Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Abnormal Levelsof γ -Glutamyl Transpeptidase (GGTP), ALT, AST in Human Immunodeficiency Virus-1(HIV-1) Infection
| Ramana KV1*, Ratna Rao1 and Sabitha2 | |
| 1Apollo Health City, Jubilee hills, Hyderabad, Andhra Pradesh, India | |
| 2Chalmeda Anand Rao Institute of Medical Sciences, Bommakal, Karimnagar, Andhra Pradesh, India | |
| *Corresponding Author : | Dr. K V Ramana Research Scholar Department of Microbiology Apollo Health City Jubilee Hills Hyderabad, India Tel: 09440704234 E-mail: ramana_20021@rediffmail.com |
| Received March 12, 2012; Accepted April 12, 2012; Published April 16, 2012 | |
| Citation: Ramana KV, Ratna Rao, Sabitha (2012) Abnormal Levels of γ-Glutamyl Transpeptidase (GGTP), ALT, AST in Human Immunodeficiency Virus-1(HIV-1) Infection. Biochem Physiol 1:101. doi:10.4172/2168-9652.1000101 | |
| Copyright: © 2012 Ramana KV. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
| Introduction |
| Human Immunodeficiency Virus infection is associated with direct inflammation of hepatocytes leading to liver damage. Antiretroviral therapy, co-infection with other hepatotropic viruses, tumors, parasitic infestation and non-antiretroviral therapeutic drugs may also cause considerable hepatic damage in Human Immunodeficiency Virus infection [1]. Mechanisms by which Human Immunodeficiency Virus infection initiates liver destruction can be attributed to apoptosis (induced by caspases 2,7), mitochondrial dysfunction either by decreasing mitochondrial DNA in various cells or by alteration in mitochondrial membrane by HIV proteins that in turn stimulate inflammatory response [2,3]. HIV infection results in cytopathic effect on cells carrying CD4 receptors including helper T cells, macrophages of various organs, microglial cells, B-lymphocytes, haemopoietic stem cells, rectal mucosal cells and liver sinusoidal epithelial cells [4]. Hepatomegaly was seen as a common feature in both HIV infected asymptomatic and AIDS cases. With the advent of HAART, though the morbidity and mortality associated with HIV infection has considerably reduced, the cause of concern is the adverse drug reactions, hepatotoxicity, dyslipidemia and disturbed metabolism. However, due to the high costs of drug regimens and the lack of healthcare infrastructure in developing countries, the widespread use of ART is currently still partial at best. Host factors may also determine whether or not an HIV-1-infected individual rapidly develops clinically overt immunodeficiency and the identification and characterization of host factors contributing to the course of HIV infection, including immunological defense mechanisms and genetic factors, will be crucial for our understanding of the immunopathogenesis of HIV infection and for the development of immunotherapeutic and prophylactic strategies [5-7]. In continuation to our previous research supporting the need to find alternatives to CD4 counts and HIV/RNA viral load which help in staging the disease and predicting the death, we impress on the need for biological markers that help in the management of HIV infection before and after initiation of highly active Antiretroviral Therapy (HAART) [8-10]. |
| γ-Glutamyl Transpeptidase (GGTP) is an enzyme that is predominantly synthesized in liver (hepatocytes) and biliary epithelial cells. Kidneys, pancreas, brain cells, spleen, seminal vesicles and cell membranes of other organs also produce GGTP [11]. The major function of GGTP is to transfer aminoacids and peptides in to cells and maintain cellular concentrations of glutathione which is a critical antioxidant defense for cell against oxidative stress [12]. The normal low level for females is less than 9 U/L, while that of men is less than 14 U/L. The moderate level for females is 18 U/L, while that of males is 28 U/L. High level is more than 36 U/L for females and more than 56 U/L for males [13]. Abnormal GGTP levels in the