Review Article Open Access
A Fine-Tuned Management between Physiology and Immunity Maintains the Gut Microbiota in Insects
| Mithilesh Kajla, Kuldeep Gupta, Lalita Gupta and Sanjeev Kumar* | |
| Molecular Parasitology and Vector Biology Laboratory, Department of Biological Sciences, Birla Institute of Technology and Science (BITS), Pilani Campus, Pilani 333031, Rajasthan, India | |
| Corresponding Author : | Sanjeev Kumar Molecular Parasitology and Vector Biology Laboratory Department of Biological Sciences Birla Institute of Technology and Science (BITS) Pilani Campus, Pilani 333031, Rajasthan, India Tel: +91 1596 515 670 Fax: +91 1596 244 183 E-mail: sanjeev@pilani.bits-pilani.ac.in |
| Received: August 27, 2015 Accepted: September 12, 2015 Published: September 19, 2015 | |
| Citation: Kajla M, Gupta K, Gupta L, Kumar S (2015) A Fine-Tuned Management between Physiology and Immunity Maintains the Gut Microbiota in Insects. Biochem Physiol 4:182. doi: 10.4172/2168-9652.1000182 | |
| Copyright: © 2015 Kajla M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The association of microbial community with the digestive system is a distinct phenomenon and the insect gut is an excellent model to understand these interactions. Insects are omnivorous, feed on all kinds of food and encounter a variety of microbes. The diversity of these large and varied microbial communities inhabiting the gut depends on the feeding behavior of insects. Insect gut is also the foremost immune organ that encounters foreign food particles and exogenous pathogenic/non pathogenic microbes. Thus, it should be equipped by some mechanism that can distinguish between the food and pathogens. In most of the insects, the synthesis of an acellular chitinous peritrophic matrix (PM) around the ingested food compartmentalizes the gut to keep exogenous/endogenous microbes containing food bolus detached from the immunoreactive gut epithelium. This barrier-like functioning of the PM blocks the induction of insect immunity against the microbes present in the gut bolus. In addition to the PM, an extensively cross-linked mucin barrier also suppresses gut immunity against soluble microbial elicitors in the mosquito. Eventually, these acellular barriers maintain ‘low immunity zone’ in the gut to support the survival and proliferation of endosymbiotic microbes. In this review, we discuss that the ‘fine-tuned’ regulation of physiological state of digestion and immunity maintains the fitness-relevant traits such as growth and fecundity in insects.
| Keywords |
| Insect; Saliva; Immunomodulators; Gut immunity; Microbiota; Peritrophic matrix; Peroxidase; Mucin layer |
| Introduction |
| Insects are among the most diverse group of animals found on the earth. They are omnipresent nearly in all environments. The dietary habits of these taxa include a large variety of behaviors. Insects feed on nectar, plant sap, rotten biomaterial, flowers, overripe fruits and animal blood [1]. Interestingly, in some insects the pattern of feeding changes with their developmental stages, age or sex. For example, in case of mosquito, larvae feed on organic matter and adult females use nectar as a flight fuel. Later on, these females switch to blood feeding to acquire excess proteins for the synthesis of vitellogenin to support the development of their eggs [2]. However, males of the same genus feed only on nectar throughout their life span. The physiological and ecological aspects are responsible for those feeding behaviors [3-6]. According to the species of mosquito, blood-feeding females may prefer to feed on the blood of amphibians, reptiles, birds, mammals or particularly humans. Interestingly, only the few mosquito species frequently feed on human blood [7]. On the other hand, some of the insects continue on one or similar type of diet throughout their life as reported in case of Drosophila that both larvae and adults feed on fermented fruits and yeast [8,9]. |
| The choice of the food depends on the desire of the insect and it is controlled by many factors. The meal size certainly depends on the phagostimulatory effects of the food and the nutritional requirements of the insect. Some studies revealed that the meal size is also controlled by the release of the internal fluids containing a mixture of stimulating chemicals [10-14]. Most of the blood-sucking insects (e.g. mosquitoes) ingest in excess even more than their body weight and digest the food in approximately 48-60 hours. The end of feeding is regulated by the degree of gut distension that stores the food temporarily [15]. |
