Research Article Open Access
8-Weeks of β-GPA Treatment Reduces Body Mass While Positively Altering Translation Initiation in Obese Skeletal Muscle
| Joshua C. Drake, Lauryn Benninger and David L. Williamson* | |
| Department of Exercise and Nutrition Sciences, School of Public Health and Health Professions, University at Buffalo, SUNY, Buffalo, NY 14214 | |
| Corresponding Author : | David L. Williamson, PhD Assistant Professor University at Buffalo SUNY School of Public Health and Health Professions Department of Exercise and Nutrition Sciences 214A Kimball Tower (office) / 5 Sherman (lab) Buffalo, NY 14214, USA Tel: (716) 829-6758 Fax: (716) 829-2428 E-mail: davidwil@buffalo.edu |
| Received November 08, 2011; Accepted November 19, 2011; Published November 21, 2011 | |
| Citation: Drake JC, Benninger L, Williamson DL (2011) 8-Weeks of β-GPA Treatment Reduces Body Mass While Positively Altering Translation Initiation in Obese Skeletal Muscle. J Obes Weig los Ther 1:101. doi:10.4172/2165-7904.1000101 | |
| Copyright: © 2011 Drake JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
The aim of this study was to determine how 3-guanidinopropionic acid (?-GPA) treatment, that reduces body mass, alters obese skeletal muscle mass and regulatory mechanisms controlling muscle mass. Lean (L) and ob/ ob (O) mice were fed either a control (C) or a ?-GPA-containing (F) diet for 8 weeks. Body mass decreased in both ?-GPA treated groups. Despite a lower plantar flexor-complex muscle mass, both ?-GPA treated groups achieved the same muscle mass. Raptor-mammalian Target of Rapamycin protein association was lower in OC muscle (vs. LC) and was not altered with ?-GPA, despite reductions in S6K1 activation (OF only). 4E-BP1 phosphorylation increased in the ?-GPA treated groups, but only the OF mice displayed an increase in eIF4E phosphorylation that corresponded with a trending increase in eIF4G-eIF4E association. Thus, long-term ?-GPA treatment augments obesity-induced dysregulation of mechanisms controlling skeletal muscle mass to that of the lean, while reducing body mass.
| Keywords |
| Translational control; eIF4E; 4E-BP1; mTOR; ob/ob |
| Introduction |
| Obesity-related health complications, such as dyslipidemia, glucose intolerance, insulin resistance, and chronic inflammation, can manifest themselves through reductions in skeletal muscle quality (i.e. skeletal muscle mass and function). This decrease in quality is partially due to an atrophying of the skeletal muscle [1-4], as a result of an imbalance in the rates of protein synthesis and degradation [5]. Complicating the situation, when obese individuals lose body mass as part of an intervention strategy, there is an accompanying reduction in muscle mass [6,7]. A loss in skeletal muscle mass would be deleterious in this situation, given its vast metabolic role in glucose and fat metabolism. |
| Muscle mass accrual relies upon protein synthesis that is primarily regulated by mRNA translation, with initiation of translation being the rate-limiting step [8-10]. Translation initiation occurs when eukaryotic initiation factors (eIFs), such as eIF4E, eIF4G, eIF3, among others, recruit the 40S ribosomal subunit to the m7 GTP cap on the 5’ end of the mRNA to be translated [11]. A major regulator of mRNA translation and cell growth is the mammalian target of rapamycin (mTOR) [12]. Although, acute increases in mTOR activation and/or recruitment to the translational complex typically result in muscle hypertrophy and growth [13,14], chronically elevated mTOR signaling, as seen in obese skeletal muscle, appear to negatively impact insulin signaling pathways and growth [3,15]. |
| mTOR is comprised of two separate multi-protein complexes, raptor containing mTOR complex 1 (TORC1) and rictor containing mTOR complex 2 (TORC2). The knockout of raptor in the TORC1 complex appears to be indispensible for skeletal muscle function and size [16-18]. TORC1 phosphorylates two downstream substrates, the eukaryotic initiation factor (eIF) 4E binding protein-1 (4E-BP1) and p70 ribosomal protein S6 Kinase-1 (S6K1) [19]. In its unphosphorylated state, 4E-BP1 binds to eIF4E, inhibiting translation initiation. TORC1 phosphorylation of 4E-BP1 promotes its release from the cap-binding protein eIF4E. EIF4E is then free to bind with the initiation factor, eIF4G, increasing eIF4F complex formation [20]. |
