Research Article Open Access
Comparison of Plaque Removal Capabilities between Two Dentifrices
Douglas Ralston1, Rey Carrasco1, Peter L. Jacobsen2 and Cherie Wink RDH3*
1Gentle Dental Milpitas, CA, USA
2University of the Pacific, School of Dentistry, San Francisco, CA, USA
3Concorde Career College, Garden Grove, CA, USA
- *Corresponding Author:
- Cherie Wink RDH
BS, Dental Hygiene Instructor
Concorde Career College
12951 Euclid Street, Suite 215
Garden Grove, CA 92840, USA
Tel: 714-742-6677
E-mail: Cwink@concorde.edu
Received Date: June 12, 2014; Accepted Date: August 14, 2014; Published Date: August 20, 2014
Citation: Ralston D, Carrasco R, Jacobsen PL, Cherie Wink RDH (2014) Comparison of Plaque Removal Capabilities between Two Dentifrices. J Oral Hyg Health 2:157. doi: 10.4172/2332-0702.1000157
Copyright: © 2014 Ralston D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Objective: To compare the efficacy of a dental gel containing 2.6% Edathamil in controlling dental plaque to that of a commercially available dentifrice containing 0.3% triclosan, 2.0% PVM/MA copolymer, and 0.243% sodium fluoride.
Materials and Methods: Following a baseline examination and index scoring for dental plaque, gingivitis, bleeding, and pocket depths, qualifying adult male and female subjects were randomized into two groups. The subject’s teeth were professionally cleaned and then they were instructed to brush their teeth twice a day (morning and evening) for two minutes with their assigned dentifrice and a soft-bristled, manual toothbrush. Examinations for dental plaque, gingivitis, bleeding and pocket depths were repeated after two weeks and four weeks of product use.
Results: Eight (8) subjects complied with the protocol and completed the study. Compared to the 0.3% triclosan/2.0% PVM/MA copolymer/0.243% sodium fluoride dentifrice group, the 2.6% Edathamil dental gel exhibited statistically significant reductions in all measured parameters, respectively, after four weeks of product use.
Conclusion: The overall results of this double-blind clinical study supports the conclusion that a dental gel containing 2.6% Edathamil is efficacious for the control of dental plaque, and that it provides a greater level of efficacy for the control of dental plaque biofilm than does a dentifrice containing 0.3% triclosan/2.0% PVM/MA copolymer/0.243% sodium fluoride.
Keywords
Fluoride; Dentifrice; Plaque removal; Edathamil; Plaque biofilm
Introduction
Fluoride dentifrices have played a significant role in reducing dental caries, and many dentifrices on the market today deliver other oral health benefits, such as improvement in periodontal health [1-5]. It is widely accepted that the control of dental plaque biofilm and gingival inflammation is the key to maintaining periodontal health. Controlling dental plaque biofilm starts with the mechanical removal by tooth brushing [6]. The American Dental Association defines the maintenance of good oral hygiene as tooth brushing for two minutes twice daily plus flossingonce a day [7]. It has been shown that selfperformed tooth brushing and flossing is not always sufficient in itself to maintain a healthy mouth [8] so, manufacturers started looking at different chemotherapeutic agents to incorporate into dentifrice formulations that would provide antiplaque and/or anti gingivitis efficacy.
One such agent is triclosan, a phenolic antibacterial agent with low toxicity and a broad spectrum of activity. It has been shown to be effective against both gram positive and gram negative bacteria [9]. Triclosan has been incorporated into a sodium fluoride dentifrice in combination with a polyvinylmethyl ether/maleic acid copolymer (PVM/MA copolymer) to ensure delivery and retention of the triclosan on oral hard and soft tissues. This dentifrice formulation (Colgate® Total® Toothpaste, Colgate-Palmolive Co., New York, NY, USA) has been clinically proven in numerous scientific publications to prevent and reduce plaque biofilm and reduce gingivitis in the adult population [10,11]. It has also been shown in long-term clinical studies to provide anticaries benefits, and has been clinically proven to prevent the progression of periodontitis [12]. Colgate Total Toothpaste is a dentifrice approved by the US Food and Drug Administration to be used for the prevention of plaque biofilm accumulation and gingivitis. It is also accepted by the American Dental Association for the prevention of plaque biofilm accumulation and gingivitis.
