Research Article Open Access
An Efficient Technique to Detect Sperm Reactive Oxygen Species: The CellRox Deep Red® Fluorescent Probe
| Maíra Bianchi Rodrigues Alves1, André Furugen Cesar de Andrade2, Rubens Paes de Arruda3, Leonardo Batissaco1, Shirley Andrea Florez- Rodriguez1, Renata Lançoni3, Bruna Marcele Martins de Oliveira1, Mariana Andrade Torres2, Gisele Mouro Ravagnani2, Tamie Guibu de Almeida1, Vinícius Silva Vellone1 and Eneiva Carla Carvalho Celeghini1* | |
| 1Laboratory of Research in Pathology of Reproduction, Department of Animal Reproduction, School of Veterinary Medicine and Animal Science, University of Sao Paulo (USP), Pirassununga, SP, Brazil | |
| 2Laboratory of Andrology and Embryo Technology, Department of Animal Reproduction, School of Veterinary Medicine and Animal Science, University of Sao Paulo (USP),Pirassununga, SP, Brazil | |
| 3Laboratory of Semen Biotechnology and Andrology, Department of Animal Reproduction, School of Veterinary Medicine and Animal Science, University of Sao Paulo (USP), Pirassununga, SP, Brazil | |
| *Corresponding Author : | Eneiva Carla Carvalho Celeghini Animal Reproduction Department FMVZ, USP, Av. Duque de Caxias Norte 225. Postal Code 13635-900 Pirassununga/SP, Brazil Tel: +55(19) 3565-4242 E-mail: celeghin@usp.br |
| Received February 28, 2015; Accepted March 25, 2015; Published April 02, 2015 | |
| Citation: Alves MBR, de Andrade AFC, de Arruda RP, Batissaco L, Florez- Rodriguez SA, et al. (2015) An Efficient Technique to Detect Sperm Reactive Oxygen Species: The CellRox Deep Red® Fluorescent Probe. Biochem Physiol 4:157. doi:10.4172/2168-9652.1000157 | |
| Copyright: © 2015 Alves MBR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Heat stress and testicular traumas increase sperm Reactive Oxygen Species (ROS) resulting in Oxidative Stress (OS) and injury of sperm. The fluorescent probe CellROX Deep Red® is not already used to detect ROS in spermatozoa. On this way, this study aims to evaluate its efficiency in detecting ROS in ram sperm. For that, it was used ejaculates from rams performing the in vitro induction of sperm OS in T0, T50 and T100. T0 was semen sample that was not submitted to OS induction, T50 was 50% induced to OS induction and T100 was entire submitted to OS induction. The production of ROS was detected by CellROX Deep Red®. Data polynomial regression analysis was performed and the result of determination coefficient was 0.728. Besides, sixteen rams were submitted to scrotal insulation during 72 hours performing the in vivo induction of OS sperm. Analyses of sperm motility, morphology, DNA fragmentation and ROS (detected by CellROX Deep Red®) were done two times before the scrotal insulation and two times afterwards. Data analysis of variance was performed from the periods (before x after) and it is observed that after scrotal insulation the total and progressive motility decrease (p<0.05) and total and major sperm defects and sperm DNA fragmentation increase (p<0.05). Moreover, after insulation, it was observed increase (p<0.05) in sperm showing moderate and intense OS. Thus, it is possible to conclude that CellROX® fluorescent probe is able to detect ROS production in ram sperm by in vitro and in vivo induction being a new technique to detect sperm OS.
