Research Article Open Access
Effect of Polygonum persicaria Agglutinin on Digestive α-amylase of Rice Striped Stem Borer
| Arash Zibaee* | ||
| Department of Plant Protection, Faculty of Agricultural Sciences, University of Guilan, Rasht, Iran | ||
| Corresponding Author : | Arash Zibaee Department of Plant Protection Faculty of Agricultural Sciences University of Guilan, Rasht, Iran Tel: 0911-237-9585 Fax: 0131-669-0281 E-mail: arash.zibaee@gmx.com, arash.zibaee@guilan.ac.ir |
|
| Received January 06, 2014; Accepted January 28, 2014; Published January 30, 2014 | ||
| Citation: Zibaee A (2014) Effect of Polygonum persicaria Agglutinin on Digestive α-amylase of Rice Striped Stem Borer. J Rice Res 2:120. doi: 10.4172/jrr.1000120 | ||
| Copyright: © 2014 Zibaee A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Rice Research: Open Access
Abstract
Results of the current study revealed that a lectin extracted from Polygonum persicaria L. had significant inhibition on digestive α-amylase of Chilo suppressalis Walker. Concentrations 0.05 to 2 mg/ml of PPA significantly decreased enzymatic activity from 5 to 72%. The highest inhibition of α-amylase was obtained at pH 9 while the optimal pH for amylolytic activity was found to be 8 and 9. Besides, optimal temperature of α-amylase activity was at 30°C but the highest inhibition of the enzyme was found at temperatures of 30-40°C. Kinetic parameters of α-amylase revealed lower and higher amounts of Vmax and Km in PPA treated enzyme indicating mixed inhibition. In vitro inhibition of α-amylase clearly showed that PPA could intervene in digestive process of C. suppressalis and decrease nutrient income of larvae. Further experiments are required to fully understand negative effects of PPA against rice striped stem borer to reach an efficient pest control via providing resistant varieties.
| Keywords | |
| Rice striped stem borer; α-amylase; Polygonum persicaria; Agglutinine | |
| Introduction | |
| Rice striped stem borer, Chilo suppressalis Walker (Lepidoptera: Crambidae) is the major pest of rice in north of Iran [1]. Larvae intensively utilize inner parts of stems and cause dead heart and white head of rice leading to severe loss of rice production [1]. C. suppressalis has 2-3 generations per year and causes significant damages to almost all rice varieties. Control procedures are basically to use synthetic insecticides like diazinon, padan and fenitrotione [2]. Although, biological control using Trichogramma spp. demonstrated positive results but insecticides still remains as the first and main tactic [2]. | |
| Digestive carbohydrases contain α-amylases, glycosidases, xylanases, chitinases and pectinases but α-amylases have crucial role in digestion of dietary starch and glycogen as the initial energy sources of insects. α-Amylases (EC 3.2.1.1) are hydrolyzing enzymes that catalyze breaking-down of inner and long α-1,4-glucan chains in starch and glycogen [3]. Several studies revealed that the enzyme has molecular weight of 48–60 kDa, pI values of 3.5–4.0, and Km values by using soluble starch ~0.1% [4]. Also, it has been reported that α-amylases are calciumdependent and those are activated by chloride with displacement of pH optimum [4]. Zibaee et al. [5] reported that activities of α-amylase in the midgut and salivary glands of C. suppressalis were 0.06 and 0.036 μmol/min/mg protein, respectively. The optimal pH and temperature of the enzyme found to be 9 and 35-40°C which is consistent with reports of other lepidopteran insects. Finally, the authors demonstrated that the α-amylase was inhibited by addition of NaCl, KCl, MgCl2, Urea, EDTA, and SDS while CaCl2 enhanced enzymatic activity [5]. | |