This approach to oral plaquebiofilm control still relies heavily on detergents, abrasives and antimicrobials. If we look at how plaque adheres to teeth, the process is intricately tied to the presence of calcium in the immediate surroundings of the plaque forming bacteria [13-16]. Bennin et. al. [17] showed that chelation of calcium caused the dispersion of Pseudomonas Aeruginosa biofilms. P. Aeruginosa is one of the bacteria species along with P. Gingivalis and T. Forsythia that have been associated with periodontal disease. Livionex uses the same metal chelator, edathamil, as used by Bennin et al [17] to remove the calcium from the plaque in order to disperse dental plaque, and prevent the bacteria from adhering to the teeth. Many dentifrices contain chelators (e.g. sodium hexametaphosphate and others) in high concentrations and amounts (far higher than in the Livionex Dental Gel) while showing little or no efficacy against plaque. According to Livionex, because edathamil is a negatively charged molecule and is repelled by the negatively charged plaque surface, it is therefore ineffective for plaque reduction as currently used. Livionex uses a proprietary method to activate the edathamil molecule and allow it to penetrate into the plaque rapidly, to remove the calcium in the plaque fluid, thereby breaking up the plaque.
The objective of this four-week clinical study was to compare the anti-plaque efficacy of two commercially available products: a dentifrice containing 0.3% triclosan, 2.0% PVM/MA copolymer, and 0.243% sodium fluoride (Colgate Total) to a dental gel containing 2.6% Edathamil (Livionex Dental Gel). Gingivitis and plaque evaluations were conducted at baseline and after two and four weeks of product use.
Materials and Methods
Plaque biofilm was measured using the Quigley-Hein, Turesky Modification Plaque Index (PI). The Gingival health was measured by using the Löe-Silness Gingival Index (GI). Gingival bleeding on probing was measured by using a modified Sulcus Bleeding Index (BOP) and periodontal health was determined by the measurement of pocket depths.
Subjects & Clinical Protocol
Ten subjects with early periodontitiswere studied. Subjects were enrolled in this randomized, controlled, double-blinded study and chosen by the presence of pocket depths greater than 4 mm in the presence of BOP, and a GI of greater than 1. Scaling and root planing was performed on each of the subjects 6 to 12 months prior to their enrollment. After signing an informed consent, the subjects were randomly assigned to brush for 28 days with either the test or control gel. Test gel was the Livionex Dental Gel, and the control gel was Colgate Total gel. The two gels were packaged in plain white sealed tubes, and marked with a four digit random number. The randomization key was kept with a third party. Both the investigator and the subject were unaware of which gel was allocated to them.
Five subjects were assigned to the test gel and 5 subjects were assigned to the control gel. Eight subjects finished the study with 5 on the active gel, and 3 on the control gel. The two dropouts were excluded from the study analysis.
The subjects, all of whom had been trained in the proper method of brushing their teeth, were asked to maintain their normal dental hygiene habits, but change their regular toothpaste with the assigned dental gel, and to brush twice daily. After the measurement of baseline data, all subjects were provided with a professional dental cleaning. Plaque levels (Quigley-Hein, Turesky Modification Plaque Index) (PI), gingival inflammation (Löe-Silness Gingival Index) (GI), Bleeding on Probing (BOP Index) and Pocket Depths were determined at baseline before the cleaning. Volunteers were evaluated at Baseline, Day 14 and Day 28 by the same blinded and experienced investigator.
Clinical Scoring Procedures
Quigley-Hein Plaque Index
Plaque was scored according to the Turesky modification of the Quigley-Hein Plaque Index. This index is the same as the Quigley-Hein Index except the criteria has been modified. With the Quigley-Hein Index, a score of 0 to 5 is assigned to the facial and lingual aspects of each tooth. Plaque was disclosed and scored on each tooth surface according to the following criteria:
0=No plaque.
1=Separate flecks of plaque at the cervical margin.
2=A thin, continuous band of plaque (up to 1 mm) at the cervical margin.
