Research Article Open Access
Effects of Citric Acid and Butylated Hydroxytoluene Alone or In Combination on Prevention of Rancidity of Rice Bran during Storage
NSBM Atapattu1*, KP Wickramasinghe1,Thaksala serasinghe1,SP Gunarathne2
1Faculty of Agriculture, University of Ruhuna, Sri Lanka
2Faculty of Veterinary Science, University of Peradeniya, Sri Lanka
- *Corresponding Author:
- NSBM Atapattu
Faculty of Agriculture
University of Ruhuna, Sri Lanka
Tel: 94-71-5303531
Fax: 94-41-2292384
E-mail: mahindaatapattu@gmail.com
Received date: October 21, 2013; Accepted date: November 13, 2013; Published date: November 15, 2013
Citation: Atapattu NSBM, Wickramasinghe KP, Srasinghe T, Gunarathne SP (2013) Effects of Citric Acid and Butylated Hydroxytoluene Alone or In Combination on Prevention of Rancidity of Rice Bran during Storage. J Rice Res 1:114. doi: 10.4172/2375-4338.1000114
Copyright: © 2013 Atapattu NSBM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Rice Research: Open Access
Abstract
Rice bran (RB), a byproduct of rice milling process is a valuable feed resource for the livestock industry. Both lipolytic and oxidative rancidity starts right after bran is removed from the grain during the milling process thereby making long term storage of RB difficult. Anti oxidants such as butylated hydroxytoluene (BHT) control the oxidative rancidity only for a short period of time. Therefore, effective rancidity control measures need to be adopted to maintain the nutritive and feeding value of RB. The objective of this study was to evaluate whether rancidity of RB could be curtailed by storing RB with citric acid (CA) and BHT alone or in combination.
RB was collected immediately after milling and stored with three levels of CA (0, 2, and 4%) and three levels of BHT (0, 200, and 400 ppm) in a 3×3 factorial arrangement. Each treatment combination had three replicates. Treated RB was stored in plastic containers with a closed lid for 82 days and analyzed for peroxide value (PV) and pH on 7, 25, 55, 82 days. The mean PV of un-treated RB on day 7 (2.85 meq kg-1) increased to 14.42meq kg-1 during 82 days of storage. When BHT or CA was not used, a significant (p<0.05) negative correlation was found between PV and pH [PV=(7.3291–pH x 0.112; R2=0.901]. Peroxide values were not significantly (p>0.05) affected by BHT or CA on day 7. The use of 0, 200 or 400 ppm BHT significantly reduced the PV during 25 days of storage time, but not thereafter. The use of 2% or 4% CA significantly (p<0.05) reduced the PV up to day 82. It can be concluded that BHT was effective in reducing rancidity of RB only for about month whereas CA controls both lipolytic and oxidative
rancidity for about three months
Keywords
Rice bran; Rancidity; Citric acid; BHT; Storage
Introduction
Rice bran (RB) is a major by-product in the milling industry of raw paddy (rough rice) into rice. A total of 40-45 million metric tons of RB are produced annually mainly in East and South Asia [1]. RB is a valuable feed resource for all the classes of livestock. The lipid content of RB is high and varies from 15% to 23% and three major fatty acids; palmitic (12%-18%), oleic (40%-50%) and linoleic (30%-35%) account for up 90% of total fatty acids [2]. One of the major restriction for the use of RB as an animal feed ingredient is its high susceptibility to rancidity during storage. It has been reported [3] that upto 50% of the fat in the bran was converted into free fatty acids within 6 weeks after milling due to rancidity. Nutritional quality of rice bran deteriorates rapidly due to rancidity [2].
Oil in un-milled rice is stable because lipolytic enzymes of intact kernel are located in the cross cells of the seed coat while most of the oil is stored in the aleurone layer and the germ [4]. After the bran layer is removed from the endosperm, the individual cells are disrupted and the bran lipids come into contact with lipases. Oxidative rancidity involves a reaction between free oxygen and lipids. The reaction attacks the double-bonds of the fatty acids and is accelerated by oxygen, free radicals, metal ions (iron, cobolt and copper), light, radiation and enzyme lipoxygenase [5]. The reaction of oxygen with unsaturated lipids involves free radical initiation, propagation, and termination processes [6].
