Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
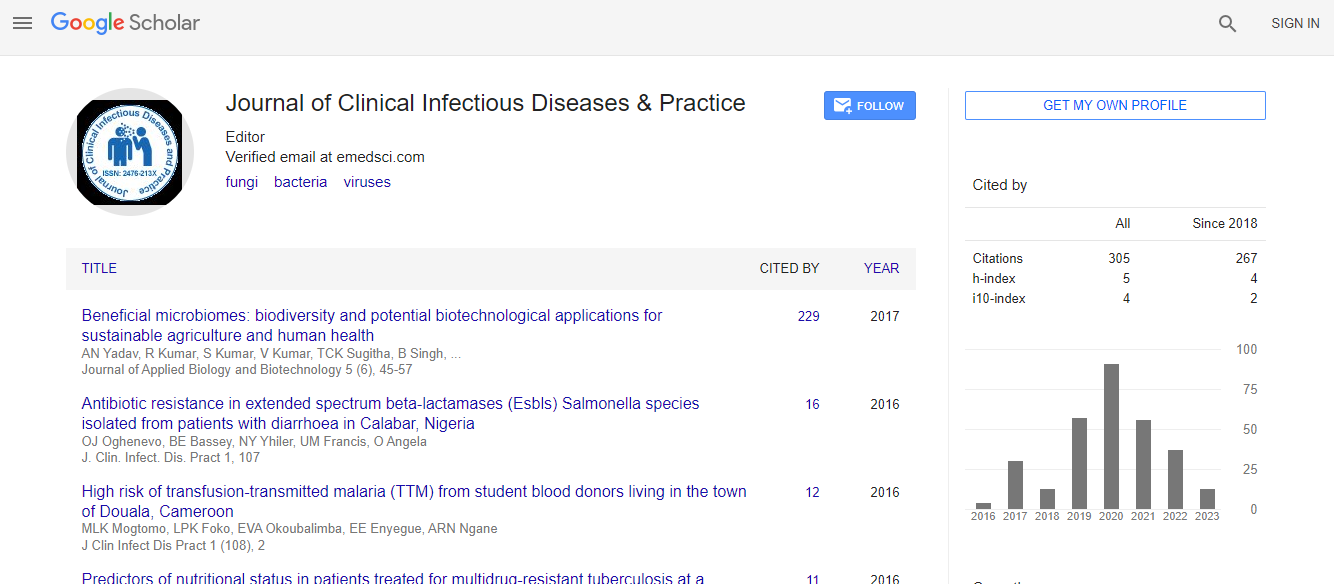
Google Scholar citation report
Citations : 531
Journal of Clinical Infectious Diseases & Practice peer review process verified at publons
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
New therapies in genetic skeletal diseases achieved through drug repurposing
9th World Congress on Rare Diseases and Orphan Drugs
Michael Darren Briggs
Newcastle University, UK
ScientificTracks Abstracts: J Clin Infect Dis Pract
Abstract
Genetic skeletal diseases (GSDs) are an extremely diverse and complex group of diseases that primarily affect the development and homeostasis of the skeleton. There are more than 450 unique and well-characterised phenotypes that range in severity from relatively mild to severe and lethal forms and although individually rare, as a group of related orphan diseases, GSDs have an overall prevalence of at least 1 per 4,000 children, which represents a large unmet medical need. Our studies have focussed on a group of clinically-related GSDs that present with disproportionate short stature and early onset OA and result from dominant-negative mutations in a range of cartilage structural proteins including cartilage oligomeric matrix protein (COMP), matrilin-3, aggrecan and types II, IX and X collagens. We have unequivocally established that endoplasmic reticulum (ER) stress, induced in chondrocytes as a result of accumulated misfolded mutant proteins, is the primary cause of growth plate dysplasia and reduced bone growth in a broad group of GSDs. Moreover, we have recently demonstrated that reducing ER-stress, through the administration of a repurposed anti-epileptic drug carbamazepine (cbz), in both cell and mouse models, restores cell homeostasis and bone growth in metaphyseal chondrodysplasia, type Schmid (MCDS) resulting from collagen X mutations.Recent Publications
1. Bell P A, Dennis E P, Hartley C L, Jackson R M, Porter A, Boot-Handford R P, Pirog K A and Briggs M D (2019) Mesencephalic astrocyte-derived neurotropic factor is an important factor in chondrocyte ER homeostasis. Cell Stress Chaperones. 24(1):159-173.
2. Mullan L A, Mularczyk E J, Kung L H, Forouhan M, Wragg J M, Goodacre R, Bateman J F, Swanton E, Briggs M D and Boot-Handford R P (2017) Increased intracellular proteolysis reduces disease severity in an ER stressassociated dwarfism. J Clin Invest. 127(10):3861-3865.
3. Gibson B G and Briggs M D (2016) The aggrecanopathies; an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J Rare Dis. 11(1):86.
4. Briggs M D, Bell P A and Pirog K A (2014) The utility of mouse models to provide information regarding the pathomolecular mechanisms in human genetic skeletal diseases: The emerging role of endoplasmic reticulum stress (Review). Int J Mol Med. 35(6):1483-92.
5. Cameron T L, Gresshoff I L, Bell K M, Piróg K A, Sampurno L, Hartley C L, Sanford E M, Wilson R, Ermann J, Boot-Handford R P, Glimcher L H, Briggs M D and Bateman J F (2015) Cartilage-specific ablation of XBP1 signaling in mouse results in a chondrodysplasia characterized by reduced chondrocyte proliferation and delayed cartilage maturation and mineralization. Osteoarthritis Cartilage. 23(4):661-70.
Biography
Michael Darren Briggs has obtained his PhD in Molecular Genetics at the MRC-Clinical Research Centre in Harrow and undertook a Genetics Fellowship at Cedars-Sinai Medical Center in Los Angeles. In 1996 he moved to the University of Manchester and in 2012 he was appointed as a Professor of Skeletal Genetics at Newcastle University. Over the last 15 years he has been instrumental in establishing four Pan-European consortia for the clinical diagnosis and research of genetic skeletal diseases. His primary research interested is focused on refining diseases mechanisms in genetic bone diseases to identify new therapies for future clinical trials.
E-mail: michael.briggs@newcastle.ac.uk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi