared deeply acidophilic with condensation and flattening of some nuclei. The more affected areas revealed wavy muscle fibers with loss of their normal architecture, condensed nuclei and congested blood vessels the same results were obtained by Soliman et al. [30]. The antioxidant system includes enzymes (superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase) as well as non-enzymatic antioxidants (alpha-tocopherol and reduced glutathione (GSH)) are responsible for heart protection as they scavenge the unpaired electrons of free radicals and prevent damage propagation [31]. Nandrolone administration has cardiotoxic effects because it disturbs normal antioxidant and induce oxidative stress via significant reduction in heart antioxidant capacity (reducing GPx, glutathione reductase and super oxide dismutase) activities leading to free radicals liberation [32,33].
Oxidative stress is defined as a disruption of the prooxidant antioxidant balance in favor of the prooxidant, leading to potential damage which may alter all function through changes in intracellular calcium or intracellular pH and eventually can lead to cell death [34]. Rodrigo et al. [35] found that ROS which released as a result of oxidative stress activate some transcription factors. The unfavourable consequences of this activation include inflammation, fibrosis or apoptosis. Nitric oxide is considered one of the most important signalling molecules regulating cardiovascular function. It regulates blood pressure by dilating blood vessels and inhibits platelet aggregation and leukocyte adhesion. It also inhibits cell proliferation in vascular smooth muscle and subsequently regulates myocardial scar formation [36,37]. In the current work, Van Geison stained sections of the nandrolone treated Group (Group II) showed increased collagen fibers the interstitium between cardiomyocytes and around blood vessels. The same findings were obtained by Franquni et al. [38] and Soliman et al. [30] who found that nandrolone administration cause a 10 fold increase in heart collagen.
Parssinen et al. [39] added that this effect tended to be dosedependent. These short-term changes in collagen metabolism may be explained by increased anabolic effects in muscle or secondary to increased working capacity. In the current work, electron microscope examination of the nandrolone treated Group confirmed the results observed by light microscope. The cardiac muscle exhibited destruction, fragmentation and lysis of some myofibrils and pyknotic nuclei with irregular nuclear envelope. Increased collagen fibers were noticed in the endomysium. Irregularly distributed intercalated discs were demonstrated in-between the adjacent cardiomyocytes. The same results were obtained by Soliman et al. [30]. Also, these findings are in accordance with Cavasin et al. [40] and Golestani et al. [41] who found anabolic steroids intake caused marked injuries in the rodent heart and led to sarcomere disruption, mitochondrial damage and apoptosis. Tokunaga et al. [42] stated that the free radicals facilitate the release of lysosomal enzymes into the cytosol with subsequent oxidation of the protein architecture of the cells causing their fragmentation. Swapnila et al. [43] added that the production of free radicals can damage DNA strands directly and induce apoptosis and expression of p53 in hepatocytes. Free radicals known to stimulate the peroxidation of membrane phospholipids causing severe damage [44].
In the present study, negative cytoplasmic immunoreaction for caspase -3 was noticed in the cardiomyocytes of control Group. While, in nandrolone treated Group strong positive cytoplasmic immunoreaction for caspase-3 was reaveled in the cardiomyocytes. On the other hand, in nandrolone treated and black seed-supplemented Group revealed weak positive cytoplasmic immunoreaction for caspase-3. Nandrolone abuse has been associated with thrombosis and arteriosclerosis, both of which predispose to myocardial ischemia and infarction. However, there are reports of sudden cardiac death in the absence of thrombus and atheroma following anabolic steroid use [45]. Programmed cell death (apoptosis) has been implicated in normal biological processes and in the pathogenesis of several diseases in humans. The characterization of genes incorporated in apoptosis has been pursued intensively and has been identified as two major classes of genes, one represented by the Bcl2 family and the other by the Caspase family. Caspases are a family of proteases that cleave their target substrates at specific peptide sequences During apoptosis, activation of caspases, variably induced by nuclear, metabolic, or externally activated stimuli, takes place in a cascade fashion, leading to nuclear engulfment and cell death [46-50].