Stabilization of RB just after milling is of utmost importance to mitigate the adverse effects of rancidity. Many experimental procedures such as lowering pH [7], use of ethanol vapour [8] and heating [4] have been used to inactivate lipases and thereby to stabilize RB and prolong its shelf-life. Microwave heating, extrusion cooking, roasting, pelleting are the other methods that can be used to stabilize RB [2]. Though physical treatments such as heating right after milling can substantially reduce the rancidity particularly the lipolytic rancidity, these methods are costly, demands high amount of energy and cumbersome [9].
Due to the inherent instability of natural antioxidants, several synthetic antioxidants have been used to stabilize fats and oils [10,11]. Butylated hydroxyanisole (BHA) and BHT compounds have been used as antioxidants in human foods since 1954 [12]. However, potential health hazards of synthetic antioxidants in foods, including possible carcinogenicity, have been reported times [11]. Prabhaker and Venkatash [7] concluded that chemical methods are not very effective in RB stabilization. Therefore, socio-economically viable rancidity prevention methods to stabilize RB are of importance.
Citric acid is a relatively cheap, readily available organic acid. CA is used in poultry and swine diets, mainly to increase the availability of phytate phosphorus [13]. The food and pharmaceutical industries utilize CA extensively because of its general recognition of safety, pleasant acid taste and chelating and buffering properties [14]. CA or citrates have been used to control oxidative deterioration of flavor or colour of a wide range of foodstuffs [15]. CA has been shown to play a synergetic role with primary antioxidants and oxygen scavengers during vegetable oil storage [16]. The objective of this study was to evaluate whether rancidity of RB could be curtailed by storing RB with CA and BHT alone or in combination.
Materials and Methods
Citric acid (2-hydroxypropane-1, 2, 3-tricarboxylic acid) and butylated hydroxytoluene were purchased from SIGMA chemicals private limited, Sri Lanka. RB was collected (un-known variety) from a local rice mill and immediately transported to the research laboratory. The experiment was a completely randomized design in 3×3 factorial arrangement. Experimental factors were three BHT (0 ppm, 200 ppm and 400 ppm) and three CA (0%, 2%, and 4%) levels. Each treatment combination had three replicates. Treated RB samples were stored in the 2 dm3 volume plastic containers with closed lids, under room temperature (27-31°C) and RH conditions (mean 85%).
Treated RB in each container was mixed well before collecting the samples for analysis. Three random samples from each treatment combination were taken on day 7, 25, 55 and 82 (Approximately one week, one month, two month and three months after storage) and were analyzed for peroxide value [17] and pH value. Data were analyzed using the GLM procedure of SAS [18]. Effects were considered significant when p<0.05. When interactions were significant LS mean comparison procedure was used to compare the means.
Determination of pH value
The pH values were obtained by using a pH meter of a glass electrode (Corning pH meter 430). Sample preparations for pH determination were performed according to method described by Dev et al. [19]. 10g of samples were mixed with 10 ml of distilled water to keep fluidity. Mixture was kept still for about 30 minutes after shaking well. Three replicates of each solution were analyzed for pH and mean value was taken as the pH value of the samples.
Determination of the Peroxide Value
10 g of RB sample was taken from each replicated. Oil was extracted by soxhlet extraction method before being subjected to chemical analyses. The peroxide value was determined using the method of AOAC [17]. The peroxide value of RB oil was determined by dissolving the oil in a solvent mixture of acetic acid and carbon tetrachloride, warmed with potassium iodide and, then titrated with sodium thiosulphate solution using starch as the indicator.
Results and Discussion
The effects of three levels of CA and BHT on the PV of RB on storage are shown in Table 1. The interaction between different levels of CA and BHT was not significant (p>0.05). The main effects; BHT and CA levels had significant (p<0.05) effect on PV of RB during storage. Both BHT and CA levels had no significant (p>0.05) effect on PV after a week of storage. The mean PV of un-treated RB on day 7 (2.85 meq kg-1) increased to 14.42 meq kg-1 during 82 days of storage. The PVs observed in the present study were slightly higher than those values reported by Mujahid et al. [20]. Njobeh et al. [21] reported that the tropical climatic conditions (temperature above 25oC and RH greater than 70%) are ideal for feed spoilage. Therefore differences in experimental conditions may be the reason for higher PVs observed in the present study.