| Since C. suppressalis utilizes inner parts of rice stems, no chemical could reach the larvae. So, insecticide spraying has been limited to wide spraying against 1st larval instar. Zibaee et al. [2] reported that rice striped stem borer has been resistant to diazinon in four different regions of northern Iran. It means that using diazinon could not be more efficient. There are six classes of α-amylase inhibitors including lectin-like, knottin-like, cereal-type, Kunitz-like, c-purothionin-like, and thaumatin-like [6]. These inhibitors show structural diversity, differ¬ent modes of inhibition and different specificity profiles against diverse range of α-amylases [7]. Lectins are the heterogeneous molecules (glycoprotein) that have been extensively distributed in nature and plays several functions in physiology of living organisms [8]. They have shown entomotoxic and inhibitory properties against insects [8]. Hence, aims of the current study were to purify a lectin from Polygonum persicaria and determine its inhibition on digestive α-amylase of C. suppressalis. | |
| Materials and Methods | |
| Insect rearing | |
| Egg patches of C. suppressalis were collected from rice fields and reared in containers provided by rice seedlings. In¬sects were reared based on a modified method of Zibaee et al. [2] at 28 ± 2°C, 80% relative humidity (RH) under conditions of 16 h light: 8 h dark. Laboratory conditions were checked, containers were cleaned and fresh stems were daily provided for larvae. | |
| Purification of PPA | |
| Stems of P. persicaria were incubated in phosphate buffer (0.1 M pH 7.1) for approximately 72 h at 4°C. Then, stems were grounded in the buffer to completely destroy the tissues prior to additional incubation for 24 h. The mixture was filtrated by a layer of cheesecloth, then it was centrifuged at 5000 rpm for 20 min. Remaining debris was removed by passing the supernatant through filter paper (Whatmann No.4) [9,10]. Supernatant was precipitated by 0-60% concentrations of ammonium sulfate and centrifuged at 5000 rpm for 20 min. Debris was eluted in Tris-HCl buffer (0.1 M, pH 7) and dialyzed in the same buffer overnight [10]. Dialyzed samples were loaded into Sepharose 4B-galactose column equilibrated with Tris-HCl buffer (0.1 M, pH 7) as affinity chromatography. The affinity column was washed with Tris- HCl buffer and buffer containing 20 mM 1,3-diaminopropane (DAP) [9,10]. Fractions showing the highest protein content were pooled and used for forthcoming step. Fractions obtained after the first affinity chromatography were loaded on DEAE-Cellulose fast flow equilibrated with DAP [9,10]. Finally, the lectin was eluted using Tris–HCl (0.1 M, pH 7.0) containing 0.5 M NaCl after DAP washing. Again, the samples showing the highest protein content were dialyzed against water and purified molecule was analyzed by SDS-PAGE stained with commassie brilliant blue [11]. | |
| Sample preparation | |
| Larvae of C. suppressalis were randomly selected and their midguts were removed by dissection under a stereomicroscope in ice-cold saline buffer (NaCl, 10 mM). The midguts were rinsed in ice-cold distilled water, placed in a pre-cooled homogenizer and grounded before centrifugation. Equal portions of larval midgut and distilled water were used to have desirable concentration of the enzyme (W/V). Homogenates were separately transferred to 1.5 ml tubes and centrifuged at 13,000 rpm for 20 min at 4°C. The supernatants were pooled and stored at −20°C for subsequent analyses. | |
| α-Amylase assay | |