| The blood feeding habits of insects, on the contrary, also expose them to microbes. These microbes may be non pathogenic or pathogenic to the insect (the primary host) or human (the secondary host). The non blood feeding insects such as, Drosophila consuming the rotten food also ingest a variety of microbes, however, these microbes are not transferred to the human host. It is simply because of the non hematophagous nature of Drosophila. On the other hand, several hematophagous insects serve as the carriers for numerous pathogenic microbes. These microbes are potent human pathogens such as, parasites (Plasmodium, Babesia bigemina, Wuchereria bancrofti, Leishmania and Trypanosoma), viruses (dengue, yellow fever and Chikungunya) and bacterium (Bartonella bacilliformis) [16]. The diseases caused by these microbial pathogens affect millions of people all over the world and are important in terms of public health. It is of note that only selected insects serve as vectors for specific pathogen(s). For example, the pathogenic agents causing malaria, dengue, and trypanosomiasis are transmitted by Anopheles, Aedes and tsetse fly, respectively [16]. Numerous factors such as, vector immunity and its internal body environment may determine these specific microbe-vector associations. |
| A variety of microbes such as, bacteria, fungi, viruses and nematodes colonize different body compartments of the insects to establish an association ranging from parasitism to mutualism. For example, the insect salivary glands, reproductive organs, head, muscles, malpighian tubules and hemolymph have been reported to encounter microbes that they acquire from the surrounding environment [17-19]. Experimental evidence suggests that the mutual association of these microbes provide numerous benefits to the insects such as, availability of essential amino acids, help in digestion (E.g. digestion of cellulose in some wood-feeding insects), tolerance to abiotic stresses and degradation of insecticide molecules [20-23]. The insect colonized microbial community also protects the host against pathogens. Interesting examples are obtained from several research reports where the endosymbiotic bacterium Wolbachia interferes the replication of Chikungunya and dengue viruses in Aedes mosquito. In addition, removing bacterial communities in antibiotics fed Anopheles mosquitoes increases their susceptibility to malaria parasite infection [20,24]. These observations also provide the opportunity to manipulate the microbial flora of the insects to control their vectorial capacity. However, further studies are required to elucidate the direct or indirect role of these microbes in the regulation of pathogenic development. In addition, understanding the organization of microbial mutualism in terms of its interaction with the insect immunity is also an important subject in this field. In other words, how the insect immune system distinguishes between beneficial and harmful microbes? Does insect immunity recognize these microbes as immune targets or it develops some mechanism to remain non reactive against them? The maintenance of microbial associations in the different insect compartment is still underway. However, we discuss the details of these phenomena in the mosquito gut, where the interaction of innate immunity with naturally acquired endogenous microbial flora, foreign food particles, food-borne exogenous microbes and pathogens takes place in close vicinity. |
| The gut of both the hematophagous and non hematophagous insects is generally housed by a large variety of microbes. Some of the important microbes present in the insects gut are Serratia, Klebsiella, Pseudomonas, Enterobacter spp, Bacillus, Chryseobacterium, Pantoea, Bordetella, Serratia spp., Gluconobacter spp., Asaia spp., Gluconacetobacter spp., and Acetobacter [20]. In addition, both useful and harmful microbes also enter the gut along with the ingested food. The insect gut is a complex organ that is not only involved in food digestion, but also serves as a central organ of immunity. Because the major aim of food ingestion and digestion is related to physiology and energy metabolism, then encountering the microbes at the same time may induce an immune reaction in the insect gut. These immune reactions can also be lethal to gut natural microbial community, which will certainly hamper their benefits in food digestion and nutrition [23]. Thus, the insect gut must be equipped with a mechanism to fine-tune its biochemical processes to maintain the balance between its physiology and immunity in parallel so foreign food particles and associated microbes never undergo an immune attack. This kind of management process will produce a hospitable environment for the propagation of natural microbial flora in the insect gut. This review will discuss how insect midgut, particularly emphasizing the blood feeding insects such as mosquito, maintains the fine balance between their physiological state of digestion and immunity to minimize the immune reactions against food particles or endogenous bacteria and at the same time protecting the system from pathogenic microbes. |