| BHT (ppm) | CA (%) | Peroxide value (mean ± SE) | |||
|---|---|---|---|---|---|
| on day 07 | on day 25 | on day 55 | on day 82 | ||
| 0 | 0 | 2.85 ± 0.01 | 5.55 ± 0.02 | 6.48 ± 1.21 | 14.42 ± 0.35 |
| 0 | 2 | 2.16 ± 0.23 | 4.80 ± 0.17 | 5.97 ± 2.19 | 7.10 ± 0.69 |
| 0 | 4 | 2.46 ± 0.12 | 3.46 ± 0.01 | 4.53 ± 0.40 | 5.15 ± 0.29 |
| 200 | 0 | 2.20 ± 0.98 | 4.15 ± 0.01 | 7.48 ±0.69 | 12.33 ± 0.87 |
| 200 | 2 | 2.09 ± 0.23 | 4.26 ± 0.06 | 4.22 ± 0.52 | 5.82 ± 0.12 |
| 200 | 4 | 2.53 ± 0.29 | 3.22 ± 0.35 | 2.99 ± 0.17 | 7.37 ± 0.01 |
| 400 | 0 | 2.23 ± 0.23 | 4.38 ± 0.29 | 6.26 ± 0.06 | 10.72 ± 1.56 |
| 400 | 2 | 2.39 ± 0.01 | 3.53 ± 0.40 | 4.37 ± 0.06 | 5.47 ± 0.98 |
| 400 | 4 | 2.29 ± 0.35 | 2.72 ± 0.12 | 5.33 ± 0.52 | 6.72 ± 0.06 |
| Main effects means ± SE | |||||
| 0 | 2.49 ± 0.1 | 4.60a ± 0.3 | 5.66 ± 0.8 | 8.89 ± 1.8 | |
| 200 | 2.27 ± 0.3 | 3.88b ± 0.2 | 4.89 ± 0.8 | 8.51 ± 1.2 | |
| 400 | 2.31 ± 0.1 | 3.54b ± 0.3 | 5.32 ± 0.3 | 7.64 ± 1.1 | |
| 0 | 2.43 ± 0.3 | 4.69a ± 0.2 | 6.74a ± 0.5 | 12.49a ± 0.8 | |
| 2 | 2.22 ± 0.1 | 4.19a ± 0.2 | 4.86ab ± 0.8 | 6.13b ± 0.5 | |
| 4 | 2.43 ± 0.1 | 3.13b ± 0.1 | 4.28b ± 0.4 | 6.41b ± 0.4 | |
| Level of significance | |||||
| BHT | NS | 0.0038 | NS | NS | |
| CA | NS | 0.0003 | 0.05 | 0.0001 | |
| BHT×CA | NS | NS | NS | NS | |
a-c Means within a column with no common superscript differ significantly (P < 0.05).
Table 1: Peroxide value of rice bran during storage as affected by three levels of CA and BHT. SE = standard error.
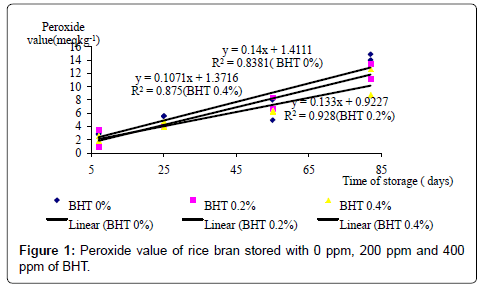
BHT reduced the PV of RB until day 25 but not thereafter. On day 25, both 200 ppm (3.88 meqkg-1) and 400ppm (3.54 meqkg-1) of BHT significantly (p<0.05) lowered the PV compared to 0% BHT (4.6 meqkg-1). Several authors [4,12,22,23] have also reported that oxidative rancidity can be controlled by adding antioxidants such as Pyrocatechol, Homocatechol, Propylgallate, BHA and BHT. Interestingly, on day 25, 4% CA also reduced PV (3.13 meqkg-1) significantly (p<0.05), compared to 2% (4.19 meqkg-1) and 0% (4.69 meqkg-1) CA. Similar effects of CA have been reported by others as well [15,16].
Results of the present study suggest that BHT is effective in controlling oxidative rancidity of RB only for about 25 days. Mujahid et al. [20] have also reported that anti oxidants did not prevent the free fatty acid formation and increase of PV during long storage of RB and thus addition of antioxidant to rice bran was not an effective mean to stabilize rice bran. Several other studies [21,22] have also shown that anti oxidants are effective only for about a month. Rapid auto oxidation of antioxidants and/or leaching with time and low penetration of antioxidants in fibrous RB are suggested as the possible reasons for shorter period of effectiveness of BHT in RB.
