Special Issue Article Open Access
Effects Of Menstrual Cycle On Sweating During Exercise Performed In Hot And Dry Environment
| Mariella G Lacerda1, Alessandra MC Garcia1, Cynthia Dela Cruz2, Juliana C Calcagno2, Fernando M Reis2* and Emerson Silami-Garcia1 | |
| 1Laboratory of Exercise Physiology, Department of Physical Education, Federal University of Minas Gerais, Brazil | |
| 2Department of Obstetrics and Gynecology, Federal University of Minas Gerais and National Institute of Hormones and Women´s Health, Belo Horizonte, Brazil | |
| *Corresponding Author : | Fernando M Reis, M.D., Ph.D. Division of Human Reproduction Department of Ob/Gyn Hospital das Clínicas UFMG, Av. Alfredo Balena, 110 9° andar 30130-100 Belo Horizonte, MG, Brazil Tel: 55 31 3409-9485 Fax: 55 31 3409-9299 E-mail: reis@medicina.ufmg.br |
| Received February 18, 2013; Accepted April 23, 2013; Published April 26, 2013 | |
| Citation: Lacerda MG, Garcia AMC, Cruz CD, Calcagno JC, Reis FM, et al.(2013) Effects of Menstrual Cycle on Sweating During Exercise Performed In Hot and Dry Environment. Biochem Physiol S3:001. doi:10.4172/2168-9652.S3-001 | |
| Copyright: © 2013 Lacerda MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The aim of this study was to compare the local and global sweat rates between the follicular and luteal phases of ovulatory cycles during a progressive exercise until exhaustion, held in a warm and dry environment. Eight women found to be healthy after undergoing a physical examination and with regular, ovulatory menstrual cycles participated in this study. The exercise was performed on a cycle ergometer according to the American College of Sports Medicine protocol. The test began with a power of 50 watts in increments of 25 watts every 2 minutes until exhaustion, while maintaining a speed of 50 rpm. The local sweating rate was measured using filter paper and the global sweating rate was estimated using body weight. Ovulation was detected both by serial transvaginal ultrasonography and serum progesterone measurement. Rectal temperature was higher in the luteal phase (37.54 ± 0.03ºC) when compared with the follicular phase (37.28 ± 0.05ºC, p<0.05) and increased significantly following exercise at both phases of menstrual cycle. The local and global sweat rates during exercise did not differ between the follicular phase (local 0.368 ± 0.111; global 3.03 ± 0.17 g.m-2.min-1) and the luteal phase (local 0.240 ± 0.063; global 3.20 ± 0.39 g.m-2.min-1) of menstrual cycle. Also in the recovery period after exercise, there were no differences in the sweat rate according to menstrual cycle phase (p>0.05). In conclusion, under the environmental conditions tested in this study, the local and global sweat rates elicited by progressive exercise to exhaustion were not influenced by the phases of the menstrual cycle.
| Keywords |
| Sweat rates; Menstrual cycle; Progressive exercise; Thermoregulation |
| Introduction |
| During the menstrual cycle, the internal temperature at rest is on average 0.3 to 0.5ºC higher in the luteal phase compared to the follicular phase. This rise in temperature observed in the luteal phase is due to an increase in the hypothalamic set point that is due to increased secretion of progesterone in this phase of the menstrual cycle [1,2]. |
| This increase of internal temperature in the luteal phase has been the subject of several studies on the menstrual cycle and thermoregulation. The aim of these studies was to determine whether this difference in temperature between the follicular and luteal phases could affect the thermoregulatory responses of women during exercise [3-5]. |
| The results of these studies are contradictory and it is clear that there is still no consensus in the scientific literature. While some studies have reported no difference in sweat rate between the follicular and luteal phases of the menstrual cycle [3,6,7] others indicate a greater [5] or smaller [7], sweat rate in the luteal phase. |
| This variety of results can be explained by the fact that the experimental procedures of the cited studies differ in relation to exercise intensity, environmental conditions and hydration protocols. Moreover, in some of the studies cited, the “luteal” phase groups included women who had not ovulated [5,8,9] whereas the rise in internal temperature observed at rest in the luteal phase occurs only in ovulatory cycles [1,2]. |