3=A band of plaque wider than 1 mm, but covering less than 1/3 of the side of the crown of the tooth.
4=Plaque covering at least 1/3, but less than 2/3 of the side of the crown of the tooth.
5=Plaque covering 2/3 or more of the side of the crown of the tooth.
Subject-wise scores were determined by averaging the values obtained overall scoreable surfaces in the mouth.
Löe-Silness Gingival Index
Gingivitis was scored according to the Löe-Silness Gingival Index. Each tooth was divided into two surfaces, facial and lingual. Those teeth with cervical restorations or prosthetic crowns were excluded from the scoring procedure. The gingiva adjacent to each tooth surface was scored as follows:
0=Absence of inflammation.
1=Mild inflammation: slight change in color and little change in texture.
2=Moderate inflammation: moderate glazing, redness, edema, and hypertrophy.
3=Severe inflammation: marked redness and hypertrophy. Tendency for spontaneous bleeding.
Subject-wise scores were determined by averaging the values obtained over all scoreable surfaces in the mouth.
Gingivitis was evaluated at the same location points that were utilized for the assessment of plaque. In the clinical examination, the degree of gingival inflammation was always determined prior to the plaque assessment.
Bleeding on Probing Index
Gingival bleedingon Probing was scored using a modified Sulcus Bleeding Index. A score of 0 to 3 is assigned to each facial and lingual nonrestored surface of all the teeth according to the following criteria:
0=No bleeding when periodontal probe is passed along the gingival margin
1=Isolated bleeding spots visible
2=Blood forms a confluent red line on the gingival margin
3=Heavy or profuse bleeding
Probing Depths
Probing Depth Measurement
1. Six Sites per Tooth
Probing depth measurements are recorded for 6 specific sites on each tooth: (1) distofacial, (2) facial, (3) mesiofacial, (4) distolingual, (5) lingual, and (6) mesiolingual.
2. One Reading per Site
Only one reading per site is recorded. If the probing depths vary within a site, the deepest reading obtained in that site is recorded. For example, if the probing depths in the facial site were to range from 2 to 6 mm, only the 6 mm reading would be entered on the chart for that site.
3. Full Millimeter Measurements
Probing depths are recorded to the nearest full millimeter. Round measurements to the next higher whole number; for example, a reading of 3.5 mm is recorded as 4 mm, and a 5.5 mm reading is recorded as 6 mm.
Results
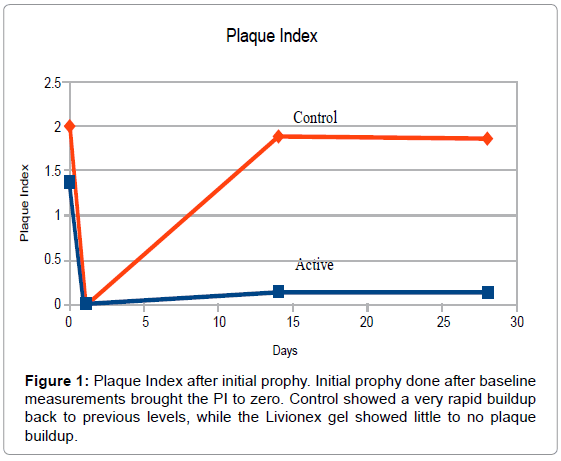
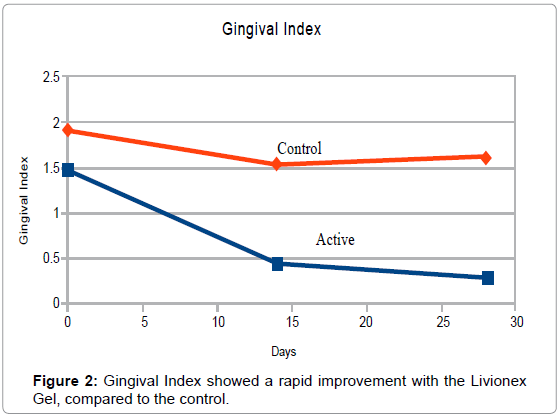
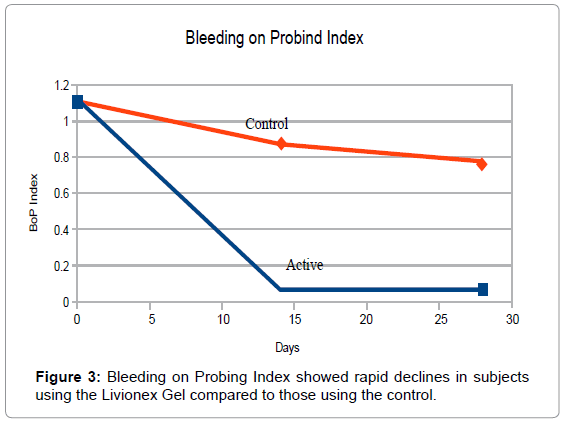
At Baseline, PI averaged 1.4 in the test group and 2.0 in the control group. The plaque index was brought down to 0 for both groups by means of a professional cleaning and teeth polishing after the baseline measurements had been taken. Mean GI measured 1.5 in the test group and 1.9 in the control group. BOP measured 1.1 in the test group and 1.7 in the control group. The small size of the sample was the primary reason for the differences in the indices.