| Insect saliva inactivates blood components and manipulates host immunity |
| The animal blood feeding is an essential event associated with the life cycle of many insects in a way similar to the mosquitoes. The hematophagous insects apply specific strategies for sucking blood from their hosts and play major tricks to keep them unaware of this process. The insects inject saliva at the site of piercing the host skin before drawing the blood. The saliva is the soup of a complex array of pharmacological agents that perform numerous important functions to facilitate the process of blood feeding [16]. These pharmacological agents also counteracts the vertebrate host responses triggered during the blood feeding, such as vasoconstriction, initiation of the clotting cascade, platelet aggregation and immunological reactions [25-34]. The roles of these insect saliva-mediated modulations of host molecular machinery are very crucial and not only augment the success of blood feeding, but also minimized its downstream side effects on the insect body system after the blood meal. |
| During blood feeding, the damage caused to the host blood vessels usually results in vasoconstriction that may increase insect feeding time due to restricted blood flow to their mouthparts. To overcome these effects, saliva components promote vasodilation. Vasodilators such as, a peptide tachykinin has been characterized in Aedes aegypti that facilitate efficient blood feeding [35]. The presence of vasodilators also reported in the saliva of other blood feeding insects such as, maxadilan in sand flies Lutzomyia longipalpis and simulium vittatum erythema protein (SVEP) in black flies Simulium vittatum [36,37]. It is believed that vasodilation not only facilitates efficient blood feeding, it also creates passage for pathogens and parasites to enter the mosquito gut reluctantly. |
| Mosquito saliva exhibits anti-histamine activity that acts like an antagonist to vasoconstriction in a way similar to other blood sucking insects such as, Rhodnius prolixus [25,32,38]. Thus, the period of blood feeding can be extended before the inflammatory reactions and itching draw the host attention against insect biting [25,38]. It is also found that the mosquito Aedes aegypti and Culex quinquefasciatus secrete adenosine deaminase in their saliva in a manner similar to the sandfly Lutzomyia longipalpis. This enzyme removes adenosine, a molecule associated with both the initiation of pain perception and the induction of mast cell degranulation, from the site of the insect bite [39,40]. |
| Anopheles and Aedes mosquito saliva also contains inhibitors of platelet aggregation that is mediated through an enzyme called apyrase. Apyrase catalyses the conversion of ADP (adenosine diphosphate), the platelet-aggregating factor, into a non-active form AMP (adenosine monophosphate) [28,29,41]. Interestingly, the amount of apyrase injected into the host skin by the mosquito directly determines its feeding time. Studies also found that an A. stephensi protein inhibits collagen-induced platelet aggregation and termed as anopheline antiplatelet protein (AAPP) [42]. It is common in several insects that their saliva not only counteracts the host hemostatic responses, it also has the capability to suppress the host immune system [43,44]. Salivamediated downregulation of the proinflammatory cytokines TNF-α, IL-2 and IFN-γ avoids early immune reactions in the vertebrate host. Thus, the downregulated immune responses will appear in the host after the insect has taken the blood meal [25,45-47], Figures 1 and 2 in reference [48] |
| The alteration of host immunity by insect saliva has consequences in terms of pathogenic attack in the vertebrate host and it may determine the vectorial capacity of insects to categorize them as major or minor vectors. A comparative study carried with West Nile virus (WNV) revealed that inoculation of the arbovirus by Culex mosquitoes potentiate infection in hosts compared to viral inoculation by infection needle. In the former case, the host developed near about 10 fold higher viremia than needle inoculation of WNV [49]. In addition, needle inoculation of virus with mosquito salivary gland extract (SGE) also produced higher viremia in the host. These effects were caused by mosquito saliva mediated alterations of cytokines and other components of host innate immunity that lead to immunosuppression or immune dysregulation [49,50]. Similar conclusions were also drawn in case of dengue and Rift Valley fever causing viruses, which are also transmitted by the Aedes mosquito. We believe that these immunosuppressive practices of mosquito saliva minimize the risk of immune attack on its gut system that may be caused by the host blood. |