| Vermesh et al. [10] conducted a study aiming to compare the accuracy of some methods used to predict and detect ovulation. The results showed that, although the measurement of serum estrogen, progesterone and luteinizing hormone concentrations are reliable methods for ovulation detection, only serial transvaginal ultrasound was capable of detecting ovulation in all volunteers. |
| On the basis of these considerations, the aim of this study was to compare the local (forearm) and global sweat rates, between the follicular and luteal phases of ovulatory menstrual cycles. With the purpose of avoiding that the sample be composed by women who have anovulatory menstrual cycles, this study used transvaginal ultrasound to confirm ovulation. In addition, to provide a great stimulus to the sweat gland, the exercise protocol proposed by Vimieiro-Gomes et al. [11] was used in this work for measurement of sweat rate. To enhance the production of sweat, the exercise was conducted in a hot environment. |
| Methods |
| Sample |
| This study was approved by the Ethics Committee of the Federal University of Minas Gerais (protocol number 475/04). All procedures adopted in this study are consistent with the “Guidelines and Norms Regulating Research Involving Human Beings” of the National Health Council (Resolution Number. 196/1996). Participants received information about the objectives and procedures of the study and signed an informed consent by agreeing to participate voluntarily in the study. |
| Eight women found to be healthy after undergoing a physical examination participated in this study. The mean (± SD) age was 24.38 ± 2.33 years, body weight was 55.72 ± 6.82 kg, height was 159.75 ± 8.03 cm, body surface was 1.57 ± 0.13 m2 and body fat was 24.69 ± 5.50%. All women were eumenorrheic, had regular menstrual cycles (assessed over a minimum of 4 months) and did not use hormonal contraceptives for at least six months. The average length of menstrual cycles of the volunteers was 29 ± 2 days. |
| The confirmation of ovulation by transvaginal ultrasound was performed using a vaginal scanner (Aloka→ ECHO CHAMBER SS- 500) of 5 MHz. Follicle tracking was initiated from the 8th day of the menstrual cycle and the tests were performed every other day until ovulation was confirmed according to the criteria proposed by Vermesh et al. [10]. The measurement of serum progesterone in the luteal phase was made by the method of Chemiluminescence (Diagnostic Products Corporation, Los Angeles, CA, USA). All samples were run in the same assay, with a detection unit of 0.6 ng/ml and an intra-assay coefficient of variation of 7%. |
| Experimental situations |
| The volunteers were subjected to two experimental sessions: one in the follicular phase (between the 5th and 9th day of the cycle) and another in the luteal phase of the menstrual cycle. In the luteal phase, the experiments were performed between six and ten days after confirmation of ovulation by transvaginal ultrasound (between the 23rd and 26th day of the cycle). The order of experiments was randomized, and the sessions were carried out in the same cycle if the order was follicular-luteal phase or in subsequent menstrual cycles if the order was luteal-follicular phase. |
| Experimental protocol |
| Data collection occurred in the months between the summer and autumn in the Southern Hemisphere, in the morning (08:00 to 12:00), to minimize the effects of circadian rhythm on the sweating rate [1,2]. |
| On the day scheduled for each experiment, the volunteers drank 500 ml of water two hours before exercise to ensure that they would be euhydrated [12]. Before the exercise, the volunteers remained 15 min at rest sitting in a room located next to the environmental chamber. In this place, the room temperature was maintained between 21°C and 24°C and relative humidity between 50 and 60%, which is considered a thermoneutral environment [13]. After this initial rest, the volunteers were directed to empty the bladder and then weighed in order to initiate the exercise. |