| S6K1 phosphorylation by TORC1 at T389 releases eIF3, allowing for the binding of the 40S ribosomal subunit to the translation initiation complex [21]. S6K1 phosphorylation recruits eIF4B to the eIF4F complex [21,22]. The eIF4F complex then binds with the mRNA and 40S ribosomal subunit so that together with a 60S subunit, forms a functional 80S monosome [8,11]. At this point, mRNA can be translated and peptides are being formed. |
| One of the ways that mTOR can be regulated is by the ubiquitous Ser/Thr kinase heterotrimer, 5’ AMP-activated protein kinase (AMPK) [23]. When phosphorylated on the T172 site of the catalytic a-subunit, ATP production in the cell is initiated, while ATP consuming pathways in the cell are inhibited (e.g. translation and protein synthesis) [23]. Activated AMPK can phosphorylate the tuberous sclerosis complex 2 (TSC2) gene product Tuberin on T227 and S1345 [24]. When active, a complex with Hamartin (aka TSC1) forms to function as a GTPaseactivating protein (GAP) to the Rheb G protein [25]. When TSC2-TSC1 complex formation increases, Rheb is converted to its inactive GDP form, decreasing mTOR activity [25]. AMPK activation also causes the phosphorylation and subsequent inactivation of acetyl-CoA carboxylase (ACC), the rate- limiting enzyme in fatty acid synthesis [26]. |
| Previously, we reported [27] that short-term (2 week) treatment with the AMPK agonist, AICAR, normalized metabolic indices (e.g. circulating insulin, glucose, and lipid, and muscle glycogen and lipid) and dysregulated/hyperactive growth pathways (e.g. mTOR) in an obese mouse model, independent of a change in body weight. Though it remains unclear how obese skeletal muscle adapts to long term 3-guanidinopropionic acid (β-GPA), a known AMPK agonist, treatment that may cause a decrease in body mass. Therefore, determining the regulatory processes involved in muscle growth that are altered in the obese and in the obese that have undergone body mass loss, will be important in developing strategies that limit skeletal muscle loss during body mass loss. We hypothesized that long-term treatment with 3-guanidinopropionic acid (β-GPA) would normalize mTOR signaling and translation-related proteins, promoting positive adaptations of obese skeletal muscle relative to body mass. |
| Methods and Procedures |
| Materials |
| β-GPA was purchased from Sigma-Aldrich (#G6878; St. Louis, MO). ATP Determination Kit (A22066) was purchased from Invitrogen Detection Technologies (Carlsbad, CA). All antibodies were purchased from Cell Signaling Technology (Danvers, MA). Biomag beads were purchased from Qaigen (Valencia, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). |
| Animals |
| All experimental procedures were approved by the Institutional Animal Care and Use Committee of the West Virginia University School of Medicine. Lean and ob/ob (#000632) male mice from Jackson Laboratories (Bar Harbor, ME) were used for the proposed experiments. All animals were maintained on a 12:12, light:dark cycle and control mice were fed a standard chow, while the experimental treatment groups were fed a standard chow diet containing 1% β -GPA (see following section for detail). The experimental groups consisted of 8 C57BL/6 (lean; LF) and 8 ob/ob mice (OF) fed a standard chow containing 1% β -GPA, and 8 lean (LC) and ob/ob (OC) mice fed standard chow only. Food and water were available ad libitum . Body and food weight was measured every third day (2x week). The average body of each mouse was calculated at the end of each week to track body mass. After the 8 week treatment, and following a 12 hour fast, a final body weight was determined to the nearest milligram and then tissues were collected while the animal was under anesthesia (vaporized 4% isoflurane). The plantar flexor complex (soleus, plantaris, medial and lateral head of the gastrocnemius) muscle groups were removed from the right and left limbs and weighed, then the right muscle group was immediately homogenized and the left leg was frozen in liquid nitrogen for subsequent analysis. The right tibia was removed and immediately placed at -20° C for subsequent analysis. |