| Keywords |
| Heat stress; Reactive oxygen species; Ram; Male; Infertility; Oxidative stress induction |
| Introduction |
| The global environment temperature is growing and it is already established that it prejudices the male reproduction function. High temperature leads increase of reactive oxygen species (ROS) and, consequently, oxidative stress [1]. Sperm cells are highly susceptible to oxidative stress, which results in fewer ROS neutralizing enzymes in their characteristic small cytoplasm. This leads to a diminished cellular protection. In addition, sperm contain large amounts of polyunsaturated fatty acids, proteins and DNA, which are substrates for ROS [2,3]. Besides, sperm mitochondria produces the most ROS [4], which trigger lipid peroxidation of polyunsaturated fatty acids in the plasma membrane [3,5]. Lipid peroxidation, in turn, reduces plasma membrane fluidity and, as a result, it prejudices sperm function, culminating in decreased sperm motility [6-8] and increased rates of sperm DNA fragmentation [9], cellular apoptosis [2,8,10,11] and morphologically abnormal sperm [2,6]. |
| The detection of ROS in sperm samples is critical for diagnosing infertility. Chemiluminescence is the most commonly used technique for this purpose, but it does not differentiate between ROS produced in sperm and ROS produced in leucocytes. Additionally, results with chemiluminescence have been inconsistent [12]. A widely used technique for measuring oxidative stress, on the other hand, is measuring malondialdehyde with the thiobarbituric acid reaction technique (TBARs) in spectrophotometry [6,13]. However, this technique may produce overestimated values because other substances may also react to TBARs and result in products with an absorbance similar to that of malondialdehyde [14]. Moreover, pH and temperature alterations can induce peroxidation and produce a reading with higher malondialdehyde values [14]. |
| Various fluorescent probes are available for oxidative stress analysis, such as C11-BODIPY [15], MitoSOX Red® [5], and H2DCF [16]. C11- BODIPY has affinity for the sperm plasma membrane. It acts as a polyunsaturated fatty acid and, consequently, it is peroxidized by ROS. Thus, in this condition, the fluorescence emitted by C11-BODIPY is altered from red to green [17,18]. Therefore, peroxidation values obtained with this technique are indirect because they express the cell’s susceptibility to oxidative stress. On the other hand, Mito SOX Red® and H2DCF detect the ROS compounds. They are able to detect the superoxide anion and hydrogen peroxide, respectively [16,19]. None of the fluorescent probes used in routine detect hydroxyl radical. On this way, researches of alternative methods for analyzing oxidative stress in sperm are necessary. They must show methods that are easy to apply and interpret. |
| CellROX Deep Red Reagent® probe (CAT 10422 – Molecular Probes) can be analyzed by spectrophotometry, fluorescence microscopy, or flow cytometry. In addition, it is used in samples preserved in formaldehyde [20] and in combination with other probes [21]. CellROX can be used to measure cellular ROS in the cytoplasm because it detects hydroxyl better than superoxide [22]. It is oxidized during cellular oxidative stress [23] and shows maximum excitation/emission at 640/655 nm. In the absence of ROS, it remains in its reduced state and emits no fluorescence. CellROX is not commonly used in cell biology, and to the best of our knowledge, there are no published studies reporting its use in sperm. |
| On this way, this study evaluated the efficiency of the use of the CellROX Deep Red Reagent® fluorescent probe to detect ROS production in ram sperm. The use of the probe was evaluated in two steps. The first step was performed in experiment 1 that evaluated the efficiency of CellROX to detect sperm oxidative stress induced in vitro. In the second experiment, it was evaluated the efficiency of CellROX to detect sperm oxidative stress induced in vivo. Both of experiments were done to evaluate the efficiency of CellROX Deep Red Reagent® fluorescent probe to detect ROS in ram sperm. |
| Materials and Methods |
| Experiment 1: Evaluation of CellROX Deep Red Reagent® fluorescent probe efficiency in detect sperm |