serum indicate liver disease mainly because of biliary obstruction rather than hepatocellular damage. Acute pancreatitis (pancreatic disease), myocardial infarction, chronic obstructive pulmonary disease, renal failure, obesity, chronic alcoholism, anorexia, hyperthyroidism, muscle disease, neurological disorders, congestive heart failure, and diabetes are other clinical conditions where serum GGTP levels are raised [14]. Drugs such as phenytoin (Dilantin) and barbiturates and carbamazepine (Tegretol), that is used to control seizures and use of many prescription and non prescription drugs including non-steroidal anti inflammatory drugs (NSAIDS), lipid-lowering drugs, antibiotics, histamine blockers (used to treat excess stomach acid production), antifungal agents, antidepressants, hormones like aldosterone, oral contraceptives and clofibrates may alter serum GGTP [15]. Abnormal levels of GGTP may be due to the leak of GGTP from the cells in response to oxidative stress. Studies in the past have related GGTP to cardiovascular and cerebrovascular disease. Abnormal GGTP which is normally considered as a risk factor for liver function in chronic alcoholics has also been related to hypertension and incident diabetes [14]. Prospective studies performed previously have positively related serum GGTP to white blood cell count, red blood cell count, the hematocrit and hemoglobin. Previous studies have demonstrated the role of GGTP itself in the generation of reactive oxygen species in response to altered iron metabolism [15]. In the present study we have evaluated the levels of serum γ-Glutamyl Transpeptidase (GGTP), ALT and AST in HIV seropositive patients who are treatment naïve and those who are on HAART for at least 3-4 months. |
| Materials and Methods |
| The study was carried out at Apollo Health City, a tertiary care hospital between June 2010 to May 2011, which included 36 HIV seropositive and antiretroviral therapy naive individuals and 21 HIV seropositive patients presently on HAART since 3-4 months attending Integrated Counseling and Testing Centre (ICTC) situated at Area hospital Siddipet were enrolled in the study. A total of 25 Normal healthy individuals are included in the study as controls. The Mean ± SD of age group included is 36.33±11.67 for cases and 35.92±12.98 for controls. Gender of the cases (54.4% of male and 45.6% females) were matched with control group. The study group were screened for Hepatitis B and C viruses and excluded from the study if found positive for any of the hepatotropic viruses. All the subjects included in the study were provided with a Proforma with details of the study and an informed and written consent was obtained. Blood samples were collected following standard laboratory procedures. Their HIV status was confirmed following NACO guidelines using three different ELISA methods [16]. Serum γ-Glutamyl Transpeptidase (GGTP), Serum Alanine aminotransferase (SGOT) and Asparate aminotransferase (SGPT) were measured by using Diasys kits in an automatic analyzer. |
| Statistical Methods |
| The Statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1 ,Systat 12.0 and R environment ver.2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc. Descriptive statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean ± SD (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5 % level of significance. The following assumptions on data is made, Assumptions: 1.Dependent variables should be normally distributed, 2.Samples drawn from the population should be random, Cases of the samples should be independent. |
| Analysis of variance (ANOVA) has been used to find the significance of study parameters between three or more groups of patients, Student’s t-test (two tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups Inter group analysis) on metric parameters. Leven1s test for homogeneity of variance has been performed to assess the homogeneity of variance. Chi-square/ Fisher Exact test has been used to find the significance of study parameters on categorical scale between two or more groups. |
