Research Article Open Access
Aural Stimulation as Add-on to Diet for Weight Loss: A Preliminary Clinical Study
| Anna Esposito1, Gregorio Fistetto1, Alessandro Di Cerbo1* and Beniamino Palmieri2 | |
| 1Poliambulatorio del Secondo Parere, Modena, Italy | |
| 2Dipartimento di Chirurgia Università di Modena e Reggio Emilia, Modena, Italy | |
| Corresponding Author : | Dr. Alessandro Di Cerbo Poliambulatorio del Secondo Parere Modena, Italy E-mail: alessandro811@hotmail.it |
| Received November 21, 2012; Accepted December 26, 2012; Published December 28, 2012 | |
| Citation: Esposito A, Fistetto G, Di Cerbo A, Palmieri B (2012) Aural Stimulation as Add-on to Diet for Weight Loss: A Preliminary Clinical Study. J Obes Wt Loss Ther 2:155. doi:10.4172/2165-7904.1000155 | |
| Copyright: © 2012 Esposito A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
We investigated the efficacy of bilateral electric auricular nerve stimulation in combination with hypo caloric or high-protein diet in 40 overweighed and obese healthy volunteer patients (4 groups of 10 individuals each) vs. diet alone. After 2 months of simultaneous treatment with electric stimulation and diet there was an average weight loss of 7.07 kg in the hypocaloric group and 9.48 kg in the high-protein one, whereas an average weight loss of 5.9 kg and 7.17 kg were observed with hypocaloric and high-protein diet alone, respectively. These results suggest that bilateral auricular nerve stimulation can help increase the weight loss in patients when used in combination with high-protein diet. Thus weight loss induced by nutritional strategies can safely and effectively be enhanced by neuro-reflexological aural stimulation.
| Keywords |
| Weight loss; Auricular nerve stimulation; Diet; Hypocaloric; Protein |
| Introduction |
| Obesity is an epidemic disease affecting the population worldwide. Obesity is commonly defined as body index mass (BMI=weight (in kg) divided by height (in metres) squared) of 30 kg/m2 or higher. On the other hand being overweight is defined by a BMI of between 25 – 29.9 kg/m2 (World Health Organization, 2000). It has been observed that more than 80% of obesity-attributable deaths occurred in individuals with a BMI index >30 kg/m2 [1]. Furthermore obesity is frequently associated with cancer, type 2 diabetes and cardiovascular diseases [2] as well as with mental disorders such as “binge eating” [3]. So far verylow- calorie diet is a well established method for short-term weight loss, with concomitant improvements in obesity-related medical conditions in obese patients [4]. |
| Complementary therapies are increasingly becoming popular choices for obese people. For example acupuncture has been used effectively to support dietary restrictions. The first scientific publications dealing with auricular acupuncture for obesity management appeared between the middle 1970 and early 1980 [5-7]. These publications, even if not without criticism from a methodological point of view, indicated that auricular nerve stimulation could be advantageously used for modulating feeding behaviour, when administered through well identified auricular zones [8]. Both hunger, often expressed as compulsive behaviour [9], and satiety, usually up-regulated by neuroendocrine imbalance in the obese people [10], affect the food intake and could be modified, for instance, by electric auricular nerve stimulation. In fact feeding behaviour is the result of the close interaction amongst environmental, cognitive, emotional and biological factors connected in a complex neuro-psychological network. |
| Hypothalamic control |