| Insect gut natural microbiota help in food digestion |
| The digestion of ingested blood takes place inside the mosquito midgut. Generally, the mosquitoes, like other insects, also harbor natural commensal bacteria in their gut. This natural gut microbiota plays an important role in food digestion and nutrition [23]. In addition, these endogenous bacteria also regulate the development and maturation of the mosquito gut innate immune system [51]. It is also interesting to understand how this microbiota play an important role in mosquito digestion (physiology) and gut immunity. |
| Insects consuming rotten biomaterial (such as Drosophila) also ingest these microbes along with the food. These microbes colonize the gut and then become part of the commensal flora [52]. As we discussed before that mosquito also takes animal blood as food, which is generally sterile. Therefore, the chances of microbial ingestion with the food are minimized. However, the commensal bacteria already colonize the mosquito gut during nectar feeding at some stages of development at least, before blood feeding. Inside the mosquito gut a diverse community of bacteria from several phylogenetic classes has been reported. Interestingly, the types of these bacteria vary in both laboratoryreared and wild populations of mosquitoes [18]. The common natural bacteria present in mosquito gut are Serratia marcescens, Klebsiella ozaenae, Pseudomonas aeruginosa, Enterobacter spp., Cryseobacterium meninqosepticum, Enterococcus faecalis, Proteobacteria, Bacteroidetes, Enterobacteriaceae and Flavobacteriaceae [18,53-57]. It is noteworthy to mention that the number and type of bacteria also change markedly depending on both the stage of development and the blood feeding status of the mosquito. |
| Intestinal microbes contribute to blood digestion, producing essential vitamins for the host and keep out potentially harmful microbes. Bacteria contribute to the nutrition of insects in different ways. Midgut bacteria can produce compounds that are directly assimilated by the host or they can improve digestion through production of degradation enzymes that facilitate the assimilation of complex molecules [20]. In addition, it is also expected from the gut microbiota that they should provide dietary supplements to complete the limitations of nutrients in the ingested food. Studies carried in non blood- or blood-feeding insects are endowed with such evidences to support this belief. In case of the plant feeders, microbiota generally provides vitamins, amino acids and sterol that complement the insect diet. One such bacterium named Buchnera has been identified in the gut of aphids [58]. In another example, bacteria Wigglesworthia morsitans provide vitamin B to the tsetse fly because of its absence in vertebrate blood [59]. The bacterial species Asaia bogorensis is also found to provide vitamins supplements to Anopheles stephensi mosquito [60]. |
| Gut bacteria also interact with host system to regulate the physiology of insect. In Drosophila, the Lactobacillus plantarum bacterium acts on host nutrient sensing system and controls hormonal growth signalling [61]. Some studies have identified the molecular aspect of the above relationship between the development of the host and the flora relationships. In Drosophila the pyrroloquinoline quinonedependent alcohol dehydrogenase (PQQ-ADH) of the commensal bacterium Acetobacter pomorum interacts with insulin/insulin-like growth factor signalling (IIS) of the host to maintain the gut-microbe mutualism [62]. In mosquitoes, evidences suggest that bacteria could also be involved in such processes. For instance, two bacteria Serratia and Enterobacter contain hemolytic enzymes and play a role in blood digestion [19,63,64]. In Aedes albopictus mosquitoes Acinetobacter johnsonii and A. baumannii bacteria are reported to be involved in both blood digestion and nectar assimilation [65]. Evidences from recent studies reveal that Acinetobacter strains isolates from the mosquito gut metabolize the amino acids α-keto-valeric acid and glycine (these are animal blood components) as well as 4-hydroxy-benzoic acid and xylose (the common plant sap constituents) [60]. These findings provide evidences that insects-acquired microbes are equally competent to support their surviving on either the blood- or non-blood meal and serve as the source of important nutritional components. |