| Oxidative stress induced in vitro: The experiment was conducted with three semen treatments: T0 (semen sample that was not submitted to oxidative stress induction), T50 (semen sample that was 50% induced to oxidative stress and 50% not induced) and T100 (semen sample that was entire submit to oxidative stress induction); and two variables: sperm under mild or no oxidative stress and sperm under moderate or intense oxidative stress. |
| Semen collection and preparation: Three White Dorper rams (Ovis aries) with an average age of 15.3 ± 2.3 months and 66.3 ± 7.5 kg were used. The rams were housed in paddock. Hay and concentrate were provided to attend the NRC (1998) [23]. The analyses were conducted on four replicates of semen samples from each of rams (n=12). Each of the four replicates was collected as two ejaculates from the same animal harvested within a 90-minute interval; therefore, a total of 24 ejaculates were collected. Samples were collected using an artificial vagina, and only ejaculates showing subjective motility ≥70% and ≤10% total defects were used in the study. The experiment agree with Ethical Principles in Animal Research adopted by “Ethic Committee in the use of animals” of the School of Veterinary Medicine and Animal Science of University of São Paulo, protocol number 2467/2012. |
| The first ejaculate of each replicate was diluted in TALP media [24] for the preparation of two samples: control samples without oxidative stress induction (T0) and samples with induced oxidative stress (T100). Oxidative stress was induced by adding 50 μl of iron sulfate (4 mM) and 50 μl of sodium ascorbate (20 mM) to 100 μl of semen diluted in TALP and incubated at 37 °C for 90 min. The second ejaculate, collected after the T100 sample incubation, was used for the preparation of the third sample, which contained 50% semen from the second ejaculate diluted in TALP (without oxidative stress induction) and 50% semen from the T100 sample (after oxidative stress induction). |
| Oxidative stress analysis: The CellROX Deep Red Reagent® fluorescent probe (2.5 mM; Life Technologies, New York, USA) was diluted in dimethyl sulfoxide (DMSO; 472301, Sigma-Aldrich, St. Louis, USA) for a final concentration of 1 mM (working solution) and stored at -20 °C in the dark. During use, this working solution was kept in the dark at 37 °C. |
| Two hundred microliters (25×106 sperm/mL) from each semen sample (T0, T50, and T100) were added to 0.5 μL of CellROX® (1 mM) and 2 μL of Hoescht 33342 (in dPBS; 2.5 mg/mL; Life Technologies, New York, USA) and incubated at 37ºC for 30 min. After incubation, each sample was centrifuged for 5 minutes at 2,000 g, the supernatant was removed, and the pellet was resuspended in 200 μL of TALP. |
| An aliquot of 4 μL of the solution stained with CellROX® was placed between a slide and coverslip and read under epifluorescence microscopy (Nikon, model 80i) at 1,000x magnification using a triple filter (D/F/R, C58420) featuring the UV-2E/C (340-380 nm excitation and 435-485 emission), B-2E/C (465-495 excitation and 515-555 emission), and G-2E/C (excitation 540-525 and 605-655 emission) sets. |
| In each sample was counted 200 cells by the same technique in all analyses. The cells were classified in three categories, as follows: sperm under mild or no oxidative stress (unstained midpiece), sperm under moderate oxidative stress (midpiece stained pale red), and sperm under intense oxidative stress (midpiece stained strong red), as showed in the Figure 1. |
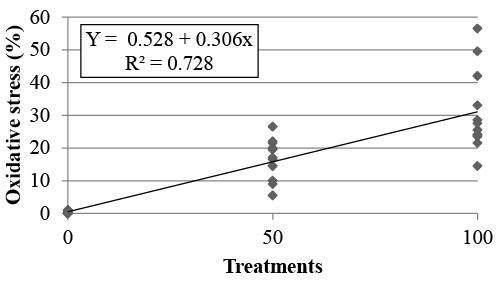
| Statistical analysis: We conducted a polynomial regression analysis using the Statview software (Stat view 1998, SAS Institute Inc., Cary, NC, USA). The experimental model was: Y = a + bX, wherein Y is the estimative of the oxidative stress as a function of the treatment, a is the polynomial regression coefficient corresponding to the value of Y when X is 0, b is the regression coefficient for the percent of X on the response Y, and X is the treatment. |
| Experiment 2: Oxidative stress induction by testicular degeneration in ram sperm |
