Research Article Open Access
Evaluation of Metabolic State in Striatal Rat Model of Parkinson's Disease: before and after Deep Brain Stimulation
| Joana Guimarães1,2,5*, Eduardo Moura3,5, Elisabete Silva4,5, Carolina Garrett1,2,5, Maria Augusta Vieira-Coelho3,5 | |
| 1Neurology Department, Hospital de São João, Portugal | |
| 2Neurology and Neurosurgery Unit of Clinical Neurosciences and Mental Health Department, Faculty of Medicine, University of Porto, Portugal | |
| 3Pharmacology and Therapeutics Department, Faculty of Medicine, University of Porto, Portugal | |
| 4Experimental Biology Department, Faculty of Medicine, University of Porto, Portugal | |
| 5Institute for Molecular and Cell Biology, University of Porto, Portugal | |
| Corresponding Author : | Joana Guimarães Neurology Department Hospital de São João, Porto, Portugal Tel: +351 22 5505640 E-mail: jguimraes9@hotmail.com |
| Received October 21, 2013; Accepted January 22, 2014; Published January 24, 2014 | |
| Citation: Guimarães J, Moura E, Silva E, Garrett C, Vieira-Coelho MA (2014) Evaluation of Metabolic State in Striatal Rat Model of Parkinson’s Disease: before and after Deep Brain Stimulation. J Obes Weight Loss Ther 4:205. doi:10.4172/2165-7904.1000205 | |
| Copyright: ©2014 Guimarães J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Weight loss occurs during the natural history of Parkinson’s disease (PD). This non-motor manifestation of the disease is reversed by deep brain stimulation of the sub thalamic nucleus (DBS-STN) therapeutics which is often associated with weight gain. Although it has been proposed that PD is associated with alterations in central energy metabolism the mechanisms responsible for this weight variation remain unknown. This study evaluates the weight profile and nutritional state of the 6-hydroxydopamine (6-OHDA) rat model of PD subjected to DBS-STN. Rats were rendered parkinsonian by bilateral injections of 6-OHDA into the striatum and electrodes were implanted bilaterally at the level of the STN. Rats were placed in metabolic cages for evaluation of weight, food and liquid intake and urine and fecal volume before and 2 and 4 weeks after the beginning of stimulation. Before stimulation began (2 weeks after 6-OHDA lesion), weight and metabolic parameters were similar between parkinsonian rats with and without electrodes and matched control rats. Two weeks after stimulation began (4 weeks after 6-OHDA lesion) and at the end of the study, 4 weeks after stimulation began (6 weeks after 6-OHDA lesion), body weight and the metabolic parameters evaluated remained unaltered between animal groups. Furthermore, at the end of the study, no statistically significant differences were found in efficiency of eating (change in weight/amount of food eaten) or weight gain between groups. In conclusion, in the rat model of PD with striatal dopaminergic neurodegeneration neither induction of PD or DBS-STN influenced weight variation or metabolic state. Additional mechanisms may be required to induce the altered metabolic state observed in PD patients before and after STN-DBS.