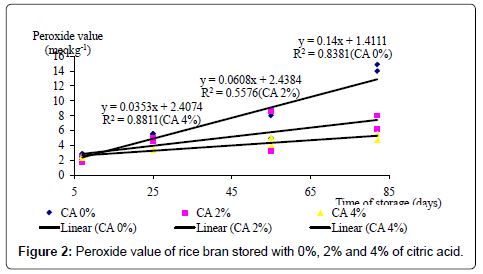
In contrast to BHT, CA significantly (p<0.05) reduced the oxidative rancidity as determined by PV of RB over a longer period of storage. When measured after 25, 55 and 82 days of storage, 4% CA significantly (p<0.05) reduced the PV compared to the 0% CA. 2% CA did not significantly (p>0.05) reduce the PV compared to 0% CA on 25 days. But, but later on day 82, the PV of the RB treated with 2% was significantly lower than that of RB with 0% CA.
The changes of PV of RB stored with BHT or CA are shown in Figures 1 and 2, respectively. The PVs of the RB treated both with BHT and CA increased over the storage period. The rate of increase in PV of the RB stored with 4% CA was lower than those of the BHT treated RB. Furthermore, throughout the storage period, the PVs of the RB stored with CA were lower than those of the RB stored without CA (Figure 2). Above findings suggest that CA controls the rancidity of RB and anti-oxidative properties of CA in controlling oxidative rancidity were more powerful than BHT. This hypothesis is further supported by the findings of Tao [24] who reported that 0.1 to 2% acetic acid having antioxidative properties, maintained the stability of the parboiled RB for at least 6 months.
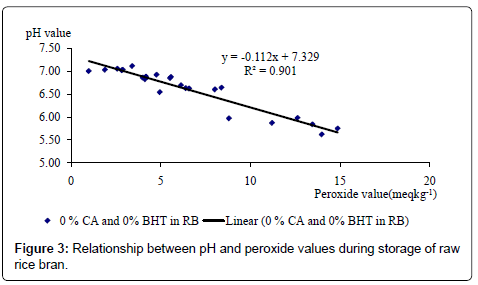
The effect of different BHT and CA levels on pH value of RB is shown in Table 2. The interaction between different levels of CA and BHT was not consistent. Significant (p<0.05) interactions were found on day 25 and day 82 but not on day 55. A clear explanation could not be found for this inconsistency. When BHT or CA was not used (Control samples), a significant (p<0.05) negative correlation (Figure 3) was found between PV and pH [PV=(7.3291-pH×0.112; R2=0.901]. This suggests that when facilities are not available for PV determination, PV can be predicted fairly accurately by measuring the pH value of the RB, stored for 82 days.
| BHT (ppm) | CA (%) | Mean pH ± SE | |||
|---|---|---|---|---|---|
| on day 07 | on day 25 | on day 55 | on day 82 | ||
| 0 | 0 | 7.02 ± 0.001 | 6.86 ± 0.006 | 6.57 ± 0.023 | 5.68 ± 0.052 |
| 200 | 2 | 5.93 ± 0.006 | 5.82 ± 0.012 | 5.88 ± 0.012 | 5.34 ± 0.023 |
| 400 | 4 | 5.17 ± 0.046 | 5.34 ± 0.029 | 5.26 ± 0.040 | 4.81 ± 0.012 |
| 0 | 0 | 7.06 ± 0.046 | 6.85 ± 0.023 | 6.63 ± 0.006 | 5.86 ± 0.012 |
| 200 | 2 | 5.98 ± 0.040 | 6.14 ± 0.092 | 5.90 ± 0.069 | 5.30 ± 0.040 |
| 400 | 4 | 5.38 ± 0.040 | 5.26 ± 0.012 | 5.18 ± 0.012 | 4.82 ± 0.017 |
| 0 | 0 | 7.04 ± 0.006 | 6.89 ± 0.023 | 6.66 ± 0.023 | 5.98 ± 0.001 |
| 200 | 2 | 5.74 ± 0.069 | 5.74 ± 0.069 | 5.78 ± 0.001 | 5.16 ± 0.001 |
| 400 | 4 | 5.36 ± 0.069 | 5.28 ± 0.001 | 5.10 ± 0.001 | 4.84 ± 0.001 |
| Main effects means ± SE | |||||
| 0 | 6.04 ± 0.34 | 6.01ab ± 0.28 | 5.90 ± 0.24 | 5.28 ± 0.16 | |
| 200 | 6.14 ± 0.31 | 6.08a ± 0.29 | 5.90 ± 0.26 | 5.32 ± 0.19 | |
| 400 | 6.04 ± 0.32 | 5.97b ± 0.30 | 5.84 ± 0.28 | 5.32 ± 0.21 | |
| 0 | 7.04a ± 0.01 | 6.87a ± 0.01 | 6.62a ± 0.02 | 5.84a ± 0.05 | |
| 2 | 5.88b ± 0.05 | 5.90b ± 0.08 | 5.85b ± 0.03 | 5.26b ± 0.03 | |
| 4 | 5.31c ± 0.05 | 5.29c ± 0.01 | 5.18c ± 0.03 | 4.82c ± 0.01 | |
| level of significance | |||||
| BHT | NS | NS | NS | NS | |
| CA | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| BHT×CA | NS | 0.0080 | NS | 0.0005 | |
NS= non significant (p>0.05)
a-cMeans within a column with no common superscript differ significantly (P < 0.05).