| Chow containing β-Guanidinopropionic Acid (β-GPA) |
| β-GPA was provided to Harlan Laboratories (Somerville, NJ) by our laboratory and mixed with standard chow (Cat# 2018) for a final concentration of 1% β-GPA [28-31]. |
| Muscle size |
| The plantar flexor complex was dissected tendon-to-tendon, fat around the tissue was removed, and then the muscle was weighed to the nearest milligram. Muscle samples were then immediately homogenized or frozen in liquid nitrogen for subsequent analysis. Due to the possibility that the β-GPA treatment caused a change in body and muscle mass, muscle mass was normalized to either body mass or tibial length. |
| Muscle tissue preparation |
| Similar to our previously described methods of muscle tissue homogenization [27], a CHAPs-containing buffer (40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA, 10 mM pyrophosphate, 10 mM glycero-phosphate, 40 mM NaF, 0.3% CHAPS) with the addition of 10 µl/ml protease inhibitor, 1 µl/ml DTT, 5 µl/ml Benzamidine, 2.5 µl/ml Sodium Vanadate, was used to homogenize the skeletal muscle. After homogenization and prior to centrifugation, 50 µl aliquot of the homogenate was placed in a separate tube with 50µl of an equal volume of 2X SDS- polyacrylamide gel loading buffer to analyze total lysate protein expression. The remaining homogenate was centrifuged at 1000xG for 10min at 4°C. This cytosolic-rich supernatant was then combined with an equal volume of 2X SDS-polyacrylamide gel loading buffer, and the remaining pellet will be discarded. |
| Protein determination |
| A Coomassie Stain Assay (Thermo Scientific) was used to determine protein concentration of the muscle lysate samples. Once diluted and combined with the Coomassie reagent, the samples were analyzed (595nm; Biotek Synergy 2 Microplate Reader) to determine the absorbance. The protein concentration was calculated based upon a bovine serum albumin standard provided with the kit. |
| Western blot analysis |
| Protein expression of total (S6K1, 4E-BP1) and phosphorylated (AMPK (P) T172, ACC (P) S79, rpS6 (P) S240/244, eEF2 (P) T56) forms of the respective proteins from plantar flexor complex muscle homogenates was determined from equal protein by Western blot analysis, as previously described [27]. Protein expression following Western analysis was visualized using enhanced chemiluminescence (ECL) and captured by a camera-integrated system (SynGene), then expression was assessed by ImageJ software (version 1.44). Results were normalized to β-Tubulin, unless where specified, and expressed as a percent of the lean control group. At least two samples from each group were run on each gel for analysis. |
| Immunoprecipitation |
| As previously described [27], the sample was homogenized in a CHAPS-containing buffer (see previous section for details), and then 500 µg of lysate was combined with eIF4E or mTOR antibody and mixed overnight at 4°C. Immune complexes were isolated with a goat anti-rabbit or –mouse BioMag IgG beads (Qaigen), using a magnetic stand. Precipitates were eluted in SDS sample buffer, boiled, pelleted by magnet, collected, and subjected to standard Western analysis for eIF4E, phosphor eIF4E (S209), eIF4G, and 4E-BP1 (eIF4E IPs only) or mTOR and raptor (mTOR IPs only). The ratios of 4E-BP1, phosphoeIF4E, and eIF4G to eIF4E, and raptor to mTOR were calculated and expressed as percentage of lean control value. At least two samples from each group were run on each gel for analysis. |
| ATP determination |
| An Invitrogen Detection Technologies ATP Determination Kit was used to measure ATP concentrations from muscle lysates, per the manufacturer’s instructions. This is a bioluminescence assay for quantitative determination of ATP with recombinant firefly luciferase and its substrate D-luciferin. The concentration of ATP for each sample was calculated based upon ATP standards provided with the kit. ATP concentrations were expressed in µM/g wet weight of tissue. |
| Statistics |