| The method using dinitrosalycylic acid (DNS) was used to assay α-amylase activity [12]. Ten microlitres of the enzyme was incubated for 30 min at 35°C with 50 μl of phos¬phate buffer (0.02 M, pH 7.1) and 20 μl of soluble starch as substrate. The reaction was stopped by adding 80 μl of DNS and heating in boiling wa¬ter for 10 min prior to read the absorbance at 545 nm. One unit of α-amylase activity was defined as the amount of enzyme required to produce 1 mg maltose in 30 min at 35°C. The negative control contained all reaction mix¬tures with pre-boiled enzyme (for 15 min) to prove the enzyme presence in the samples. | |
| Inhibition of α-amylase by different concentrations of PPA | |
| Inhibition experiment was carried out using 50 μl of PBS (phosphate buffer solution) (0.02 M, pH 7.1) 20 μl of starch 1% and 20 μl of different concentrations of PPA (0, 0.1, 0.5, 1, 1.5, and 2 mg/ml) Then, 10 μl of the enzyme was added and the reaction continued as described earlier. The blank con¬tained PBS, starch 1%, and each concentration of PPA. | |
| Effect of pH on α-amylase inhibition by PPA | |
| pH dependency of the PPA inhibition was deter¬mined at different pH values using Tris-HCl buffer (20 mM) with pH set at 5, 6, 7, 8, 9, 10, 11, and 12. Α-Amylase activity was assayed after incubation of the reac¬tion mixture containing Tris-HCl buffer (in the given pH value), starch 1%, PPA (2 mg/ml) and enzyme. | |
| Effect of temperature on α-amylase inhibition by PPA | |
| Effects of temperature regimes on α-amylase inhibi¬tion by PPA were made by the reaction mixture containing Tris-HCl (20 mM pH, 9), Starch 1%, PPA (2 mg/ml) and enzyme at different temperatures set at 15, 20, 25, 30, 35, 40, 45, 50, and 60°C. | |
| Kinetic Studies | |
| Kinetic parameters of inhibition were carried out using different concentrations of starch as substrate (0.5–2.0%) in the presence of PPA (2 mg/ml). Lineweaver-Burk plot analysis was made to find Km and Vmax values by Sigma-Plot software (version 12). | |
| Protein Assay | |
| Protein concentrations were assayed according to the method described by Lowry et al. [13]. The method recruits reaction of Cu2+, produced by the oxidation of peptide bonds with Folin–Ciocalteu reagent. In the assay, 20 μl of sample was added to 100 μl of reagent, and incubation was made for 30 min prior to read the absorbance at 545 nm (Recommended by Ziest Chem. Co., Tehran-Iran). | |
| Statistical Analysis | |
| All data were compared by one-way analysis of vari¬ance (ANOVA) followed by Tukey’s studentized test when significant differences were found at p ≤ 0.05, and marked in figures with letters. | |
| Results and Discussion | |
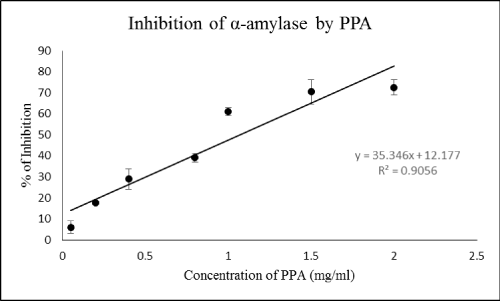
| Lectins are the heterogeneous proteins that are able to recognize carbohydrates in physiological media [14]. In facts, lectins reversibly bind to carbohydrates without altering their covalent structure [15]. These molecules may have several functions in living organisms but plant lectins have been shown entomotoxic effects and enzymatic inhibition [8]. Certain tissues of plants like seeds, bark and bulbs contains lectins that might indicate their roles as storage proteins [8]. In the current study, different concentrations of PPA significantly affected digestive amylolytic activity of C. suppressalis (Figure 1). Although the highest inhibition was found to be 72% but concentrations of 1, 1.5 and 2 mg/ml show somehow same inhibition (Figure 1). Among six types of α-amylase inhibitors, lectin-like one has demonstrated various effects. For example, α-AI1 from Phaseolus vulgaris L. inhibited digestive α- amylases of Callosobruchus maculatus Fabricius (Coleoptera: Bruchidae) and C. chinensis while α-AI2 had no inhibitory effects on these insects [16,17]. In a current study, different concentrations of extracted lectin, Citrullus colocynthis agglutinin (CCA), inhibited digestive amylolytic activity in Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) by 22-49% [18]. Direct inhibition of digestive α-amylase by PPA and well-known function of lectins as binding moelcules to epithelial cells of midgut point out entomotoxic and inhibitory effects that definitely disrupt digestive processes of insects lead to mal-nutrition and death of pest without other effects on non-target organisms and environmental pollutions. | |