Light and electron stained sections of rat cardiac muscle fibers of Group III ( N. sativa and nandrolone) revealed marked protection of myofibers against the deleterious changes induced by nandrolone, however, minimal changes in their histological features were noticed. Little intercellular spaces and mild congested blood vessels were observed in between muscle fibers. Some cardiomyocytes nuclei were vesicular and others appeared dark. Van Geison stained sections of nandrolone treated and black seeds supplemented rats revealed few fine collagen fibers between the cardiomyocytes and around blood vessels. Highly statistically significant increase in the mean area % of collagen fibers was detected in Group II when compared with the control (Group I) and NS-treated (Group III) Groups. Highly statistically significant increase in the mean area percent of caspase 3 immunoreaction was noticed in Group II when compared with Group I and Group III. No statistically significant difference was detected between Group I and Group III. In inflammation-induced fibrosis, NS reduced cardiac fibrosis and inflammatory markers (serum and tissue) possibly through its antioxidant activity. The cardiovascular health benefits of NS have been established in several studies. These include hypolipidemic, antidiabetic, hypotensive, antiplatelet, and bradycardiac effects and improving endothelial function [51-53].
Thymoquinone, the biologically active compound of NS seeds, reduces liver and pulmonary fibrosis and inflammation, and suggested it as a potential candidate for fibrosis therapy. NS had a dosedependent antioxidative property. NS oil and thymoquinone protect gastric mucosa, which is partly attributed to their free-radical scavenging activity. Moreover, the ethanolic extract of NS protects radiation induced oxidative damage in mice. Several mechanisms for antioxidative properties of NS have been suggested. It is demonstrated that it preserves the activity of catalase, glutathione peroxidase, and glutathione-S-transferase. It also inhibits microsomal lipid peroxidation [54,30]. Verhagen et al. [55] proved that vitamin E and other antioxidants have a protective action against the damaging effects of free radicals. Free radicals destroy the cells and may contribute to the development of cardiovascular diseases and cancer.
Conclusion
In conclusion, this current work proved that the high dose of nandrolone not only elicits measurable increase in cardiac muscle performance, but also cause many adverse effects in cardiac muscle fibers at the histological, histochemical and ultrastructural levels. However, N. sativa supplementation has a great role in maintenance of muscle strength and doesn't cause any harmful effects to the cardiac muscle fibers. The application of N. sativa to the nandrolone treated rats may have a possible protective role against the deleterious effects of nandrolone injection. It seems that it acts through alteration of oxidative/antioxidative balance and raising antioxidative enzymes. More studies are guaranteed to clarify the beneficial effects and the underlying mechanisms of NS seed. So, nandrolone must be used under complete clinical supervision particularly in young misinformed athletes.
References
- Nilsson S, Allebeck P, Marklund B, Baigi A, Fridlund B (2004) Evaluation of a health promotion programme to prevent the misuse of androgenic anabolic steroids among Swedish adolescents. Health Promot Int 19: 61-67.
- Socas L, Zumbado M, Pérez-Luzardo O, Ramos A, Pérez C, et al. (2005) Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: A report of two cases and a review of the literature. Br J Sports Med 39: e27.
- Kostic TS, Stojkov NJ, Bjelic MM, Mihajlovic AI, Janjic MM, et al. (2011) Pharmacological doses of testosterone upregulated androgen receptor and 3-Betahydroxysteroid dehydrogenase/delta-5-delta-4 isomerase and impaired leydig cells steroidogenesis in adult rats. Toxicol Sci 121: 397-407.
- Shahidi NT (2001) A review of the chemistry, biological action and clinical applications of anabolic androgenic steroids. Clin Ther 23: 1355-1390.
- Bagchus WM, Smeets JM, Verheul HA, De Jager VDVSM, Port A, et al. (2005) Pharmacokinetic evaluation of three different intramuscular doses of nandrolone deconate: Analysis of serum and urine samples in healthy men. J Clin Endocrinal Metab 90: 2624-2630.
- Yesalis CE (2000) Anabolic steroids in sport and exercise. Champaign, I. L., (2nd edtn). Human Kinetics Publishers. London, New York.
- Casavant MJ, Blake K, Griffith J, Yates A, Copley LM (2007) Consequences of use on anabolic androgenic steroids. Pediatric Clin North Am 54: 677-690.
- Marshall-Gradisnik S, Green R, Brenu EW, Weatherby RP (2009) Anabolic androgenic steroids effects on the immune system: A review. Cent Eur J Biol 4: 19-33.
- FallahHH, Amini M, Mohtashami R, Ghamarchehre ME, Sadeqhi Z, et al. (2013) Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res 27: 1849-1853.
- El-Tahir KEH, Al-Ajmi MF, Al-Bekari AM (2003) Some cardiovascular effects of dethymoquinonated Nigella sativa volatile oil and its major components a-pinene and p-cymene in rats. Saudi Pharm J 11: 104.