Table 2: The pH values of rice bran during storage as affected by three levels of CA and BHT.
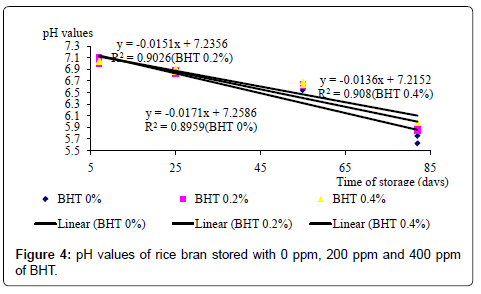
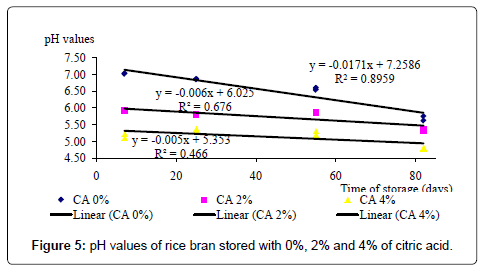
During 82 days of storage period, RB mixed with CA had significantly (p<0.05) lower pH values than the control (RB without CA) whereas BHT had no effect on pH (Table 2). Suggesting a formation of free fatty acids due to lipolytic rancidity, the pH of the RB stored with BHT and those stored without BHT or CA decreased drastically over the storage period (Figures 4 and 5). This suggests that BHT does not control the lipolytic rancidity. Meanwhile, throughout the storage period, the pH values of the RB stored with 2 and 4% of CA were significantly (p<0.05) lower than those of control samples. Apparently this may primarily be due to the acidification due to the presence of CA (Figure 5).
The changes of pH and PV suggest that BHT, being an anti-oxidant, has controlled the rancidity, but without inhibiting the formation of free fatty acids. Coppen [25] has also found that anti-oxidants could reduce the formation of peroxides but not the free fatty acids. Even though the pH value of CA treated RB also decreased over the storage period, the rate of pH reduction was lower than that of the RB stored without CA and also the RB stored with BHT. This observation indicates that apart from controlling the oxidative rancidity, CA has controlled the lipolytic rancidity that results in free fatty acid formation.
The rancidity controlling capacity of may be due to a number of reasons. Hydrolytic rancidity of RB is initiated when lipases and lipids in bran come into contact upon milling. RB lipases have pH optima of 7.5-8.0 and either increase or decrease of pH may reduce the rancidity [2]. It has been shown that complete cessation of lipase activity cannot be achieved even at pH 4 [7]. RB stored with CA always had lower pH values than the control. Therefore, it may be a possibility that reduced pH due to CA might have slowed the activity of lipase and thus the lipolytic rancidity.
Oxidative rancidity involves a reaction between free fatty acids and oxygen. The strong anti-oxidant properties of the CA may be the reason for the oxidative-rancidity controlling capacity of CA. The oxidative rancidity is accelerated by oxygen, free radicals and metallic ions such as iron, cobalt, copper as well as light and enzyme lipoxigenase [26]. CA is a strong chelating agent and thus can bind heavy metals [27]. Therefore, it can be suggested that CA binds with heavy meals in the RB, thereby preventing them acting as a catalyst in oxidative rancidity reactions. It is concluded that BHT was effective in reducing rancidity of RB only for about month whereas CA controls both lipolytic and oxidative rancidity for about three months.
References
- Farrell DJ (1994) Utilization of rice bran in diets for domestic fowl and ducklings. World's Poultry Science Journal 50: 115-131.
- Malekian F, Ramu M Rao, Prinyawiwatkul et al. (2000) Lipase and lipoxigenase activity, functionality, and nutrient losses in rice bran during storage. Lousiana State University Agriculture Centre.
