Case Report Open Access
Clinical Presentation of Oral Manifestations and Intraoral Somatosensory Changes in Fahr's Disease
| Takuya Naganawa1*, Hitoshi Sato2,3, Abhishek Kumar4,5, Takashi Iida6, Eiko Naganawa7, Toshihiro Okamoto1 and Tomohiro Ando1 | |
| 1Department of Oral and Maxillofacial Surgery, Tokyo Women's Medical University, School of Medicine, Tokyo, Japan. | |
| 2Department of Dentistry & Oral surgery, School of Medicine, Keio University, Tokyo, Japan. | |
| 3Department of Dentistry & Oral Surgery, Kawasaki Municipal Kawasaki Hospital, Kanagawa, Japan. | |
| 4Section of Oral Rehabilitation, Department of Dentistry, Karolinska Institutet, Sweden. | |
| 5Scandinavian Center for Orofacial Neurosciences (SCON). | |
| 6Department of Oral Function and Rehabilitation, Nihon University School of Dentistry at Matsudo, Chiba, Japan. | |
| 7Department of psychiatry, Tokyo Women's Medical University, School of Medicine, Tokyo, Japan. | |
| Corresponding Author : | Naganawa T Department of Oral and Maxillofacial Surgery School of Medicine, Tokyo Women's Medical University 8-1 KAwada-cho, Shinjuku-ku Tokyo 162-8666, Japan Tel: 03-3353-8111 E-mail: tanaganawa@gmail.com |
| Received October 30, 2015; Accepted November 17, 2015; Published November 20, 2015 | |
| Citation: Naganawa T, Sato H, Kumar A, Iida T, Naganawa E, et al. (2015) Clinical Presentation of Oral Manifestations and Intraoral Somatosensory Changes in Fahr’s Disease. J Pain Relief 4:214. doi:10.4172/2167-0846.1000214 | |
| Copyright: © 2015 Naganawa T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Fahr’s disease is a rare congenital disorder characterized by abnormal calcium deposition with subsequent atrophy involving the basal ganglia, cerebral and cerebellar cortical regions. Very little information is available regarding non-neurological manifestations of this disease, and almost no information is available on oral findings. Moreover, information is unavailable regarding intraoral somatosensory changes in Fahr’s disease. We report oral findings and intraoral somatosensory changes in patient with Fahr’s disease. A 62-year-old Japanese woman was referred to the Department of Oral and Maxillofacial Surgery at Tokyo Women’s Medical University Hospital with symptoms of bleeding gums. The patient had been diagnosed with Fahr’s disease by the Department of Psychiatry 1 year earlier. Intraoral examination showed poor oral and dental hygiene with gingival hyperplasia on the buccal aspects of the upper and lower incisors. Generalized attrition of the teeth was seen. Panoramic radiography showed horizontal bone resorption, but no evidence of congenital absence of any teeth. Gingival bleeding associated with poor periodontal condition was diagnosed. As an additional symptom, the patient reported intraoral burning sensation of the tongue. Qualitative sensory testing was performed for the tongue, upper and lower gingiva and lip mucosa, showing heat and cold hyperalgesia of the tongue. Mechanical allodynia located in the upper and lower lip mucosa was also reported. In accordance with these clinical findings, we diagnosed primary and/or secondary burning mouth syndrome related to Fahr’s disease. This case represents only the second instance for which intraoral findings of Fahr’s disease have been reported. Somatosensory changes were also found to be associated with the present case of Fahr’s disease. Intraoral somatosensory changes related to Fahr’s disease may be due to progressive lesions associated with this disease. Continuous follow-up and qualitative sensory testing to assess disease progression may be needed for clarification of these issues.