- Beheshti F, Khazaei M, Hosseini M (2016) Neuropharmacological effects of Nigella sativa. Avicenna J Phytomed 6: 104-116.
- Boskabady MH, Keyhanmanesh R, Khamneh S, Ebrahimi MA (2011) The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. Clinics (Sao Paulo) 66: 879-887.
- Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA (2008) Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad 20: 25-27.
- Soliman HM, Elseweidy MM, Taha MM (2011) Effect of Nigella sativa oil versus amoxicillin in induced chronic fungic gastritis in adult female albino rats: A histological, immunohistochemical, and biochemical study. Egyptian Journal of Histology 34: 674-686. â€
- Abdelhafez HM (2014) Histological, histochemical and ultrastructural study on the effect of Deca Durabolin and whey protein isolate on cardiac muscle in adult male albino rats. Int J Adv Res 2: 164-181.
- Bancroft J, Layton C (2013) The Hematoxylin and eosin. In: Suvarna SK, Layton C and Bancroft JD (ed). Theory and practice of histological techniques. (7th edtn). Churchill Livingstone of Elsevier, Philadelphia.
- Drury RA, Wallington EA (1980) Carlton’s histological techniques. (5th edtn). Oxford: Oxford University Press, USA
- Glauert AM, Lewis PR (1998) Biological specimen preparation for transmission electron microscopy. (1st edtn) Portland Press, London.
- Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, et al. (2008) Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest 20: 393-413.
- Sretenovic J, Zivkovic V, Srejovic I, Milosavljevic Z (2016) The effects of high doses of nandrolone deconate on cardiac muscle tissue. Ser J Exp Clin Res17: 303-308.
- Kicman AT (2008) Pharmacology of anabolic steroids. Br J Pharmacol 154: 502-521.
- Dillon EL, Durham WJ, Urban RJ, Sheffield-Moore M (2010) Hormone treatment and muscle anabolism during aging: Androgens. Clin Nutr 29: 697-700.
- Tylicki A, Kawalko A, Sokolska J, Strumilo S (2007) Effect of anabolic steroid nandrolone decanoate on the properties of certain enzymes in the heart, liver, muscle of rats and their effect on rats' cardiac electrophysiology. Horm Metab Res 39: 268-272.
- Tanno AP, das Neves VJ, Rosa KT, Cunha TS, Giordano FC, et al (2011) Nandrolone and resistance training induce heart remodeling: Role of fetal genes and implications for cardiac pathophysiology. Life Sci 89: 631-637.
- Montisci M, El-Mazloum R, Cecchetto G, Terranova C, Ferrara SD, et al. (2012) Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci Int 217: e13-18.
- Franquni JV, do Nascimento AM, de Lima EM, Brasil GA, Heringer OA, et al. (2013) Nandrolone decanoate determines cardiac remodeling and injury by an imbalance in cardiac inflammatory cytokines and ACE activity, blunting of the Bezold-Jarisch reflex, resulting in the development of hypertension. Steroids 78: 379-385.
- Frankenfeld SP, de Oliveira LP, Ignacio DL, Coelho RG, Mattos MN, et al. (2014) Nandrolone decanoate inhibits gluconeogenesis and decreases fasting glucose in Wistar male rats. J Endocrinol 220: 143-153.
- Frati P, Busardo FP, Cipolloni L, Dominicis ED, Fineschi V (2015) Anabolic androgenic steroids (AAS) related deaths: Autoptic, histopathologycal and toxicological findings. Curr Neuropharmacol 13: 146-159.
- Nikolic T, Zivkovic V, Jevdjevic M, Djuric M, Srejovic I, et al. (2016) The effects of chronic administration of nandrolone decanoate on redox status on exercised rats. Mol Cell Biochem 411: 95-105.
- Soliman ME, El-Saify GS, Badawy KNS, Soliman MAM, Abo-Habsa SS (2017) Effect of nandrolone on rat cardiac muscle and the possible protective role of Vitamin E: A light and electron microscopic study. J Am Sci 13.
- Shao D, Oka S, Brady CD, Haendeler JP, Eaton J, et al. (2012) Redox modification of cell signalling in the cardiovascular system. J Mol Cell Cardiol 52: 550-558.
- Sadowska-Krepa E, KÅ‚apcinska B, Jagsz S, Sobczak A, Chrapusta SJ, et al. (2011) High-dose testosterone propionate treatment reverses the effects of endurance training on myocardial antioxidant defenses in adolescent male rats. Cardiovasc Toxicol 11: 118-127.