- Warren BE, Ferrell DJ (1990) The nutritive value of full-fat and defatted Australian rice bran. I. Chemical composition. Animal Feed Sci Technol 27: 219-228.
- Sounders RM (1985) Rice bran: Composition and potential food sources. Food Rev Int 1: 465-495.
- Takahama U (1985) Inhibition of lipoxygenase-dependent lipid peroxidation by quercetin: Mechanism of antioxidative functions. Phytochem 24: 1443-1446.
- Frankel EN (1984) Lipid oxidation: Mechanisms, products and biological significance. J Am Oil Chemists Soc 61: 1908-1916.
- Prabhakar JV,Venkatesh KVL (1986) A simple chemical method for stabilization of rice bran. J Am Oil Chemists Soc 63: 644-646.
- Champagne ET, Robert J. Hron Sr, Abraham G (1992) Utilizing ethanol to produce stabilized brown rice products. J Am Oil Chemists Soc 69: 205-208.
- Atapattu NSBM (2005) Rice bran: potential and constraints as a poultry feed ingredient. Proceedings of the 1st international symposium of Sabaragamuwa University.
- Hilton JW (1989) Antioxidants: function, types and necessity of inclusion in pet foods. Can Vet J 30: 682-684.
- Sharafi SM, Rasooli I, Owlia P, Taghizadeh M, Astaneh SD (2010) Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn Mag 6: 147-153.
- Hilton JW (1989) Antioxidants: function, types and necessity of inclusion in pet foods. Can Vet J 30: 682-684.
- Kopecky J, Hrncar C, Weis J (2012) Effect of organic acids supplement on performance of broiler chickens. Scientific Papers Animal Science and Biotechnologies 45.
- Soccol CR, Vandenberghe LP, Rodrigues C, Pandey A (2006) New perspectives for citric acid production and application. Food Technol Biotechnol 44: 141-149.
- Milsom PE (1987) Organic acids by fermentation, especially citric acid. In Food Biotechnology-1. Springer Netherlands 273-307.
- Santiago PA, Francisco PA, Jose MG (2004) Studies on rancidity inhibition in frozen horse mackerel (Trachurus trachurus) by citric and ascorbic acids. Eur J Lipid Sci Technol 100: 232-240.
- Association of Official Analytical Chemists (1980) Official Methods of Analysis. 14th ed. Association of Official Analytical Chemists. Washington, DC, USA.
- SAS Institute (1986) SAS user’s guide statistics, SS Institute, USA.
- Dev CN, Carol C, Donna D, Yvon-louis T (2003) Determination of the pH of foods including foods in hermitically sealed containers. Food section, Ottawa laboratory (Carling) Canadian food inspection Agency.
- Mujahid A, Ikram ul Haq, Asif M, Gilani AH (2005) Effect of various processing techniques and different levels of antioxidant on stability of rice bran during storage. J Sci Food Agric 85: 847-852.
- Njobeh PB, Iji PA, Nsahlai IV (2006) Influence of composition and storage conditions on the concentrations of free fatty Acids and peroxides in broiler diets. Int J Poult Sci 5: 279-283.
- Connor TP, Brien NM (1991) Significance of lipoxygenase in fruits and vegetables. In Food Enzymology, Fox, P.F. Elsevier Science Publishing.
- Cabel MC, Waldroup PW (1989) Research note; Ethoxyquin and ethylenediaminetetra acetic acid for the prevention of rancidity in rice bran stored at elevated temperature and humidity for various lengths of time. Poult Sci 68: 438-442.
- Tao J (2001) Method of stabilization of rice bran by acid treatment and composition of the same. U.S. Patent No. 6,245,377. Washington, DC: U.S. Patent and Trademark Office.
- Coppen PP (1989) The use of antioxidants. In: rancidity in Foods. Eds. Allen JC and Hamilton, J Elsevier applied science. London and Newyork.
- Ramezanzadeh FM, Rao RM, Windhauser M, Prinyawiwatkul W, Tulley R, et al. (1999) Prevention of hydrolytic rancidity in rice bran during storage. J Agric Food Chem 47: 3050-3052.
- Boling-Frankenbach SD, Snow JL, Parsons CM, Baker DH (2001) The effect of citric acid on the calcium and phosphorus requirements of chicks fed corn-soybean meal diets. Poult Sci 80: 783-788.
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 19790
- [From(publication date):
December-2013 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 14361
- PDF downloads : 5429