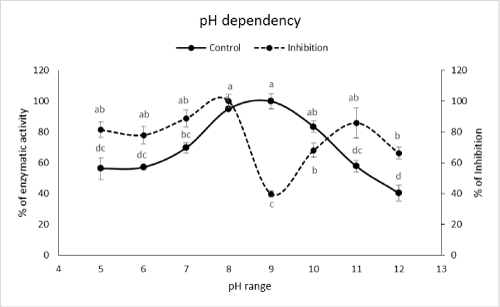
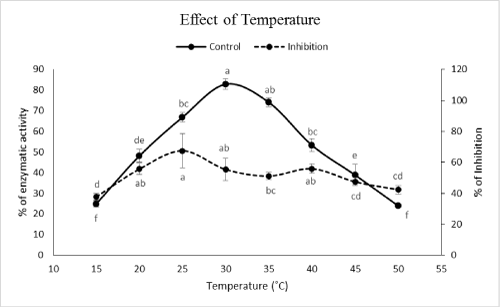
| Although optimal pHs of the enzyme were found at pHs 8 and 9 but the highest inhibition occurred at pH 9 (Figure 2). In case of temperature, the highest inhibition obtained at temperatures of 15, 35, 45 and 50Ã?Â?C (Figure 3). Although these temperatures showed the highest inhibitions but the correct value could be 35Ã?Â?C because other values have been shown lower amylolytic activity in both control and PPA treated enzymes (Figure 3). On the other hand, lower activity in 15, 45 and 50Ã?Â?C might be due to lower enzymatic velocity or its denaturation not PPA inhibition. Other reports imply on correlation between optimal values of pH and temperature for enzymatic activity versus inhibition. For example, Mehrabadi et al. [7] reported pHs of 5 and 6 for inhibition of salivary and midgut α-amylase in Eurygaster integriceps Puton (Hemiptera: Scutelleridae). Barbosa et al. [19] showed that αAI extracted from P. vulgaris inhibited porcine pancreatic α-amylase at pH 5.5 depending on the strain of bean used. Finally, Ramzi and Sahragard [18] demonstrated the highest inhibition of E. ceratoniae α-amylase by CCA at pH values of 8 and 9. Meanwhile, inhibition of the enzymes was found at 40°C as control optimal value. | |
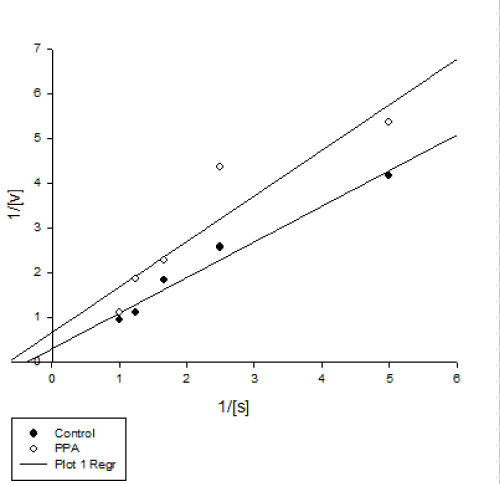
| Enzyme kinetic parameters revealed lower and higher amounts of Vmax and Km in PPA treated enzyme that is a mixed inhibition (Figure 4 and Table 1). This kind of inhibition is considered as a combination of competitive and uncompetitive processes leading to decrease in both Km and Vmax values. Vmax and Km are the two basic parameters to calculate enzyme behavior in biochemical media. In details, Km shows affinity of an inhibitor or a substrate to an enzyme. Mixed inhibition has been reported in pancreatic amylase inhibition P. vulgaris seeds [20], and α-amylase of Rhyzopertha dominica by wheat α-amylase inhibitors [21]. | |
| Synthetic chemicals mainly organocholorous and orhanophosphorous have been made several concerns on environmental pollutions, residue in non-target organisms, resistance and etc. Since 1972 that rice stripes stem borer was introduced to Iran, chemical insecticides, mainly diazinon, were the unique control against the pest [2]. In 2009, resistant of the pest has been reported indicating necessity of using an alternative control procedure. Resistant varieties have shown well results against herbivorous insects when biotechnological approaches were adopted to express an entomotoxic protein or inhibitor in host plants. A traditional example could be Galanthus nivalis L. agglutinin that has been expressed in several crops showing well results. Since, a low concentration of PPA showed inhibitory effects against α-amylase of rice striped stem borer, it could be a nice candidate to provide resistant varieties of rice expressing the molecule. Although this objective requires comprehensive studies in both biotechnological and pest control aspects. | |
References |
|
|
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 14913
- [From(publication date):
February-2014 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 10224
- PDF downloads : 4689