| Results are means ±standard error of the mean for eight mice per treatment group, except where stated (data for one OC mouse was not used for the analysis because the data were more than 2 standard deviations away from the mean).Comparisons were made for each variable using a one-way or two-way (body weight data only (Figure 3) ANOVA with a Tukey or Bonferroni post hoc test, respectively, to establish significant differences between groups, only after the F statistic indicated an overall significance in the data via Prism (version 3.0, GraphPad Software, La Jolla, CA). Significance level was set a priori at P< 0.05. |
| Results |
| ATP Concentration and AMPK phosphorylation |
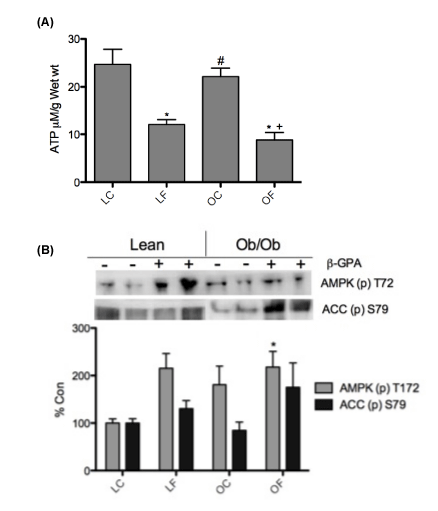
| A reduction in ATP concentration has previously been reported following β-GPA administration [28], therefore confirmation of the treatment was determined. Following 8 weeks of consuming a chow-containing β-GPA and a 12hr fast, ATP concentrations were significantly less (p<0.05) in the skeletal muscle of both β-GPA treated groups compared to the control groups (Figure 1A). In support of this, Western analysis of skeletal muscle samples showed a significant increase (p<0.05) in AMPK phosphorylation in the OF versus LC mice (Figure 1B). However, no significant difference was observed in the AMPK substrate, ACC, between groups (Figure 1B). |
| Body mass |
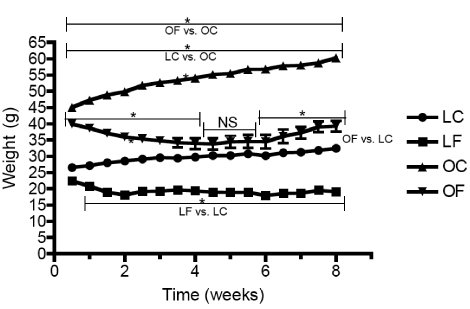
| Body mass of the OC mice were significantly different (p<0.05) from the LC mice throughout the 8 week experimental period (Figure 2). OC mice had a final weight 86.5% heavier than their LC counterparts (Table 1). LF mice were significantly different (p<0.05) from the LC mice after week 0.5 on (Figure 2) with a final weight 42.2% lighter (Table 1). The body weight for the OF mice decreased (p<0.05) to a similar level of the LC mice in the middle of week 4 (Figure 2). This reduction in weight remained until week 6, at which point the OF mice gained weight through to the end of the 8 week study. Despite this gain, the OF remained 29.3% lighter than the OC mice and 32.0% heavier than LC mice (Table 1) after 8 weeks on the β-GPA-containing diet. |
| Plantar flexor complex muscle characteristics |
| The absolute weight of the plantar flexor complex was significantly lower (p<0.05) in the OC mice compared to the LC group (Table 1). When muscle weight was expressed relative to body mass, the OC mice were 57.4% lower (p<0.05) than that of the LC mice (Table 1). After 8 weeks of β-GPA feeding, the OF group’s absolute plantar flexor complex muscle weight was still 43.3% lower (p<0.05) than the LC mice (Table 1) and 28.6% less (p<0.05) than the OC mice (Table 1). However, the LF group’s absolute muscle weight loss (-41.7%; vs. LC) was similar to the OF loss (vs. OC). Thus, the loss (i.e. delta) in muscle mass was less for the OF versus LF (Table 1). When expressing muscle weight relative to tibial length, the OC were significantly lower (p<0.05) than LC mice (Table 1). Following the β-GPA treatment, the muscle weight relative to tibial length for the LF and OF groups were 39.8% and 23.5% lower (p<0.05) than LC mice, respectively (Table 1). Though statistical significance was found in both treated groups, it should be noted that there is very little variance for tibial length between groups. |
| TORC1 |
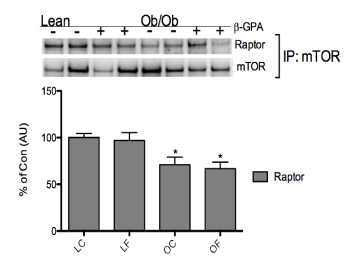
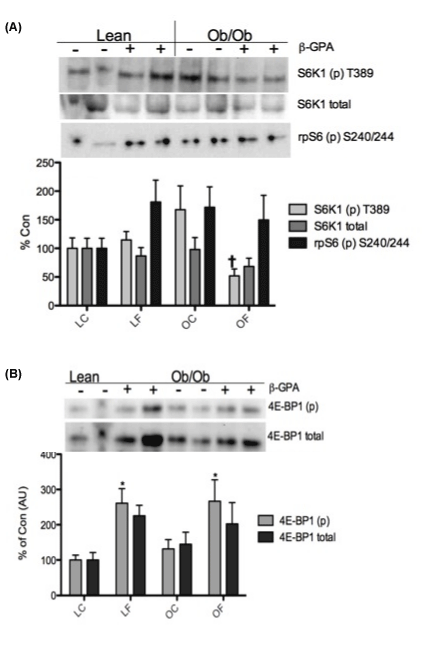
| After 8 weeks of β-GPA treatment, the rapamycin-sensitive mTOR complex (TORC1) protein, raptor, had lower (p<0.05) association with mTOR in mTOR immunoprecipitates in OC mice compared to LC, and remained at the same level following the β-GPA treatment (Figure 3). Activation status of TORC1 substrates, S6K1, S6K1’s substrate, ribosomal protein S6 (rpS6), and 4E-BP1, was also determined from cytosolic-rich fractions. Following a 12 hour fast, S6K1 phosphorylation at T389 was lower (p<0.05) in OF mice versus OC mice returning its activation to a level comparable to lean levels (Figure 4A), independent of a change in total protein expression of S6K1. No significant difference was found in rpS6 phosphorylation between groups (Figure 4A). However, the phosphorylation of 4E-BP1 was significantly higher (p<0.05) in the LF and OF groups compared to LC mice (Figure 4B), independent of a change in total 4E-BP1 protein expression. |