| Exercise and post-exercise were conducted in an environmental chamber (Russells WMD-1150-5s, Holland, MI, USA) at 37.5ºC of dry temperature and 32% relative humidity. During the data collection, volunteers wore top, shorts, socks and sneakers throughout the procedures, and there was no fluid replacement. The exercise was performed on a cycle ergometer (Monark-824E), according to the protocol of the American College of Sports Medicine [14], as previously described [11]. Briefly, the test began with a power of 50 watts in increments of 25 Watts every 2 minutes until exhaustion, while maintaining a speed of 50 rpm. The following criteria were considered for discontinuation of exercise: a voluntary request to stop the exercise, stabilization of heart rate even with increased exercise intensity, and reach of a grade 20 on a scale of a perceived subjective exertion American College of Sports Medicine [14]. Immediately after exercise, the volunteers were weighed again and sat for 15 minutes at rest (period called post-exercise). |
| Variables |
| The body surface area (m2) was calculated based on the equation proposed by Dubois and Dubois [15]. Percent fat was estimated by the skinfold method using a skinfold caliper (Lange®), graduated in millimetres. The equation proposed by Brozek et al. [16] was used to calculate the percentage of fat. |
| The local sweating rate was estimated using absorbent filter papers of 16 cm2 (J Prolab→, weight 250) that was placed on the right forearm in the medial region, near the elbow joint. The filter paper was placed in contact with the skin at the beginning of exercise and was removed immediately after completion. During the post-exercise, another filter paper was used and also remained in contact with the skin during this period. |
| While the papers were in contact with the skin, they were covered by a plastic affixed onto to the skin with waterproof tape (Cremer→) to prevent sweat evaporation. After sweat collection, each filter paper was stored in a plastic bag, sealed and identified, so it could be weighed. |
| The papers were weighed before and after each experimental session and the local sweat production (mg) was calculated by the change in the weight of the paper. This value, in mg was divided by the area of the paper (cm2) that stayed in contact with the skin to obtain the local sweat rate in mg.cm-2.min-1. The paper was weighed on an analytical digital balance (Mettler→-AB 204) with an accuracy of 1 mg. |
| The total amount of sweat produced was estimated by change in weight of each volunteer before and after exercise and before and after the post-exercise [17], using a digital balance accurate to 0.02 kg (Filizola®-MF 100). The global sweating rate was calculated by subtracting the final body weight from the initial body weight (g). This value was then divided by the time span and by the body surface area (m2) to obtain the overall rate of sweating in g.m-2.min-1. |
| Rectal temperature was measured using a disposable rectal probe (Yellow Springs Instrument→-4491E) inserted 10 cm beyond the anal sphincter. The temperature of the forearm was measured by a temperature sensor (Yellow Springs Instrument→-409 B). The heart rate (HR) was registered using a digital monitor (Polar® Fitwatch). Rectal and forearm temperatures and HR were recorded every minute during the initial rest, exercise and post-exercise. |
| The rate of perceived exertion was assessed during the exercise using the Borg´s scale [18]. The specific gravity of urine was measured before and after each experiment using a refractometer (Uridens®- IOC) [19]. The VO2max (mLO2.kg-1.min-1) was estimated according to the protocol of the American College of Sports Medicine [14], the same protocol used for measurement of sweat rate. Maximum HR (bpm) was considered as the highest heart rate recorded during exercise performance. Maximum power (Watts) was determined as the higher power achieved in the progressive maximum test. The total exercise time (min) was recorded using a stopwatch. |
| Statistical analysis |
| The paired Student t test was used to compare the local sweat rate, the global sweat rate, the specific gravity of urine, the VO2max, HRmax, maximum power and total exercise time. |
| The rate of perceived exertion was not normally distributed and, therefore, was presented as median and tested by Wilcoxon test. |
| Rectal temperature, forearm temperature and heart rate were compared by two-way ANOVA with repeated measures. The Bonferroni test was employed as post hoc. The variables analysed during the exercise were compared until five minutes, which was the maximum time of exercise done by all volunteers. |
| The data are expressed as mean and standard deviation or mean and standard error of the mean, when appropriate. In all analysis the level of significance was p<0.05. The sample size was calculated to allow the detection of differences of at test 1.2 standard deviation in the sweating rates between the two phases of menstrual cycle, with 95% confidence and 80% statistical power. |