| Some research findings gathered direct evidences for the involvement of endogenous gut microbes in food digestion and physiology. Studies carried in tsetse fly revealed that a gut associated bacterium Wigglesworthia genome encodes for proteins that regulate the biosynthesis of several group B vitamins [66,67]. Elimination of the bacteria Wigglesworthia from tsetse fly by lysozyme treatment revealed that the fecundity of these flies was greatly reduced. In some experiments when the diet of the tsetse fly was supplemented with B vitamins, it partially restored the fecundity [66]. In another study, oral administration of antibiotics in Aedes aegypti females affected the lysis of red blood cells (RBCs) as well as slowed down the digestion of blood proteins. Although the antibiotics treatment did not affect the survival of mosquitoes however, it reduced their ability of egg production significantly [68]. These findings revealed that gut microbes not only donate the diet supplements to the insect host, rather they also regulate some other physiological events that are helping in food digestion and ultimately the fecundity. |
| In conclusion, the acquisition of microbial community from the environment is an essential part of insect life, digestive process and survival. The above examples also provide evidences that the acceptance of endogenous microbes by the insect gut is although a common process, however it is specific too. The internal environment of the insect may allow only certain types of microbes to establish their community inside the gut. On the other hand, it may be also possible that the availability of common microbes from particular external environment regulate the gut internal environment to support their growth. Further studies are required to elucidate the details of microbialgut relationship in the insects. This knowledge will enhance our ability to control insect physiology and survival through the manipulation of their gut environments. |
| Insect gut finely balances the blood digestion and immunity against foreign particles |
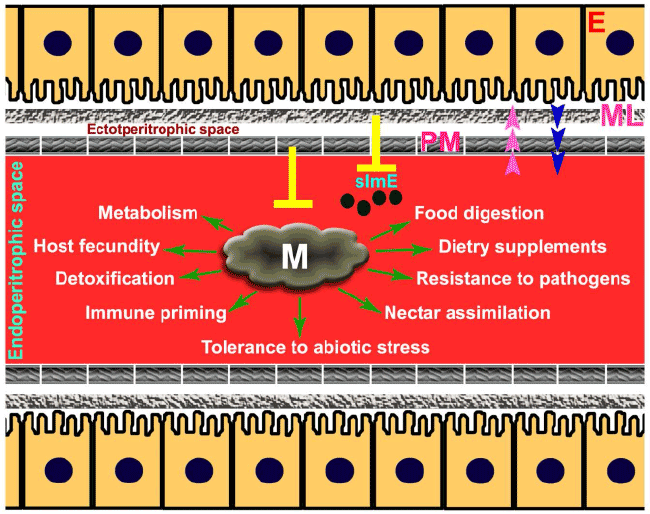
| As we have seen in case of mosquitoes that the colonized native microbes play an important role in gut homeostasis in a way similar to Drosophila gut. It is important for these insects to maintain low levels of ‘immune zone’ in the gut to permit the structure and composition of the flora. It is noteworthy to mention that blood feeding induces proliferation of these bacteria [69,70]. Thus, management of gut immunity against foreign food particles and endogenously proliferating bacteria along with food digestion in parallel requires a fine balance. This management will positively support physiology and stop unnecessary activation of immunity because immune reactions produce toxins which have negative effect on mosquito fecundity [70,71]. The mechanisms that maintain low immunity for securing the existence of gut microbiota or commensal gut homeostasis in case of blood-feeding insects (such as Anopheles mosquito) is discussed below (Figure 1). |
| Physical barriers modulate gut immunity against foreign food particles and antigens |
| An interesting phenomenon is apparent in non- or bloodfeeding insects where the gut lumen side is lined by non cellular chitinous material called the peritrophic matrix (PM). PM serves as a shield to protect microvilli from direct contact with ingested food and the abrasion caused by the food particles. Drosophila has a type II PM that is continuously produced by a ring of specific cells at the cardia, a specialized organ at the anterior of the midgut and PM grows posteriorly, it encloses the food passing through it all along the digestive tract [72]. Mosquitoes have type I PM, which is produced in direct response to blood feeding, forming a thick bag-like structure that completely surrounds the ingested meal [73,74]. In other words, the PM compartmentalizes the gut environment where the food particles are enclosed by the matrix in endoperitrophic space and leaving an ectoperitrophic space between midgut epithelium (Figure 1). |