| Translation initiation and elongation |
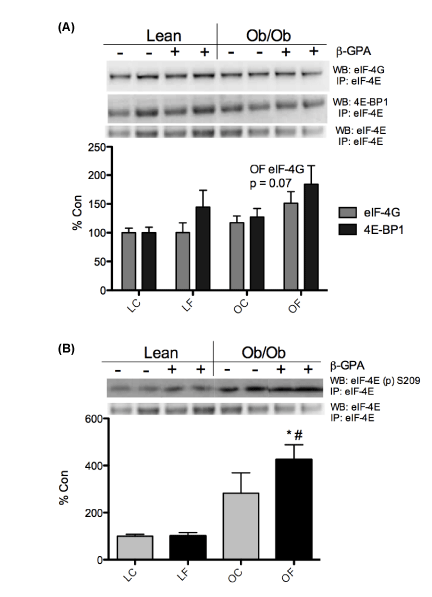
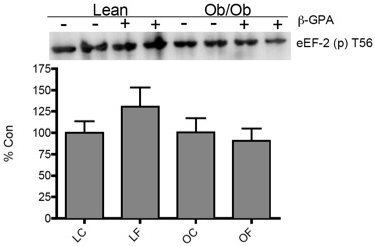
| The formation of the translation initiation complex (i.e. eIF4F) was examined in eIF4E immunoprecipitates from control and treated, lean and obese (ob/ob) plantar flexor complex muscle lysates. The association of eIF4G with eIF4E showed a trend towards significance (p=0.07) in the OF group (Figure 5A). However, 4E-BP1 associated with eIF4E was not different between any of the groups (Figure 5A). Interestingly, the phosphorylation status of the mRNA cap-binding protein, eIF4E, on S209 (from eIF4E immunoprecipitate samples) was significantly elevated (p<0.05) in OF mice (Figure 5B). Elongation did not seem to be affected by body weight or treatment in this study, given a lack of change in the phosphorylation of elongation factor 2 (eEF2) (Figure 6). |
| Discussion |
| Determining alterations in obese skeletal muscle mass and regulatory mechanisms controlling muscle mass, while losing total body mass/ weight, was the impetus for the current study. The results of the present study show that long term β-GPA feeding of ob/ob mice can normalize aspects of hyperactive mTOR-related signaling seen in obesity, and promote portions of translation initiation, such as the phosphorylation of 4E-BP1 and eIF4E. The findings of the current study confirm and extend our previous findings [27], in that long-term β-GPA treatment, a known AMPK activator, normalizes and/or promotes regulatory mechanism of muscle growth in the obese during weight loss. |
| Reduced skeletal muscle size has been demonstrated in obese animals [1] and humans [4], despite hyperactive growth-promoting pathways [1,32,33]. A reduction in body mass has been and currently is used as a means to negate the negative effects of obesity, but this reduction is often associated with a loss in lean body mass (i.e. skeletal muscle). Given the importance of skeletal muscle mass for proper metabolism to occur, we sought to normalize dysregulated mechanism of muscle growth, using a treatment that would elicit a reduction in body mass. As anticipated, there was a dramatic reduction in body mass observed in the OF mice over the 8 week feeding period (Table 1), that did not differ significantly in body weight from lean control mice between 4-6 weeks of treatment (Figure 2). When normalized to body weight, the muscle mass was similar between treatment groups (control vs. β-GPA) for the respective lean and obese group, but when normalized to tibial length, the muscle mass-to-tibial length ratio for the LF and OF was similar. It is possible that had we examined muscle weight between 4-6 weeks as opposed to 8 weeks, we would have seen a larger muscle mass to body mass ratio. A reduction in muscle mass during a loss in body weight is similar to studies in humans that lose body weight during caloric restriction [7], but the muscle mass is maintained with the combination of exercise and weight loss. Intriguingly, the reduction in skeletal muscle mass in both LF and OF groups achieved approximately the same absolute level (Table 1), but what relevance this holds in the current investigation remains unclear. |
| For proper skeletal muscle growth to occur, coordination of growthpromoting mechanisms (i.e. mTOR signaling, mRNA translation, and protein synthesis) is required. Muscle-specific knockout of mTOR or raptor mice [16] results in severely dystrophic and metabolically insensitive. Consistent with our previous work [27], raptor’s association with mTOR was lower in the obese mice, but did not increase with the β-GPA treatment (Figure 3). This was surprising given our previous finding that after 2 weeks of treatment with AICAR, raptor-mTOR association increased. This may suggest differences in type and/or duration of treatment. One of the few studies to examine raptor in obese muscle used soleus overload as a means to stimulate muscle growth [34]. The authors did not show any changes with total raptor protein following overload, despite alterations in mTOR signaling and muscle mass. |