| Food intake control is regulated by a manifold network of signals which collect information from the periphery. They are then elaborated at the hypothalamic level and eventually integrated at cortical level initiating food searching behaviour. The arcuate nucleus, between the 3rd ventricle and the median eminence plays a key role in such processes [11]. It is made of two important 1st order neural sets: Npy/ AgRP [12,13], and Pomc/Cart [13,14] which are reciprocally inhibited and related to 2nd order neurons of hypothalamic paraventricular nuclei (ventromedial and lateral). Such systems receive further information and control from cortical and subcortical regions through multilevel release of cerebral amines such as noradrenaline, dopamine and serotonin. It is believed that serotonin is able to affect the food choice by stimulating the organism towards protein-rich food instead of carbohydrate-rich aliments [15-17]. During dieting, decreased food intake induces a reduction of the intracerebral serotonin causing episodes of “binge eating” or “craving” for food [18]. The hypothesis that central neuromediators such as serotonins can be modulated by other neuromediators (such as GABA) is strongly supported by the neurophysiological evidence [19]. In 1995 Shiraishi et al. detected the both inhibition of the lateral hypothalamic neuronal activity and excitation of the ventromedial hypothalamic activity. While auricular acupuncture stimulation clearly modulated feeding-related hypothalamic neuronal activity in both normal and experimental (hypothalamic and dietary) obese rats, the effects upon weight were highly related with the obesity degree. The animal experiments suggested that auricular acupuncture stimulation rather than modify eating habits was more effective on earlier satiety awareness [20]. |
| The current study aimed to clarify the influence of transcutaneous, bilateral auricular nerve stimulation on body weight in healthy, mildlyobese and obese volunteer patients. We used the “FoodWatcher™”, a low-intensity medical device believed to act by vagus nerve inhibition to reduce the gastric secretion and motility through the hypothalamic pituitary axis. In fact The FoodWatcher™ has two conical shaped earpieces which make them easy to insert into the external auditory canal allowing the stimulation via the vagus nerve. |
| Materials and Methods |
| The present study was conducted at “Second opinion medical office” (Modena, Italy). It was approved by the Internal Review Board and it was conducted in accordance with the declaration of Helsinki. |
| The overall trial required two months of follow-up but it was not registered anywhere since the methodology used is approved in the EU. Patient’s compliance with the study protocol was good, no dropouts were observed. |
| Exclusion criteria were: subjects wearing implanted stimulators (pacemakers, prosthesis, etc.), epileptics and pregnants due to the risk of possible adverse reactions, patients affected by vertigo, headache or nausea after the first day of treatment. |
| Stimulation protocol |
| The FoodWatcher™ (http://www.themicrocurrentsite.co.uk/), a medical device, was connected to small (80 mm × 1.1 cm) auricular ear plug-like electrodes (Figure 1b). They were inserted bilaterally into both the auditory meatuses, an area innervated by the vagus nerve. The device generates a signal with amplitude of 40 V, frequency of 50 Hz and current of 40 mA through the ear plugs. |
| Measurements and experimental timeline |