| Animals: Semen samples from 16 White Dorper rams with an average age of 17.62 ± 2.6 months and 67.12 ± 7.2 kg were analyzed. The rams were housed in paddock. Hay and concentrate were provided to attend the NRC (1998). Prior to the beginning of the study, sperm motility was ≥60% and total defects were ≤20%. The animals were submitted to scrotal insulation for 72 hours through the use of heating bags to induce testicular degeneration and loss of testicular thermoregulation. We performed four semen collections with 15-day intervals in between; two were performed before the scrotal insulation and two afterwards. Semen samples were collected using an artificial vagina. The experiment agree with Ethical Principles in Animal Research adopted by “Ethic Committee in the use of animals” of the School of Veterinary Medicine and Animal Science of University of São Paulo, protocol number 2467/2012. |
| Semen preparation and evaluation of testicular degeneration: For examination of the testicular degeneration induction was performed evaluation of total and progressive motility, sperm defects and sperm DNA fragmentation. The total and progressive motility was assessed by computer analyses system. The software was the Sperm Class Analyzer (SCA, Microptics, Spain) and the set up was adjusted by ram sperm. It was chosen five fields for the analyses and it was used the Makler® chamber to perform the evaluation. The sperm defects were evaluated by differential interference contrast microscopy (DIC). It was counted 200 cells that were classified in major and minor sperm defects. DNA fragmentation was determined by commercial kit Halomax® (Halotech, Spain) and it was determined the fragmentation in 1,000 cells. All evaluation was done by competent techniques. |
| Semen preparation and evaluation of oxidative stress: Two hundred microliters of semen samples diluted in TALP media [25] (25×106 sperm/mL) were added to 0.5 μL of CellROX (1 mM in DMSO) and 2 μL of Hoescht 33342 (2.5 mg/mL; in dPBS), and incubated for 30 minutes at 37ºC. After incubation, the solution was centrifuged for 5 minutes at 2,000 g, the supernatant was removed, and the pellet resuspended in 200 μL of TALP. An aliquot of 5 μL was placed between a slide and coverslip and read under epifluorescence microscopy (Nikon, model 80i) at 1,000x magnification using a triple filter (D/F/R, C58420) featuring the UV-2E/C (340-380 nm excitation and 435-485 emission), B-2E/C (465-495 excitation and 515-555 emission), and G-2E/C (540- 525 excitation and 605-655 emission) sets. |
| It was counted 200 cells and they were classified as sperm under mild or no oxidative stress (unstained midpiece), sperm under moderate oxidative stress (midpiece stained pale red), and sperm under intense oxidative stress (midpiece stained strong red) (Figure 1). |
| Statistical analysis: Nine variables were analyzed in this experiment (total motility, progressive motility, total sperm defects, minor sperm defects, major sperm defects, DNA fragmentation, sperm under mild or no oxidative stress, sperm under moderate oxidative stress, and sperm under intense oxidative stress) in two moments (before insulation/ control period, and after insulation/degenerated period). |
| An analysis of variance was conducted (ANOVA). Differences between treatments were considered significant at p<0.05 (Stat view 1998, SAS Institute Inc., Cary, NC, USA). |
| Results |
| In the first experiment, the percentage of cells with moderate or intense oxidative stress was compared between treatments T0, T50 and T100. T0, T50 and T100 showed, respectively, 0.25 ± 0.11%, 16.37 ± 1.75% and 30.83 ± 3.57% of moderate or intense oxidative stress. Results from the polynomial regression analysis (R²=0.728) are shown in Figure 2. |
| In the second experiment, the testicular degeneration and oxidative stress were induced by scrotal insulation and it caused a decrease in total (p<0.0001) and progressive motility (p<0.0001), and an increase in total (p< 0.0001) and major sperm (p<0.0001) defects and DNA fragmentation (p=0.001) (Table 1). Besides, it caused a reduction in sperm with mild or no oxidative stress (p=0.02), and an increase in sperm showing moderate (p=0.03) and intense (p=0.03) oxidative stress (Table 2). |
| Discussion |