| TORC1 complex formation regulates cap-dependent translation through the activation of its substrates S6K1 and 4E-BP1 [21]. In the present study, raptor’s association with mTOR was decreased in OC mice compared to LC and LF mice (Figure 3). This is consistent with the hyperphosphorylated TORC1 signaling classically seen in obesity and in our previous data [27]. The phosphorylation of S6K1 on T389 was reduced in OF mice vs. OC mice to levels equivalent to those of the lean control mice (Figure 4B). Elevated S6K1 phosphorylation results in an increase in IRS-1 phosphorylation through negative feedback, decreasing insulin sensitivity and ultimately giving rise to insulin resistance in chronic situations [35]. In mice that are S6K1deficient, there is a protective effect against obesity and insulin resistance because of an up-regulation in oxidative phosphorylation and insulin sensitivity [15]. Therefore, the lower activation of S6K1 after 8 weeks of β-GPA in ob/ob mice in the current study is suggestive of more normalized mTOR signaling and a possible protective effect against insulin resistance. |
| Another TORC1 substrate, 4E-BP1, displayed significantly increased T37/46 phosphorylation in ob/ob mice fed β-GPA (Figure 4B). This finding was unexpected and contrary to the reduction in mTOR signaling and increase in AMPK-related signaling. Although, this is consistent with mice lacking 4E-BP1/2, which are more sensitive to a high fat diet resulting in insulin insensitivity [36]. 4E-BP1 regulates 40S ribosome binding to mRNA by regulating the availability of eIF4E [11]. When 4E-BP1 is phosphorylated it releases eIF4E, allowing eIF4G to bind with eIF4E and subsequently recruiting the 40S ribosomal subunit [11]. Increased 4E-BP1 phosphorylation suggests increased availability of eIF4E, which would imply an increase in eIF4G’s association with eIF4E. To assess this possibility we examined the eIF4F complex using eIF4E immunoprecipitation, to determine the association with 4E-BP1 and eIF4G. No significant change was found in 4E-BP1’s association with eIF4E following 8 weeks of feeding with β-GPA in ob/ob mice (Figure 5A). This is contrary to our previous data using AICAR as the means to stimulate AMPK [27], which may suggest a difference in mechanism by which β-GPA versus AICAR act upon 4E-BP1, and possibly TORC1 signaling. Although 4E-BP1 is phosphorylated by TORC1, it is not the only signaling pathway that influences 4E-BP1. Protein kinase C (PKC ) and ERK1/2 have also been shown to phosphorylate 4E-BP1 [37,38]. Insulin has also been shown to regulate the phosphorylation of 4EBP1 through the phosphatidylinositol 3-kinase (PI 3-kinase) signaling pathway, which is dependent on the phosphorylation and activation of protein kinase B (PKB)/Akt [39]. Had we analyzed skeletal muscle under fed conditions, we may have observed lower 4E-BP1 in the eIF4E immunoprecipitations, providing an alternative perspective to our treatment. However, the exact role of 4E-BP1 in obese skeletal muscle remains unclear at this time. |
| The formation of the eIF4F complex is essential for proper translation initiation to occur. In the current study, the association of eIF4G with eIF4E trended towards significance (p=0.07) (Figure 5A), following β-GPA treatment, suggested improved translation initiation in the obese. Elongation did not seem to be affected by body weight or treatment in the current study (Figure 6), as was observed in our previous short- term study [27]. This trending change in eIF4G association with eIF4E supports our previous results [27], but may suggest a variation of type and/or duration of treatment from our previous findings with AICAR. To further understand translational control in obese skeletal muscle, we examined the phosphorylation status of eIF4E. Interestingly, we found that eIF4E phosphorylation was significantly increased in the obese β-GPA fed mice (Figure 5B). Similar findings were reported by Svanberg et al. [11] in obese mice treated with insulin, suggesting that obesity may use alternative means of controlling mRNA translation. Phosphorylation of eIF4E has been shown to increase eIF4E’s affinity for mRNA caps by 3-4 fold compared to non-phosphorylated eIF4E [40]. However, the inability to phosphorylate eIF4E at S251 in drosophila (homolog to the mammalian S209) results in delayed development and an overall smaller size [41]. The phosphorylation of eIF4E is largely regulated by the MAP-kinaseinteracting kinase (MNK) 1/2, which appears to be primarily regulated by the ERK1/2 pathway [42]. Observing dramatic changes in eIF4E phosphorylation under physiological conditions has been limited aside from cancer/tumor studies. Thus, we feel that our model provides a unique stimulus for eIF4E and 4E-BP1 that may have profound effects on translational control mechanisms in obesity. |