| Thirty minutes before meals, all subjects that underwent auricular nerve stimulation were asked to turn on the Food-Watcher™ device for 10 minutes and two sessions a day. Glycemia was measured at the beginning and at the end of the experimental diet period by Accu-Chek Active, blood glucose monitoring system (Roche Diagnostics GmbH, Germany). Fat Free Mass (FFM) as well as basal metabolism was measured before and after treatment by bio-impedance using a Body Fat Analyzer Bt-905 (Skylark Device and System Co., Ltd., Taiwan). Test current and frequency were less than 1 mA and approximately 50 KHz, respectively. This technique entails measuring total body electrical impedance. The amount of water and FFM can be accurately estimated by measuring total body electrical impedance after placing four electrodes are over metacarpals and metatarsals and introducing a single frequency (50 KHz) signal current. The detected signal level, when corrected for subject height, is an index of total body water and FFM. Also the waist circumference was measured before and after treatment by means of a tape measure. The study lasted for 2 months when a follow-up examination was performed. |
| Statistical analysis |
| Data was analysed using Stata Software (Statistics/Data Analysis, College Station, Texas). Percentage change in FFM, BMI, weight, glycemia and waist circumference for all groups was calculated as baseline – post-treatment. Pearson chi square test was applied. A oneway ANOVA with Bonferroni post-hoc test was used. A p<0.05 was considered significant. All data are reported as mean ± Standard Error of the Mean (SEM). |
| Results |
| Forty healthy overweight volunteers (male 16, female 24) averaging 42.2 ± 16.2 (range: 18-76) years of age, 93.6 ± 2.8 (range: 57-131) kg of body weight, signed the informed consent and were included into this study. They were randomized to 4 groups. The first group received hypocaloric diet (50-55% of carbohydrates, 25-30% of fats e 15-20% of protein and a calorific value ranging from 1200 to 1800 Kcal, depending on the individual basal metabolism). The second group received a high-protein diet (ketogenic diet with four stages of transition with a gradual reintroduction of carbohydrates and a calorific value ranging from 1200 to 1800 Kcal depending on the individual basal metabolism). The third group received auricular nerve stimulation plus hypocaloric diet. The fourth group received auricular nerve stimulation plus highprotein diet (Figures 1a, 1c and 1d). All groups received advice on diet regimen by a qualified nutritionist once a week. An accurate record of eating habits, behaviours and fasting glycemia were collected at the first examination. |
| The frequency of food consumption was assessed by a multiple response grid in which patients were asked to estimate how often a particular food or beverage was consumed. We arranged the food items, based on their similarities in nutrient content, into six major food groups as previously reported [21]. The food groups were: 1) Bread, cereal and starches (different types of bread, rice, pasta, porridge and bakery products); 2) Meat, eggs and fish; 3) Dairy products; 4) Vegetables (fresh and cooked vegetables excluding potatoes), and fruits (fresh and canned); 5) Beverages (fruit juice, alcoholic and nonalcoholic drinks, coffee and tea); 6) Sweets and salads (nuts, candy and cake). In order to estimate the average frequency of intake of foods during the previous year we used close-ended responses consisting of 9 categories: Never or less than once/month, 1–3/month, 1/week, 2–4/ week, 5–6/week, 1/day, 2–3/day, 4–5/day, >6/day. |
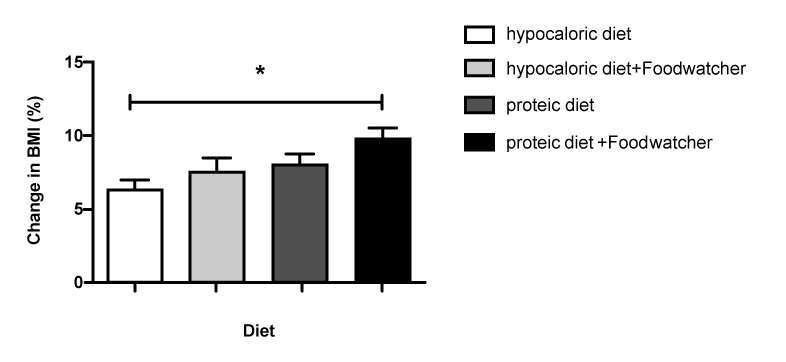
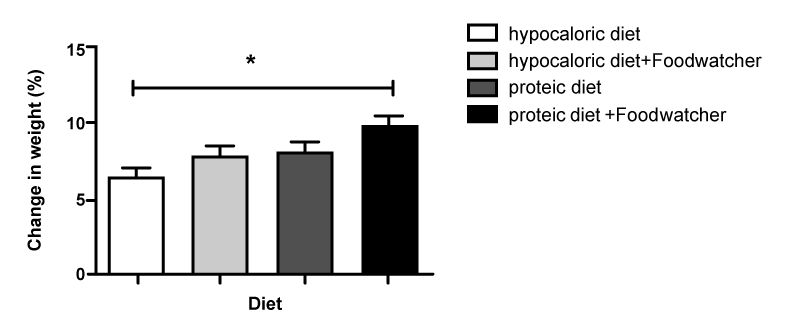
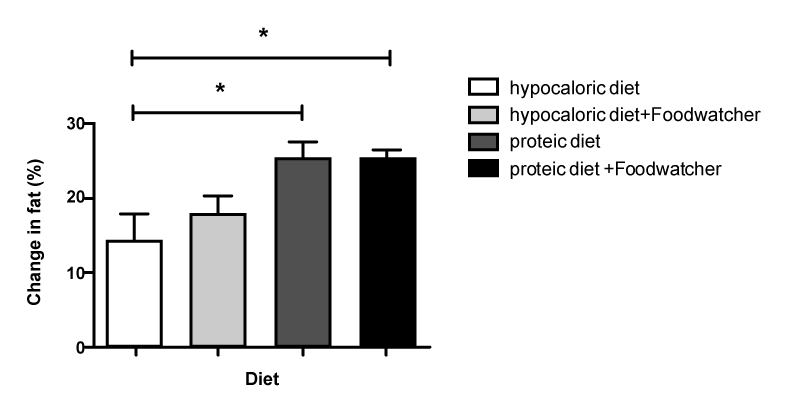