| The use of the CellROX Deep Red® probe in the analysis of sperm oxidative stress using ovine species as the experimental model was evaluated and it was successfully validated for the detection of oxidative stress induction. This fluorescent probe has not been previously used in sperm cells. |
| Ferrous sulfate (FeSO4) and sodium ascorbate were used to induce oxidative stress in vitro because a preliminary test using menadione, cited by the commercial guide as an inducer of oxidative stress in bovine lung endothelial cells [26], did not effectively induce oxidative stress in ram sperm. In boar sperm, the induction of oxidative stress with FeSO4 and Na ascorbate was also more efficient than with menadione and hydrogen peroxide [17]. After 30 minutes’ incubation with FeSO4 and Na ascorbate, an analysis with BODIPY showed 92.9 ± 5.9% oxidized sperm, which was significantly different from the non-induced sample (p<0.05). After 30 minutes’ incubation with menadione and hydrogen peroxide, the peroxidation rates were 0.3 ± 0.1% and 1.1 ± 0.3%, respectively. These results were not significantly different from the noninduced sample [17]. |
| In the present study, the rates of CellROX® positive sperm observed in the T100 group were lower than those reported by Guthrie and Welch (2007) [10]. These differences may be explained by the differences between the CellROX® and BODIPY methods. CellROX® assesses the amount of hydroxyl and peroxide present in the sample. BODIPY, used by Guthrie and Welch (2007) [10], assesses the lipid peroxidation caused by any ROS [18]. We know that antioxidant agents in the seminal plasma [27,28] neutralize the ROS molecules present there [3]. On this way, the BODIPY assesses more ROS than CellROX®. Nevertheless, regardless of the neutralizing action of the seminal plasma, the amounts of ROS (including non-neutralized) in T50 and T100 were sufficient to produce different results between groups. |
| The rams in experiment 1 were healthy and did not present signs of testicular degeneration, showing ≥70% sperm motility, and ≤10% sperm defects. Thus, the ROS rates were expected to be lower in T0 and T50 than in T100. The same result was expected for the two retrievals conducted prior to the use of heating bags. |
| The loss of testicular thermoregulation was induced by applying heating bags to the animals’ testes. The confirmation of thermoregulation deleterious was done evaluating the motility, sperm defects and DNA fragmentation. Loss of testicular thermoregulation leads to increase cellular metabolism, hypoxia, and a subsequent increase in ROS production, which in turn leads to oxidative stress [8,11,29,30]. Mice subjected to thermal stress at 42ºC for 30 minutes showed an increased expression of heme oxygenase-1 after 24 and 48 hours of stress exposure, and increased glutathione peroxidase 1 and alpha glutathione-S-transferase after 3 hours [11]. A significant increase in 4-HNE protein expression was observed in men diagnosed with different degrees of varicocele compared to those in the control group, while a positive significant correlation (p=0.003; r=0.49) between the increased temperature and 4-HNE protein expression was observed in the groups with varicocele [31]. Therefore, after the insulation period, we expected to see an increase in sperm cell ROS production, as we previously saw for the last two stages. |
| On this way, the results showed that the CellROX Deep Red® fluorescent probe is efficient in detecting the production of sperm ROS under oxidative stress induced in vitro and in vivo. The detection of ROS in ram sperm can be important in the diagnosis of reproductive disorders |
| Acknowledgements |
| The authors thank Dr. Marcílio Nichi and Dr. João Diego de Agostini for assistance in the performance of the oxidative stress induction. We also thank Mr. José Maria Bernardes, Márcio De Carli and João Carlos Campos for assistance to the animals. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP process numbers 2011/16744-3 and 2012/00040-0). |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 17336
- [From(publication date):
June-2015 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 12500
- PDF downloads : 4836