By the end of week 4, PI in the test group averaged 0.15 vs. 1.82 in the control group. Mean GI was reduced to 0.27 in the test group vs. 1.50 in the control group, and BOP index averaged 0.03 in the test group vs. 0.61 in the control group (Figures 1-3).
A comparison of the percentage change from baseline in each of the measurements was performed. A statistical analysis was done to see whether the differences between the Livionex Gel and the control were statisticallysignificant. A T-test was performed that accounted for the uneven size of the two arms. The Null Hypothesis was that there was now difference between the two treatments. The null hypothesis was rejected at the 0.05 level. The results can be seen in Table 1. A retrospective calculation of the power of the tests showed them to be over 95% at an alpha 0f 0.05
| Plaque Index | Gingival Index | Bleeding on Probing Index | Probing Depth | |||||
| Livionex | Control | Livionex | Control | Livionex | Control | Livionex | Control | |
| Average | -89.00% | -1.28% | -83.17% | -16.54% | -93.71% | -54.24% | -12.16% | 11.16% |
| Std Dev. | 24.17% | 39.3% | 20.29% | 1.95% | 9.86% | 5.91% | 5.71% | 6.80% |
| N | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 |
| T-Stat | -3.9943 | -5.4946 | -6.1811 | 5.2391 | ||||
| df | 6 | 6 | 6 | 6 | ||||
| P-value | 0.0072 | 0.0015 | 0.0008 | 0.0019 | ||||
Table 1: Comparison of the percentage change in 4 measures from baseline (before prophy) to 4 weeks between the two gels produced highly significant differences on all 4 measures even with the small sample size. A difference of 87.7% (89.00%-1.28%) was seen in the plaque index, a 66.6% (83.17%-16.54%) was seen in the gingival index.
A comparison of each measurement site was also performed to see whether or not there was an improvement. This procedure is somewhat similar to that adopted by Lindhe [18]. The results of that comparison are in Table 2. Again, a T test was performed. The Null Hypothesis was that there was now difference between the two treatments. The null hypothesis was rejected at the 0.05 level. A retrospective calculation of the power of the tests showed them to be over 95% at an alpha 0f 0.05.
| Plaque Index | Gingival Index | Bleeding on Probing Index | Pocket Depth | |||||
| Livionex | Control | Livionex | Control | Livionex | Control | Livionex | Control | |
| Improved | 82.69% | 30.95% | 94.23% | 41.67% | 82.31% | 76.79% | 42.69% | 2.78% |
| Not Improved | 17.31% | 69.05% | 5.77% | 17.31% | 17.69% | 23.21% | 57.31% | 97.22% |
| N | 260 | 168 | 260 | 168 | 260 | 168 | 780 | 504 |
| T-Stat | 12.119 | 12.916 | 1.371 | 20.825 | ||||
| df | 305 | 215 | 330 | 1021 | ||||
| P-value | 0.00001 | 0.00001 | 0.1713 | 0.00001 | ||||
Table 2: A comparison of the percentage of sites that improved in the 4 measures tested between the two gels were highly significant other than for the Bleeding on Probing Index. It should be noted that this just looked at whether or not there was an improvement, and paid no attention to the magnitude of improvement. The magnitude of improvement has been taken into account previously.