| Keywords |
| Fahr’s disease; Intraoral; Sensory test; Burning mouth syndrome; Bruxism; Somatosensory change; Pain; Hyperalgesia; Allodynia. |
| Introduction |
| Fahr’s disease is a rare congenital disorder characterized by abnormal calcium deposition involving the basal ganglia, cerebral and cerebellar cortical areas and subsequent atrophy [1-3]. Very little information is available regarding the number of neurological manifestations of this disease and almost no information is available regarding oral findings. Considering the various metabolic abnormalities such as stunted physical and neuropsychological development in these patients, one could also expect abnormalities in the oral tissues. Moreover, no information is available about intraoral somatosensory changes in Fahr’s disease. We therefore report oral findings and intraoral somatosensory changes in a patient with Fahr’s disease. |
| Case Report |
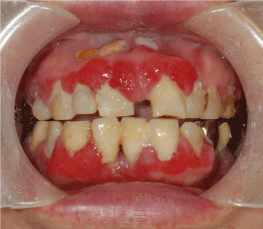
| A 62-year-old Japanese woman was referred to the Department of Oral and Maxillofacial Surgery at Tokyo Women’s Medical University Hospital with symptoms of bleeding gums. The patient had been diagnosed with Fahr’s disease by the Department of Psychiatry 1 year earlier. The patient was also on medication for diabetes, and had been previously prescribed prednisolone and bisphosphonate for lung cancer. Intraoral examination showed dental attrition, poor oral and dental hygiene with gingival hyperplasia on the buccal aspects of the upper and lower incisors (Figure 1). The patient reported difficulty in tooth brushing due to tremors and muscle rigidity related to Parkinsonism. Panoramic radiography showed horizontal bone resorption, but no evidence of congenitally missing teeth. Gingival bleeding associated with poor periodontal condition was diagnosed in this case. Periodontal treatment involving oral prophylaxis and tooth brushing instructions were given and efforts were continuously made to maintain oral hygiene. |
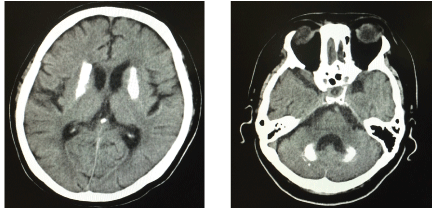
| As additional symptoms, the patient reported an intraoral burning sensation of the tongue. After dental, bacteriological and radiographic examinations, possible cases of symptoms like infection by Candida albicans and other structural changes were ruled out, with the exception of calculus deposition and without symptoms associated with dental caries. Qualitative sensory testing using a spatula (put out from hot and cold water for thermal stimuli) and Q-tip (to investigate mechanical allodynia) was performed on the tongue, upper and lower gingiva and adjacent lip mucosa [4]. The results of this qualitative sensory testing showed heat and cold hyperalgesia of the tongue and mechanical allodynia of the upper and lower lip mucosa. Moreover, paradoxical heat sensation was observed on the upper gingiva. Bilateral calcification of the basal ganglia and cerebellar dentate nucleus were observed in intracranial computed tomography (CT) (Figure 2), but no evidence of tumor or nerve dislocation was seen. In accordance with these clinical findings, we diagnosed primary and/or secondary burning mouth syndrome (BMS) related to Fahr’s disease. |
| Discussion |
| Fahr’s disease is characterized by abnormal deposition of calcium in areas of the brain that control movements including the basal ganglia, thalamus, dentate nucleus, cerebral cortex, cerebellum, subcortical white matter, and hippocampus [5]. Most cases initially present with extrapyramidal symptoms. They may also present with cerebellar dysfunction, speech difficulty, dementia and neuropsychiatric symptoms [6]. The diagnostic criteria for Fahr’s disease have been modified and derived from those described by Moskowitz et al. [3,5,6] and present case was diagnosed following the suggested diagnostic criteria. |
| • Bilateral calcification of the basal ganglia visualized on neuroimaging. Calcification in other brain regions can also be observed. |
| • Progressive neurological dysfunction, which generally includes movement disorder and/or neuropsychiatric manifestations. Age of onset is typically in the fourth or fifth decade, although this dysfunction may also present in childhood. |
| • Absence of biochemical abnormalities and somatic features suggestive of a mitochondrial or metabolic disease or other systemic disorders. |
| • Family history consistent with autosomal-dominant inheritance. |