| In conclusion, 8 weeks of β-GPA feeding caused significant decreases in body mass, especially in the obese mice. Along with this, the skeletal muscle mass decreased to the same absolute and relative levels in lean and obese β-GPA-treated mice, despite lower muscle mass in the OC versus LC. β-GPA feeding returned a downstream target of TORC1 signaling, S6K1 T389, to a level that is comparative with that of their lean counterparts. Interestingly, the OF mice showed an increase in 4E-BP1 and eIF4E phosphorylation, and a trend for an increase in eIF4G’s association with eIF4E, suggesting enhanced translational control in skeletal muscle from obese treated mice (versus OC). Some of the findings in the current study differ from our previous findings [27], which may suggest differences in treatment type and/or duration on mechanisms regulating muscle mass in obesity. Taken together, it is feasible that β-GPA feeding returns ob/ob mouse skeletal muscle to a state in which growth could be attained under the proper stimulus (i.e. exercise or nutrition), not unlike their lean counterparts, and this remains to be determined. Moreover, limiting the loss of muscle mass during a reduction in body weight may allow for a more efficient means of weight reduction and eventual homeostasis in obese individuals. |
| Disclosure |
| The authors have nothing to disclose. |
| Acknowledgements |
| The authors would like to thank Drs. Stephen Alway, Greg Dick, and John Hollander for their generosity and insight throughout the study. |
References
- Kemp JG, Blazev R, Stephenson DG, Stephenson GM (2009) Morphological and biochemical alterations of skeletal muscles from the genetically obese (ob/ob) mouse. Int J Obes (Lond) 33: 831- 841
- Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, et al. (1990) Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J 270: 397-400
- Miller AM, Brestoff JR, Phelps CB, Berk EZ, Reynolds TH, et al. (2008) Rapamycin does not improve insulin sensitivity despite elevated mammalian target of rapamycin complex 1 activity in muscles of ob/ob mice. Am J Physiol 295: 1431-1438
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, et al. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311-326
- Augert G, Monier S, Le Marchand-Brustel Y (1986) Effect of exercise on protein turnover in muscles of lean and obese mice. Diabetologia 29: 248-253
- Chomentowski P, Dubé JJ, Amati F, Stefanovic-Racic M, Zhu S, et al. (2009) Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Geront 64: 575-580
- Amati F, Dubé JJ, Shay C, Goodpaster BH (2008) Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol 105:825-831
- Rannels DE, Pegg AE, Rannels SR, Jefferson LS (1978) Effect of starvation on initiation of protein synthesis in skeletal muscle and heart. Am J Physiol 235: 126-133
- Kelly FJ, Jefferson LS (1985) Control of peptide-chain initiation in rat skeletalmuscle. Development of methods for preparation of native ribosomal subunits and analysis of the effect of insulin on formation of 40S initiation complexes. J Biol Chem 260: 6677-6683
- Sparrow MP, Earl CA, Laurent GL, Everett AW (1976) Turnover rates of muscle proteins in cardiac, skeletal, and smooth muscle: turnover rate related to muscle function. Recent Adv Stud Cardiac Struct Metab 12: 29-34
- Svanberg E, Jefferson LS, Lundholm K, Kimball SR (1997) Postprandial stimulation of muscle protein synthesis is independent of changes in insulin. Am J Physiol 272: 841-847
- Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR (2006) Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281: 39128-39134
- Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, et al. (2003) Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213-220.