| Statistical analysis was performed using statistical programs Sisvar and Statistical Package for Social Sciences (SPSS version 10.0). |
| Results |
| The serum concentration of progesterone in the luteal phase was 11.57 ± 1.37 ng.mL-1. |
| During exercise, no difference in local sweat rate between the follicular phase (0.368 ± 0.111 mg.cm-2.min-1) and luteal phase (0.240 ± 0.063 mg.cm-2.min-1) (p>0.05) was observed. Throughout the period after exercise, there were also no differences in this variable related to all phases of the menstrual cycle (0.496 ± 0.099 vs. 0.595 ± 0.181mg.cm-2. min-1 in follicular and luteal phases, respectively, p>0.05). |
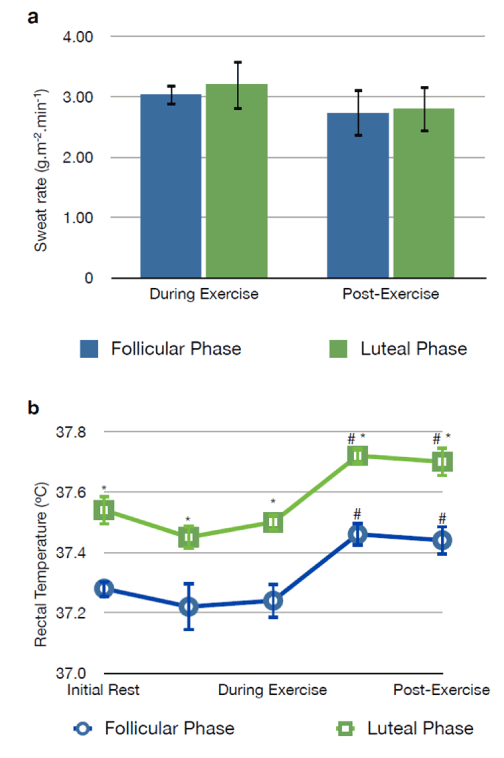
| The overall sweat rate during exercise was not different between the follicular (3.03 ± 0.17 g.m-2.min-1) and luteal phases (3.20 ± 0.39 g.m-2.min-1) of menstrual cycle (p>0.05). During the post-exercise, there were no differences between the phases of menstrual cycle (2.73 ± 0.41 vs. 2.80 ± 0.38 g.m-2.min-1, follicular and luteal phases, respectively, p>0.05) (Figure 1). |
| During the initial rest, rectal temperature was higher in the luteal phase (37.54 ± 0.03ºC) when compared with the follicular phase (37.28 ± 0.05ºC) (p<0.05). Th e difference in rectal temperature between the two phases of menstrual cycle remained constant during and after exercise (p<0.05). At both menstrual cycle phases there was a significant rise in rectal temperature immediately after the active exercise phase, which persisted during the 30 min post-exercise rest period (Figure 1). In the follicular phase, the rectal temperature increased up to 37.46 ± 0.03ºC following exercise, whereas in the luteal phase the post-exercise core temperature reached 37.72 ± 0.04ºC (p<0.05 vs. initial rest and p<0.05 vs. follicular phase, Figure 1). |
| The temperature of the forearm at the initial rest was not different between the menstrual cycle phases. During exercise, forearm temperature increased and remained higher in the period of postexercise. However, no significant difference between the follicular and luteal phases was found during the exercise or the period of postexercise. In the initial rest, there was no difference in HR between menstrual cycle phases. HR increased during exercise and recovered partly in the immediate post-exercise period. There were no HR differences in relation to the menstrual cycle during the exercise and in the post-exercise (Table 1). |
| The rate of perceived exertion increased during the exercise. However, the average grade was not different when comparing the phases of the menstrual cycle (12 vs. 12 in the follicular and luteal phases, respectively, p>0.05). The specific gravity of urine increased across the experiment from 1.006 ± 0.001 to 1.012 ± 0.002 (p<0.05) in the follicular phase and from 1.007 ± 0.002 to 1.013 ± 0.002 (p<0.05) in the luteal phase, but no differences were observed between the two menstrual cycle phases (p>0.05). The maximum oxygen consumption, maximal heart rate, maximum power attained and total exercise time were not different when comparing the follicular and luteal phases of the menstrual cycle (p>0.05) (Table 2). |
| Discussion |
| In this study, the higher internal temperature observed at rest in the luteal phase did not alter the rates of local and global sweating when exercise was performed in different phases of the menstrual cycle. We had predicted that the combination of exercise, hot and high internal temperature before exercise would produce different thermoregulatory responses in the luteal phase. In order to compensate for the higher internal temperature at rest at this stage of the menstrual cycle, the hypothesis was that there would be a greater sweat production and an increase in skin temperature in the luteal phase to make the process of dissipation of body heat more efficient. The highest rectal temperature observed at rest in the luteal phase remained in the exercise and post-exercise. However, the temperature variation during exercise was similar in both phases of the menstrual cycle. Previous studies have shown that during the luteal phase there is a reduction in the plasma volume and that this decrease may compromise the ability of women to exercise in hot conditions [20,21]. Probably, the water intake over the completion of long-term exercise in hot environments minimizes this difference and allows the body to increase sweating to compensate the high internal temperature in the luteal phase [5]. However, in situations where there is no fluid replacement, it seems that the temperature increase in the luteal phase causes an increase in the threshold of internal temperature to start the sweating, without changing the total amount of sweat produced. |