| When comparing the percentage change in BMI from baseline in the 4 treatment groups, the therapy with high-protein diet plus FoodWatcher™ resulted to be significantly more effective vs hypocaloric diet and hypocaloric diet plus Food-Watcher™ (p<0.05; (Figure 2); 3.28% increased improvement in BMI vs. group hypocaloric diet group; 2.02% increased improvement in BMI vs. hypocaloric diet plus FoodWatcher™ group; 1.59% increased improvement in BMI vs. high-protein diet group). Referring to the percentage change in weight from baseline, a comparison among the 4 groups showed a significant improvement in the high-protein diet plus FoodWatcher™ vs. hypocaloric diet and hypocaloric diet plus FoodWatcher™ (p<0.05; (Figure 3); 3.25% increased improvement in weight vs hypocaloric diet group Ð�?; 2.78% increased improvement in weight vs. hypocaloric diet plus FoodWatcher™ group; 1.49% increased improvement in weight vs. high-protein diet group). When comparing the percentage change in FFM from baseline in the 4 treatment groups, the therapy with high-protein diet alone or plus FoodWatcher™ resulted to be both significantly more effective vs. hypocaloric diet and hypocaloric diet plus FoodWatcher™ groups (p<0.01; (Figure 4);10.21% increased improvement in fat in high-protein diet group vs. hypocaloric diet; 4.99% increased improvement in fat in high-protein diet group vs. hypocaloric diet plus FoodWatcher™ group; 12.59% increased improvement in fat group 4 vs. group 1; 7.37% increased improvement in fat in high-protein plus FoodWatcher™ group vs. hypocaloric diet plus FoodWatcher™ group). Regarding waist circumference and glycemia, no significant difference was observed between groups. Glycemia, BMI, weight, waist circumference and FFM variation from baseline, before and after each diet approach, are summarized in table 1. |
| Conclusions |
| The present study evaluated the effect of hypocaloric or high-protein diet alone or in combination with FoodWatcher™ device on weight, FFM, BMI, waist circumference and glycemia. Our results support the concept that, after a 2-month therapy, auricular stimulation can produce dramatic body weight reduction in obese patients [5,6,22-26]. As to the action mechanism, a reflex activation of fat metabolism through the auditory meatus via a Yin-yang acupuncture energy balance has been supposed; nevertheless the classic neural pathway originally described by Shiraishi et al., supports the auricular stimulation effects on feedingrelated lateral hypothalamic and ventromedial hypothalamic neuronal activity in normal and experimental (hypothalamic and dietary) obese rats [20]. |
| The patients participating in this study reached satiety during auricular nerve stimulation if compared to patients treated without electrical stimulation. In addition a significant average body weight loss, FFM and BMI reduction during bilateral auricular nerve stimulation in high protein plus FoodWatcher™ diet group were achieved. In conclusion our study supports the synergistic use of the FoodWatcher™ medical device in combination with high-protein diet. No adverse side effects (such as vertigo, headache, and nausea) or negative rebounds consisting in increase in hunger and compulsive food intake were observed. In conclusion the present study supports the use of the FoodWatcher™ as add-on therapy to high protein diet program although further studies in larger cohorts of patients are required. |
| Statement of Authorship |
| The authors hereby certify that all work contained in this article is original work. The authors claim full responsibility for the contents of the article. |
| Conflict of Interest Statement |
| The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. |
References
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB (1999) Annual deaths attributable to obesity in the United States. JAMA 282: 1530-1538.