- Kimball SR, Jurasinski CV, Lawrence JC, Jefferson LS, et al. (1997) Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol 272: 754-759
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, et al. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200-205
- Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, et al. (2008) Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411-424
- Koopman R, Schaart G, Hesselink MK (2001) Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol116: 63-68
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor- mTOR complex. Science 307: 1098-1101
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM, et al. (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4EBP1. Proc Natl Acad Sci U S A 95: 1432-1437
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, et al. (1999) Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes & Dev 13:1422-1437
- Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569-580
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, et al. (2004) Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J 23: 1761-1769
- Meglasson MD, Wilson JM, Yu JH, Robinson DD, Wyse BM, et al. (1993) Antihyperglycemic action of guanidinoalkanoic acids: 3- guanidinopropionic acid ameliorates hyperglycemia in diabetic KKAy and 57BL6Job/ob mice and increases glucose disappearance in rhesus monkeys. J Pharmacol Exp Ther 266: 1454-1462
- IInoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577-590
- Ellisen LW (2005) Growth control under stress: mTOR regulation through the REDD1- TSC pathway. Cell Cycle 4: 1500-1502
- Tzatsos A (2009) Raptor binds the SAIN (Shc and IRS-1 NPXY binding) domain of insulin receptor substrate-1 (IRS-1) and regulates the phosphorylation of IRS-1 at Ser-636/639 by mTOR. J Biol Chem 284: 22525- 22534
- Drake JC, Alway SE, Hollander JM, Williamson DL (2010) AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am J Physiol 299: 1546-1554
- Fitch CD, Jellinek M, Fitts RH, Baldwin KM, Holloszy JO (1975) Phosphorylated beta-guanidinopropionate as a substitute for phosphocreatine in rat muscle. Am J Physiol 228: 1123-1125
- Fitch CD, Jellinek M, Mueller EJ (1974) Experimental depletion of creatine and phosphocreatine from skeletal muscle. J Biol Chem 249: 1060-1063
- Fitch CD, Shields RP, Payne WF, Dacus JM (1968) Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem 243: 2024-2027
- Shields RP, Whitehair CK (1973) Muscle creatine: in vivo depletion by feeding beta-guanidinopropionic acid. Can J Biochem 5: 1046-1049
- Purchas RW, Romsos DR, Allen RE, Merkel RA (1985) Muscle growth and satellite cell proliferative activity in obese (OB/OB) mice. J Anim Sci 60: 644-651
- Shargill NS, Ohshima K, Bray GA, Chan TM (1984) Muscle protein turnover in the perfused hindquarters of lean and genetically obese-diabetic (db/db) mice. Diabetes 33: 1160-1164
- Katta A, Kundla S, Kakarla SK, Wu M, Fannin J, et al. (2010) Impaired overloadinduced hypertrophy is associated with diminished mTOR signaling in insulinresistant skeletal muscle of the obese Zucker rat. Am J Physiol 299: 1666-1675
- Rivas DA, Yaspelkis BB, Hawley JA, Lessard SJ (2009) Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5’-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside. J Endocrinol 202: 441-451
- Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, et al. (2007) Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387- 396
- Kumar V, Pandey P, Sabatini D, Kumar M, Majumder PK, et al. (2000) Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J 19: 1087- 1097
- Herbert TP, Tee AR, Proud CG (2002) The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem 277:11591-11596.
- Takata M, Ogawa W, Kitamura T, Hino Y, Kuroda S, et al. (1999) Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J Biol Chem 274: 20611-20618
- Minich WB, Balasta ML, Goss DJ, Rhoads RE (1994) Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Nat Acad Sci 91: 7668-7672
- Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P (2002) Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol 22: 1656-1663
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R (2004) Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 24: 6539-6549.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14428
- [From(publication date):
December-2011 - Jul 31, 2025] - Breakdown by view type
- HTML page views : 9775
- PDF downloads : 4653