| Peritrophic matrix is like a web of chitin fibers interlocked with proteins or glycoproteins and proteoglycans. The various proteins that make the PM are called peritrophins and have chitin-binding domain. These proteins are premade in the gut cells and released soon after the entry of food particles into the gut lumen [75]. The peritrophic matrix is made around the ingested food to enwrap it in such a way that the foreign food particles never come in direct contact with the immunoreactive gut epithelium. In addition, if the microbes are present along with food particles they are also not allowed to interact the gut epithelium directly [71,76]. In other words, the recognition of ingested foreign particles by the immune receptors that are present on gut epithelium is minimized by PM-mediated compartmentalization. |
| This phenomenon not only avoids the induction of immunity against the bolus contents, but also reduces downstream consequences as we discussed before. |
| Peritrophic matrix acts like a biochemical barrier that sequesters and inactivates toxins and protects insects from oxidative damage due to the ingested allelochemicals. In case of blood feeding insects, the iron-containing heme is released during the process of hemoglobin degradation that can generate highly toxic reactive oxygen species (ROS). Thus, to prevent toxicity heme binds to the PM and later on excreted outside the body [77,78]. In conclusion, the PM compartmentalizes digestive processes to allow efficient nutrient acquisition and preventing any damage to the insect gut system by the food-generated toxins. |
| Evidences gathered from several studies reveal that the peritrophic matrix is acting like a barrier to reduce the activation of insect immune system against food particles or pathogens present in it. In case of Drosophila a protein Drosocrystallin (Dcy) contributes to PM formation and a loss-of-function mutation in the dcy gene resulted in reduction of PM width. These Drosophila mutants also revealed higher levels of expression of antibacterial peptides upon bacterial ingestion. There is also an increase susceptibility to entomopathogenic bacteria Pseudomonas entomophila and Serratia marcescens and P. entomophila novel pore-forming toxin (PFT) extract in dcy-deficient flies [79]. Similarly the disruption of PM in tsetse fly induced immunity against gut microbial antigens and exogenous bacteria (Enterobacter spp. and Serratia marcescens) [80]. These findings concluded that the PM acts as a barrier to regulate the insects immune reactions against the gut microbiota. The induction of insect immunity in the absence of PM may indicate the interaction between immunoreactive gut epithelium and microbial flora present in the food bolus. |
| Permeability of PM regulates food digestion over microbial interaction with gut epithelium |
| The firmed organization of insect PM blocks the access of food antigens to the immune system without affecting the food digestion. This indicates that epithelium-secreted digestive enzymes must cross the PM to reach the food bolus in the gut lumen. Therefore, sufficient porosity of PM must allow the hydrolytic products of digestion to traverse the barrier in the opposite direction so they can be absorbed by the gut epithelial cells [81]. A chitinase enzyme expressed after the blood meal in mosquitoes is proposed to partially degrade the PM to increase its porosity for the trafficking of digestive enzymes and digested products [82]. It is also important to mention that the major proteins participating in the formation of peritrophic matrix appear to be glycosylated, primarily by high mannose N-linked glycosyl groups [83]. This glycosylation property of proteins facilitates the activity of the digestion process and protects them from digestion. |
| In fact PM acts like a sieve around the ingested blood and its pore size is very small and varies among insects. For example, the tsetse fly PM pore size is 9 nm and allows the passage of globular molecules less than 150 KDa [84,85]. This pore size is comparable with the mosquitoes PM [86]. Thus, soluble enzymes in the endoperitrophic space digest the larger food particles and the smaller digested products reach the ectoperitrophic space through the small pores of the matrix. Further, the digestion is completed in the ectoperitrophic space with the help of gut epithelium integral enzymes [87]. This sequential breakdown of food certainly helps to facilitate digestion over the direct interaction of gut epithelium with food particles or microbes present in it. |