- Ahmed MA (2015) Amelioration of nandrolonedecanoate-induced testicular and sperm toxicity in rats by taurine: Effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway. Toxicol Appl Pharmacol 282: 285-296.
- Tulasigiriyappa Y, Potadar RR, Basappa B, Kaliwal BB (2012) Indoxacarb induces liver oxidative stress in swiss albino mice. Eur J Exp Bio 2: 180-186.
- Rodrigo R, Trujillo S, Basco C, Orellena M, Thielemann L, et al. (2002) Changes in (Na + K)-adenosine triphosphate activity and ultrastructure of lung and kidney associated with oxidative stress induced by acute ethanol intoxication. Chest 121: 589-596.
- Zaitone SA, Abo-Gresha NM (2012) Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: Role of iNOS and VEGF. Eur J Pharmacol 691:134-142.
- Sun SJ, Wu XP, Song HL, Li GQ (2015) Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. Int J Clin Exp Med 8: 22063-22072.
- Parssinen M, Karila T, Kovanen V, Seppala T (2000) The effect of supraphysiological doses of anabolic androgenic steroids on collagen metabolism. Int J Sports Med 21: 406-411.
- Cavasin MA, Tao ZY, Yu AL, Yang XP (2006) Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physio 290: H2043-2050.
- Golestani R, Slart RH, Dullaart RP, Glaudemans AW, Zeebregts CJ, et al. (2012) Adverse cardiovascular effects of anabolic steroids: Pathophysiology imaging. Eur J Clin Invest 42: 795-803.
- Tokunaga T, Morshed SR, Otsuki S, Takayama F, Satoh T, et al. (2003) Effect of antioxidants, oxidants, metals and saliva on cytotoxicity induction by sodium fluoride. Anticancer Res 23: 3719-3726.
- Swapnila C, Flora SJ (2010) Arsenic and fluoride: Two major ground water pollutants. Indian J Exp Biol 48: 666-678.
- Kannan MM, Quine SD (2013) Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism 62: 52-61.
- Phillis BD, Abeywardena MY, Adams MJ, Kennedy JA, Irvine RJ (2007) Nandrolone potentiates arrhythmogenic effects of cardiac ischemia in the rat. Toxicol Sci 99: 605-611.
- Thornberry NA, Lazebnik Y (1998) Caspases: Enemies within. Science 281: 1312-1316.
- Condorelli G, Roncarati R, Ross J, Pisani A, Stassi G, et al. (2001) Heart-targeted overexpression of caspase 3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA 98: 9977-9982.
- Palojoki E, Saraste A, Eriksson A, Pulkki K, Kallajoki M, et al. (2001) Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 280: H2726-2731.
- Liu Q, Zhang J, Xu Y, Huang Y, Wu C (2013) Effect of carvedilol ocardiomyocyte apoptosis in a rat model of myocardial infarction: A role for toll-like receptor 4. Indian J Pharmacol 45: 458-463.
- Ahmed SM, Abdelrahmana SA, Salama AE (2017) Efficacy of gold nanoparticles against isoproterenol induced acute myocardial infarction in adult male albino rats. Ultrastruct Pathol 41: 168-185.
- Kanter M, Demir H, Karakaya C, Ozbek H (2005) Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone against acute alcoholinduced gastric mucosal injury in rats. World J Gastroenterol 11: 6662-6666.
- Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al-Thani H (2013) Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc Toxicol 13: 9-21.
- Shafiq H, Ahmad A, Masud T, Kaleem M (2014) Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran J Basic Med Sci 17: 967-979.
- Wilson H, Carvalho B, Granot M, Landau R (2013) Temporal stability of conditioned pain modulation in healthy women over four menstrual cycles at the follicular and luteal phases. Pain 154: 2633-2638.
- Carvalho BM, Abdalla SMJ (2013) Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm 1:13.
- Verhagen H, Buijsse B, Jansen E, de Mesquita BB (2006) The state of antioxidant affairs. Nutr Today 41: 244-250.
Citation: Mahmoud AA, Mekawy NH, Mohammed MZ (2018) The Nandrolone Effect on Cardiac Muscle of Adult Male Albino Rat and the Possible Role of Nigella sativa: Light and Electron Microscopic Studies. J Biochem Cell Biol 1: 109.
Copyright: © 2018 Mahmoud AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 11966
- [From(publication date): 0-2018 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 10740
- PDF downloads: 1226