Conclusion
Based on this small study, it can be concluded that the Livionex Dental Gel is very effective at inhibiting the formation of new plaque biofilm when compared to triclosan based toothpaste. Livionex Dental Gel can also remove plaque biofilm, efficiently and quickly, using activated edathamil.
This small study documents that Livionex Dental Gel, with activated edathamil, is significantly better than a triclosan based toothpaste at removing plaque, improving the gingival index and improving pocket depths. These results merit further study in a larger population.
References
- Marinho VC, Higgins JP, Sheiham A, Logan S (2003) Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 1:CD002278.
- Twetman S, Axelsson S, Dahlgren H, Holm AK, Källestål C, et al. (2003) Caries-preventive effect of fluoride toothpaste: a systematic review. Acta Odontol Scand 61:347-355.
- Davies RM, Ellwood RP, Davies GM (2004) The effectiveness of a toothpaste containing triclosan and polyvinyl-methyl ether maleic acid copolymer in improving plaque control and gingival health: a systematic review. J Clin Perio dontol 31:1029-1033.
- Hioe KP, van der Weijden GA (2005) The effectiveness of self-performed mechanical plaque control with triclosan containing dentifrices. Int J Dent Hyg 3:192-204.
- Gunsolley JC (2006). A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc 2006;137:1649-1657.
- https://www.ada.org/public/topics/cleaning
- Axelsson P, Lindhe J, Nyström B (1991) On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. J Clin Perio dontol 18:182-189.
- O'Mullane D (1992) New agents in the chemical control of plaque and gingivitis: reaction paper. J Dent Res 71:1455-1456.
- Lindhe J (1990) Triclosan/copolymer/fluoride dentifrices. A new technology for the prevention of plaque, calculus, gingivitis and caries. Am J Dent 3(SpecIss):S3-S4.
- Gaffar A, Nabi N, Kashaba B, Williams M, Herles S, et al. (1990) Anti plaque effects of dentifrices containing triclosan/copolymer/NaF system versus triclosan dentifrices without the copolymer. Am J Dent 3(Spec Iss):S7-S14.
- Nabi N, Mukerjee C, Schmid R, Gaffar A (1989) In vitro and in vivo studies on triclosan/PVM/MA copolymer/NaF combination as an anti-plaque agent. Am J Dent 2 Spec No:197-206
- Panagakos FS, Volpe AR, Petrone ME, DeVizio W, Davies RM, et al. (2005) Advanced oral antibacterial/anti-inflammatory technology: A comprehensive review of the clinical benefits of a triclosan/copolymer/fluoride dentifrice. J Clin Dent 16 Suppl S1-S19.
- Electrostatic Charge and Bacterial Adhesion.
- Johnson, Michael D.L (2011) The Effect of Calcium Binding on Adhesion and Pilus Biogenesis in the PilC Family of Proteins. PhD Thesis University of North Carolina, Chapel Hill.
- Martinez-Gil M, Romero D, Kolter R, Espinosa-Urgel M (2012) Calcium Causes Multimerization of the Large Adhesin LapF and Modulates Biofilm Formation by Pseudomonas putida. J Bact 194: 6782–6789.
- Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ (2005) Calcium-Induced Virulence Factors Associated with the Extracellular Matrix of Mucoid Pseudomonas aeruginosa Biofilms. J Bact 187: 4327–4337.
- Benin E, Brady KM, Greenberg EP (2006) Chelator-Induced Dispersal and Killing of Pseudomonas aeruginosa Cells in a Biofilm. Applied and Environmental Microbiology 72:2064–2069.
- Lindhe J, Rosling B, Socransky SS, Volpe AR (1993) The effect of a triclosan-containing dentifrice on established plaque and gingivitis. J Clin Periodontol 20: 327-334.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 17864
- [From(publication date):
November-2014 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 13071
- PDF downloads : 4793