| It is clear that the nano-sized pores in the PM certainly inhibit the interaction of food-ingested pathogens with immunoreactive insect gut epithelium. Experiments with Spodoptera exigua (Hübner) larvae indicate that continuous feeding of larvae on a diet treated with chitin binding Calcofluor White M2R significantly retarded larval development and resulted in high mortality. This compound produced pores in PM and increased its permeability that also enhanced larval susceptibility to Syngrapha falcifera multiple nucleopolyhedrovirus (SfaMNPV) infections [88,89]. Results obtained in tsetse fly revealed that the exogenous bacteria (Enterobacter spp. and Serratia marcescens) proliferation was impeded in flies that lacked an intact PM after RNA interference. The reduced growth of bacteria was due to the induced expression of antimicrobial peptide gene attacin by these flies in comparison to the controls with fully developed PM. In addition, the flies lacking an intact PM were also highly susceptible to African trypanosome parasites [80]. These findings concluded that the PM acts as a barrier to regulate the insects’ ability to immunologically detect and respond to the presence of microbes in the gut bolus (Figure 1). |
| Modulation of insect gut immunity against endogenous microbes and the immune elicitors |
| The insects gut system is in constant exposure to their commensal microbiota. To maintain a fine balance between normal physiology and immunity, the gut immune system must distinguish commensal and pathogenic bacteria and avoiding the constitutive production of immune effectors, such as antimicrobial peptides (AMPs) and reactive oxygen species (ROS). Non blood feeding insect Drosophila melanogaster maintains commensal gut microbial community by modulating the immunodeficiency (IMD) pathway and dual oxidase (DUOX) system [90]. The former is suppressed by the binding of homeobox transcription factor Caudal to the promoter regions of the AMPs genes. The Caudal-deficient flies produce AMPs constitutively, alter the gut microbiota and exhibit disintegration of the epithelial cell layer [91]. Amidases secreted by the epithelial cells of D. melanogaster midgut and Pirk sequesters specific peptidoglycan-binding receptors (PGRP-LC) in their cytoplasm cleave pro-inflammatory peptidoglycans or bind them, respectively to modulate immunity against commensal bacteria [92,93]. Peptidoglycans (PGNs) or other virulent factors released by proliferating transiently colonized foreign pathogenic bacteria may activate IMD pathway-dependent AMP production to distinguish them from the friendly bacteria [94]. In D. melanogaster the proliferating pathogenic bacteria activate DUOX system through p38 signaling pathway. However, the commensal microbiota inhibits DUOX induction by MKP3 pathway with no effect on basal ROS production that is mediated through a non-PGN ligand on the epithelial cell surface [95]. Thus, the ‘fine-tuned’ regulation of the above-discussed two synergistic immune responses, contribute to commensal microbial homeostasis in the midgut of D. melanogaster. |
| Colonization of the insect gut with commensal microbes may increase the host resistance against parasites or other pathogens. This might be simply due to the competition for nutrition, space or immune priming [90]. Gut inhabitants in mosquito have been reported to exhibit an antagonistic effect against malaria parasite development [56,96,97]. In Anopheles mosquitoes, gut colonization with Gram-negative bacteria or feeding antibiotics resulted in reduced and enhanced Plasmodium infections, respectively [96,98-100]. These effects are due to the bacteria-induced antiplasmodial immune responses [99,101]. Thus, the community composition of vectors is important to regulate their vectorial capacity in nature. The immune priming may be mediated through the activation or alteration of the insect gut immune responses toward recurrent colonization of commensal bacteria or pathogens. |
| Studies carried in malaria vector revealed that the gut microbiota is essential for priming the mosquito immune system against malaria parasite [102]. Also in the tsetse fly microbial symbionts were demonstrated to be essential during larval development and making the adult flies trypanosome-refractory through influencing peritrophic matrix integrity [103]. |
| Although peritrophic matrix acts like a barrier between the foreign food particles and gut epithelial immunity, however, the soluble elicitors released by the microbes present in the food may interact with the gut epithelium and can induce an immune response. It is observed in many studies where feeding the microbial elicitors alone without blood induced immune responses in insects including mosquito [104,105]. However, the oral administration of microbial elicitors (e.g. lipopolysacharide, LPS) with blood does not induce antimicrobial activity in the mosquito midgut [71]. This may be simply explained in terms of blood feeding induced PM synthesis in the mosquito gut. However, the comparison of the pore size in mosquito gut and LPS molecular weight may reveal that LPS can easily pass through these pores and thus, may react with epithelial cells. So if PM cannot inhibit the LPS movement, then there must be another barrier between the ectoperitrophic space and midgut epithelium to block the immune responses against soluble microbial elicitors. |