- Haslam DW, James WP (2005) Obesity. Lancet 366: 1197-1209.
- Munsch S, Herpertz S (2011) [Eating disorders associated with obesity and diabetes]. Nervenarzt 82: 1125-1132.
- (1993) Very low-calorie diets. National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health. JAMA 270: 967-974.
- Soong YS (1975) The treatment of exogenous obesity employing auricular acupuncture. Am J Chin Med (Gard City N Y) 3: 285-287.
- Mok MS, Parker LN, Voina S, Bray GA (1976) Treatment of obesity by acupuncture. Am J Chin Med (Gard City N Y) 29: 832-835.
- Lei ZP (1988) Treatment of 42 cases of obesity with acupuncture. Journal of traditional Chinese medicine 8: 125-126.
- Mulhisen L, Rogers JZ (1999) Complementary and alternative modes of therapy for the treatment of the obese patient. J Am Osteopath Assoc 99: S8-S12.
- Filbey FM, Myers US, Dewitt S (2012) Reward circuit function in high BMI individuals with compulsive overeating: similarities with addiction. NeuroImage 63: 1800-1806.
- Valette M, Bellisle F, Carette C, Poitou C, Dubern B, et al. (2012) Eating behaviour in obese patients with melanocortin-4 receptor mutations: a literature review. Int J Obes (Lond).
- Mercer JG, Speakman JR (2001) Hypothalamic neuropeptide mechanisms for regulating energy balance: from rodent models to human obesity. Neurosci Biobehav Rev 25: 101-116.
- Sawchenko PE, Pfeiffer SW (1988) Ultrastructural localization of neuropeptide Y and galanin immunoreactivity in the paraventricular nucleus of the hypothalamus in the rat. Brain Res 474: 231-245.
- Neary NM, Goldstone AP, Bloom SR (2004) Appetite regulation: from the gut to the hypothalamus. Clin Endocrinol (Oxf) 60: 153-160.
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, et al. (2003) Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord 27: 289-301.
- Yang YK, Harmon CM (2003) Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes Rev 4: 239-248.
- Blundell JE, Stubbs RJ (1999) High and low carbohydrate and fat intakes: limits imposed by appetite and palatability and their implications for energy balance. Eur J Clin Nutr 53:S148-S165.
- Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, et al. (1999) Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest 104: 975-982.
- Wurtman JJ (1988) Carbohydrate craving, mood changes, and obesity. J Clin Psychiatry 49: 37-39.
- Przewlocka B, Stala L, Scheel-Kruger J (1979) Evidence that GABA in the nucleus dorsalis raphe induces stimulation of locomotor activity and eating behavior. Life Sci 25: 937-945.
- Shiraishi T, Onoe M, Kojima T, Sameshima Y, Kageyama T (1995) Effects of auricular stimulation on feeding-related hypothalamic neuronal activity in normal and obese rats. Brain Res Bull 36: 141-148.
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, et al. (1992) Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 135: 1114-1126.
- Hu JH (1974) Therapeutic effects of acupuncture: a review. Am J Acup 2: 8-13.
- Giller RM (1975) Auricular acupuncture and weight reduction: A controlled study. Am J Acup 3: 151-175.
- Lau BHS, Wang B, Wong D (1975) Effects of acupuncture on weight reduction. Am J Acup 3:335-338.
- Huang MH, Yang RC, Hu SH (1996) Preliminary results of triple therapy for obesity. Int J Obes Relat Metab Disord 20: 830-836.
- Richards D, Marley J (1998) Stimulation of auricular acupuncture points in weight loss. Aust Fam Physician 2: S73-S77.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 13952
- [From(publication date):
December-2012 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 9369
- PDF downloads : 4583