| Intraoral characteristic features of Fahr’s disease have been reported in which several missing teeth were evident, along with tooth mobility, generated gingival inflammation and recession with associated periodontitis [7]. In the present case, gingival inflammation and bleeding were observed; however, no absence of teeth was evident. A previous case report has reported radiographic findings showing partial anodontia, as well as partial development of the maxillary and mandibular regions [7]. Generalized rarefaction of bone, resembling that seen in hyperparathyroidism, was also found. In this case, horizontal bone resorption was observed without any other tooth or bone abnormalities. The poor dental hygiene in this case may be explicable by Parkinsonism (motor impairment, apathy, depression, and dementia) [8]. There is no evidence of bruxism and clenching in patients with Fahr’s disease. However, an earlier study showed an association of bruxism and clenching with motor symptoms of Parkinson’s disease [9]. The dental attrition in the present case could thus have been due to bruxism associated with Parkinsonism on this patient with Fahr’s disease. Although, tooth attrition during oral examination was evident in the present patient there was no evidence of involuntary movement or mandibular dystonia during clinical examination. |
| Bruxism is defined as a repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible [10]. Since some researchers have proposed alternatives to the so-called gold standard tool for diagnosis about sleep bruxism (e.g. polygraphic and audio-video record-ing) [11,12], it is necessary to measure masticatory muscle activities with polysomnography to diagnose about sleep bruxism. Further studies may be needed to investigate the correlation between sleep bruxism and Fahr’s disease using polysomnography. |
| Moreover, somatosensory abnormalities were reported as nonmotor symptoms of Parikinson’s disease. In general, abnormalities in nociceptive and mechanical thresholds have been described in Parkinson’s disease [13]. These have been attributed to anomalies of central nociceptive processing and sensorimotor integration through the affected basal ganglia and dopaminergic pathways [14]. |
| In the present case, we identified heat and cold hyperalgesia and mechanical allodynia of the intraoral region. BMS was also diagnosed by taking into account the findings from intraoral qualitative sensory testing and other clinical findings. No previous reports have described abnormalities of sensory loss or gain, or burning sensation in the mouth in patients with Fahr’s disease. In this case, because of the lack of evidence for peripheral organic changes in the oral mucosa, the sensory changes and burning mouth sensations may be best explained by anomalies of central nociceptive processing in the basal ganglia and/ or dopaminergic pathways. We therefore think that an association may exist between these reported somatosensory changes and Fahr’s disease. |
| To the best of our knowledge, this case represents only the second instance for which intraoral findings of Fahr’s disease have been reported. Somatosensory changes were also found to be associated with the present case of Fahr’s disease. Intraoral somatosensory changes related to Fahr’s disease may be due to progressive lesions associated with this disease. Continuous follow-up and qualitative sensory testing to assess disease progression may be needed for clarification of these issues. |
| Conflict of Interest |
| None of the authors have any conflicts of interests to declare. |
References
- Bilateral striopallidodentate calcinosis (1980) 'http://www.orph.net/consor/cgibin/OC' before (1980).
- Manyam BV, Walters AS, Narla KR (2001) Bilateralstriopallidodentatecalcinosis: clinical characteristics of patients seen in a registry. MovDisord 16: 258-264
- Ellie E, Julien J, Ferrer X (1989) Familial idiopathic striopallidodentate calcifications. Neurology 39: 381-385.
- Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, et al. (2013) Chairside intraoral qualitative somatosensory testing: reliability and comparison between patients with atypical odontalgia and healthy controls. J Orofac Pain 27: 165-170.
- Ahad MA, Bala C, Karim S. Fahr’s syndrome (2013) Bang Med J (Khulna) 45: 33-35.
- Chiu H, Lam L, Shum P, Li K (1993) Idiopathic calcification of the basal ganglia. Postgrad Med J 69: 68-70.
- Aditya A, Lele S, Aditya P (2012) Fahr’s disease with oral manifestations: report of a rare case. Med PrincPract 21: 395-397.
- Hanaoka A, Kashihara K (2009) Increased frequencies of caries, periodontal disease and tooth loss in patients with Parkinson’s disease. J ClinNeurosci 16: 1279-1282.
- Srivastava T, Ahuja M, Srivastava M, Trivedi A (2002) Bruxism as presenting feature of Parkinson’s disease. J Associ Physician India 50: 457.
- Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, et al. (2013) Bruxism defined and graded: an international consensus. J Oral Rehabil 40: 2-4.
- Walters AS, Lavigne G, Hening W, Picchietti DL, Allen RP, et al. (2007) The scoring of movements in sleep. J Clin Sleep Med3:155-167
- Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K (2008) Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil Jul 35:476-494.
- Guieu R, Pouget J, Serratrice G (1992) Nociceptive threshold and Parkinson disease. Rev Neurol ( Paris)148:641-644.
- Lewis GN, Byblow WD (2002) Altered sensorimotor integration in Parkinson’s disease. Brain 125:2089-2099.
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 11044
- [From(publication date):
November-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10119
- PDF downloads : 925