| Results |
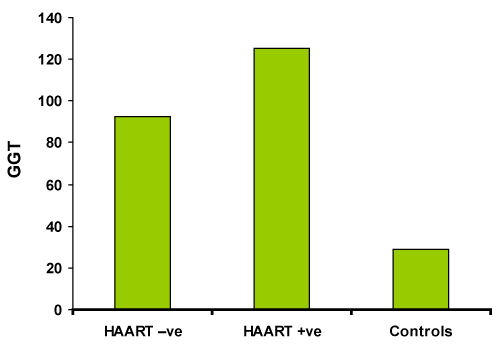
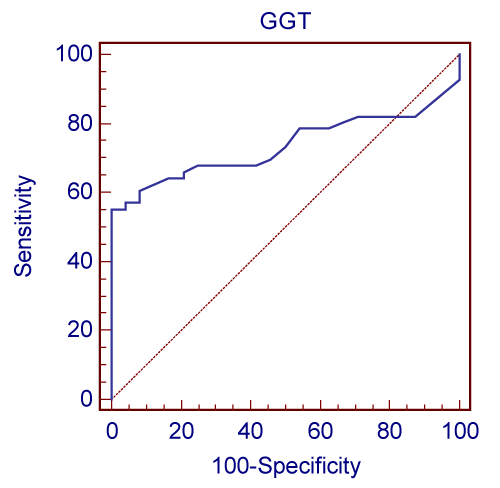
| The study results showed serum concentrations of γ-Glutamyl Transpeptidase (GGTP), AST and ALT in HIV seropositive patients who are antiretrotherapy naïve patients as 60.57±33.58, 22.25±11.85 and 35.14±30.11 respectively. After HAART the serum levels of the same parameters along with the controls and AST/ALT ratio are listed in table 1. Graphical representation of GGT in three study groups is shown in Graph1. Graph 2 represents ROC curve analysis of GGT showing 100% specificity. |
| Discussion |
| The study results have clearly shown abnormal liver function tests in HIV patients who are not on HAART and revealed raised levels of serum GGTP, ALT and AST and AST/ALT(>1) ratio compared to the normal healthy individuals. Results also have implicated the role of HAART in initiating liver damage even in the absence of other hepatotropic viruses as revealed by other studies [17,18]. Recent reports have indicated abnormal liver function tests in a multisystem disorder [19]. Liver function has been influenced by various non-infectious factors such as smoking, diabetes, body mass index and hypertension [20-22]. What is very interesting here is that HIV, with its complex disease progression where the infected patients can survive even up to 20 years are at risk of developing multiple organ dysfunction. This can be attributed to various mechanisms of HIV pathogenesis including chronic inflammation, recurrent infections and drug related toxicities [23]. Liver is one such organ which is most affected and if proper intervention is not done may cause severe morbidity and mortality. |
| As evidenced from previous reports most of the HIV infected patients are in the age group of 20-40 years with much of their productive life is at the danger due to the complex disease progression. Though availability of HAART has significantly reduced the mortality of HIV infected persons, various other factors also play an important in reducing the morbidity of HIV infected persons including proper nutrition, avoiding alcohol, smoking etc [24,25]. HIV related liver disease as evidenced from recent reports should be considered as a major concern for physicians’ treating HIV patients [26,27]. HIV patients should be carefully monitored for liver dysfunction and appropriate surgical and patient care must be provided. |
| In conclusion this study highlights the role of HIV in liver disease and significance of monitoring HIV infected and those on HAART for possible hepatic destruction. Physicians treating HIV infected patients including the gastroenterologists can assess the extent of liver damage by measuring in patient’s serum GGT, ALT and AST which are cost effective and easily performed non invasive methods [28]. The further course of the HIV-1 pandemic, therefore, mainly depends on how and to what degree developing countries with a high HIV-1 prevalence are able to take advantage of the medical progress achieved in Europe and North America, and whether an effective prophylactic vaccine will become available in the near future. An understanding of the immunopathogenesis of HIV-1 infection is a major prerequisite for rationally improving therapeutic strategies. The influence of these and other factors, on the clinical progression of HIV infection should be reviewed in detail, both preceding and following treatment initiation [29]. |
| Acknowledgements |
| I sincerely thank the Dr NTR University of Health Sciences, Vijayawada, Andhrapradesh, India and also Dr K. P. Suresh, PhD (biostatistics) for reviewing the statistics |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 11544
- [From(publication date):
May-2012 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 6943
- PDF downloads : 4601