| Recent finding from Kumar et al. [69] in Anopheles gambiae mosquitoes indentified the presence of another barrier at the luminal surface of the midgut epithelium where mucins are cross-linked with the help of two heme peroxidases called Immunomodulatory peroxidase (IMPer) and DUOX. This barrier further compartmentalizes the ectoperitrophic space and reduces the possibility of interaction between microbial immune elicitors and midgut epithelium. These authors tested this hypothesis after silencing the IMPer gene and analyzed its effect on the immune responses in normal blood fed mosquito midguts. The RNA interference mediated silencing of IMPer gene induced an array of anti-bacterial immune markers, such as cecropin, peptidoglycan recognition protein–S3 (PGRP-S3) and PGRP-LB. These immune genes collectively suppressed the bacterial load in the blood bolus. When the same experiment was carried in IMPer silenced and antibiotics fed mosquitoes, these anti bacterial immune markers were not induced [69]. These findings clearly indicate that the presence of peroxidases-mediated mucin crosslinked barrier blocks the immune activation against bolus bacteria and supports their growth in the gut lumen. This report also has an interesting outcome that the mosquito immunity is capable of killing malaria parasites significantly in the IMPer silenced mosquitoes against controls [69]. In this case the killing of malaria parasite was mediated through the activation of nitric oxide synthase (NOS) pathway rather the classical antibacterial immune genes as discussed above. Thus, microbial specific immune pathways are activated after the removal of peroxidases crosslinked mucin barrier in mosquitoes. |
| We emphasize that the barrier-mediated temporary compartmentalization blocks the interactions of microbes and their immune elicitors with pathogen recognition receptors present on the surface of midgut cells. This kind of management is necessary to minimize the immune activation and maintaining a ‘zone of low immunity’ in the gut that will support the survival of commensal microbes and their benefits to the host. If the immunity is not controlled, it may engage the gut in the war against food all the time. The questions remain unanswered whether these mechanisms are specific to A. gambiae mosquito or more widely distributed among other insects and that demands future studies. |
| Conclusion |
| The feeding behavior of insects exposes them to a variety of microbes. Many of these microbes harbor their gut and remain inside throughout the life of the insect. Because these microbes are beneficial to the insect host in terms of digestion and providing dietary supplements thus, it is essentially required that their niches should be defined inside the gut. In addition, these microbes must have easy access to the food and remain protected against digestive process and insect immunity. |
| The area of microbes-insect host interactions has been extensively studied in numerous commonly found insects. Due to the disparity in the feeding behavior (for example, non blood- and blood-feeding), insects harbor many different kind of microbes. These microbes adapt the gut environment and strengthen their association with the host system in a way that both of them can mutually receive their benefits. The existence of host-microbes association also demands their protection from each other. If microbes affect the host system, then innate immunity regulates their growth. However, in general, the microbiota remains protected by the host immunity through various mechanisms. One important mechanism is the synthesis of protective acellular matrices between the foreign food particles and gut epithelium. These matrices act like a barrier and block the interactions of microbes and immunoreactive gut epithelium. Thus, the insects can continue the digestion of food without the activation of immunity. This fine balance between the two phenomena stabilizes the insect-microbial association. |
References
|
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15504
- [From(publication date):
December-2015 - Aug 25, 2025] - Breakdown by view type
- HTML page views : 10805
- PDF downloads : 4699