| In this study, the experiments performed in the luteal phase occurred only after confirmation of ovulation by transvaginal ultrasound. Thus, we ensured that all women had rest, internal temperatures higher in the luteal phase compared to follicular phase of the menstrual cycle. Furthermore, the progesterone concentrations of all the volunteers in the luteal phase were higher than 3 ng.mL-1, which is compatible with ovulation [10,22,23]. |
| We did not observe any difference related to the phases of the menstrual cycle in the temperature of the forearm. It is likely that the internal temperature threshold to start vasodilation is similar to the temperature threshold to start sweating, and is even greater in the luteal phase compared to the follicular phase [24,25]. Additionally, Frascarolo et al. [26] reported that blood flow and skin thermal conductance were lower in the luteal phase. Probably, this decrease in thermal conductance at this stage may be a physiological mechanism that contributed to maintain a higher internal temperature in the luteal phase of the menstrual cycle. |
| During rest, exercise and post-exercise, there were no menstrual cycle related differences in HR, perceived exertion, VO2max, maximum power and total exercise time. Previous studies also found no differences in HR [3,5,27], PSE [5], VO2max [3,4,28] maximum power and total exercise time [27] when comparing the different phases of the menstrual cycle. These results demonstrate that the greater internal temperature observed in the luteal phase does not represent a burden on the cardiorespiratory system, at least in the experimental conditions evaluated. |
| In the current study, we chose to challenge the mechanisms of thermoregulation thorough a short, maximal load exercise followed by a post-exercise rest, rather than a longer steady state exercise. Importantly, the physiological parameters were recorded not only during the exercise phase but also for 30 min during post-exercise recovery, in order to evaluate the thermal and metabolic impact of the exercise beyond the active phase of the test. Actually, the heart rate and core temperature remained elevated in the post-exercise rest and part of the metabolic heat produced was dissipated by sweating during the recovery period. |
| In conclusion, the present study shows that there is no difference in local and global sweat rates between the follicular and luteal phases of ovulatory cycles during a progressive exercise until exhaustion, held in a warm and dry environment. Core temperature was higher in the luteal phase when compared with the follicular phase not only at baseline, as expected, but also throughout exercise and post exercise. These findings suggest that in the luteal phase of menstrual cycle there is an adjustment to tolerate the progesterone-induced temperature rise not only at rest but also during and after exercise. These observations, however, are restricted to the environmental conditions (warm and dry) and fluid replacement protocol (no fluid intake during exercise) tested here. In concert with previous observations made in a humid environment with free water intake, where the basal temperature rise of luteal phase was mitigated by the increased sweating and reduced urinary rates [5], we hypothesize that fluid replacement during exercise may help to adjust body temperature and compensate for the menstrual cycle variation. This hypothesis, as well as its implications for the endurance and performance, remains to be investigated. |
| Acknowledgements |
| We would like to thank the volunteers for their dedication, availability and great assistance in conducting this study. |
| Research supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Brazilian National Institute of Hormones and Women’s Health. The authors declare that there are no conflicts of interest. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14865
- [From(publication date):
September-2015 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 10208
- PDF downloads : 4657