| Keywords |
| Striatal rat model of Parkinson’s disease; Deep brain stimulation; Metabolic state; Weight variation |
| Introduction |
| Parkinson’s disease (PD) is a progressive neurodegenerative disorder that affects several regions of the central nervous system [1]. Although motor signs are the dominant feature of the disease, the involvement of several neural systems leads to non-motor symptoms that are initially eclipsed by the obvious movement impairment [2]. Some of these non-motor symptoms occur early in PD and may even precede clinical diagnosis [3]. This group of non-motor symptoms of PD includes several clinical manifestations and weight variation has been described as a non-motor manifestation of PD. The weight loss that occurs in the natural history of the disease has been considered a key clinical manifestation that renders disability and significantly influences of the quality of life of patients [4,5]. |
| Deep brain stimulation of subthalamic nucleus (DBS-STN) offers an additional therapeutic possibility in most patients with idiopathic PD [6]. Despite extensive research, there is still no unified concept explaining the mechanism of DBS-STN, namely the non-motor effects. Body weight variation has been reported in many studies as a non-motor secondary effect of DBS-STN [7-10]. Studies have shown that weight loss in patients with PD before surgery is a continuous and progressive process that starts years before a formal diagnosis is made, and that surgery modifies this state [11-14]. Some authors have proposed that PD is associated with profound alterations in energy metabolism that are normalized after DBS-STN [13,15]. |
| Several studies discuss the non-motor effects of DBS-STN and the mechanism underling DBS-STN appears to be more complex than a simple inactivation of the structure [16-19]. In recent years, several studies have been performed to try to address this issue, namely experimental studies [20,21]. The use of toxin-induced animal models has been crucial to the elucidation of the pathophysiology underlying PD. The toxin-induced nigrostriatal degeneration produces measurable motor impairments and triggers a succession of events within the basal ganglia that parallels many of those characteristic of PD [20,22,23]. The 6-hydroxydopamine (6-OHDA)-lesioned rat has contributed enormously to translate animal experimentation into clinical practice, including the effects of DBS-STN [24-27]. |
| 6-OHDA lesions of nigrostriatal pathway with bilateral lesion of the medial forebrain bundle (MFB) have been associated to a behavioral syndrome characterized by aphagia and adipsia [28,29]. In the recent years the nigrostriatal dopamine denervation in the rat that results from intra-striatal 6-OHDA lesion, has been used as a rat model to evaluate the effects of STN-DBS [23,29-31]. Interestingly, the feeding behavior and weight variation have not been described in this striatal model of more selective destruction of the nigrostriatal dopaminergic pathway. As weight variation in PD is an important non-motor manifestation of the disease, the effects of striatal 6-OHDA lesion on the nutritional state of the parkinsonian rats, before and after bilateral STN stimulation, deserves further investigation. |
| To study weight variation and feeding behavior in PD, we used the rat model of PD with bilateral injection of 6-OHDA into striatum which leads to retrograde degeneration of dopaminergic neurons in substantia nigra and bilateral DBS-STN stimulation [17,32]. |
| Materials and Methods |
| Animals |
| Male rats (Sprague-Dawley) (N=48) weighing approximately 300 g at the time of surgery were obtained from Charles River (Barcelona, Spain). The experimental protocols used in this study were in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and the experiments were performed according to the Portuguese law on animal welfare. All animals were kept under controlled environmental conditions, housed in standard cages at constant temperature (20-25°C) and humidity (30-50%), with a 12 h light–dark cycle (lights on 8:00 h-20:00 h), and with free access to water and food. |
| 6-Hydroxidopamine lesion and electrode implantation |
| An experimental group of Parkinsonian animals with 6-OHDA striatal lesion with 4 intrastriatal injections of 6-OHDA (2 per hemisphere) as previously described with DBS-STN stimulation was constituted in order to analyze the effect of striatal lesion and neurostimulation on feeding behavior and weight variation [17,27,32]. |
| Rats were randomly assigned to one of the following groups: |
| -- Sham-operated rats (Sham) (n=8); |
| -- Sham-operated rats with electrodes but without stimulation (Sham+EL) (n=8); |
| -- Sham-operated rats with electrodes and with stimulation (Sham+EL+DBS) (n=8); |
| -- Parkinsonian animals (only intrastriatal injection of 6-OHDA) (6-OHDA) (n=8); |
| -- Parkinsonian animals (intrastriatal injection of 6-OHDA) only with electrodes (6-OHDA+EL) (n=8); |
| -- Parkinsonian animals (intrastriatal injection of 6-OHDA) with electrodes and stimulation (6-OHDA+EL+DBS) (n=8). |
| Rats were anesthetized with a mixture of ketamine hydrochloride (Ketalar; 60 mg/kg) and medetomidine hydrochloride (Domitor; 0.25 mg/kg), and the skull were fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Bregma was taken as a landmark for the stereotaxic coordinates. A midline longitudinal incision was performed, the skin retracted and the skull exposed. After making burr holes in the skull, rats of the 6-OHDA groups received stereotaxic injections of 2 μl 6-OHDA (5 μg/μl dissolved in 0.9% saline and 0.2% ascorbic acid) at four sites (two per hemisphere) in the striatum [coordinates from Bregma: anterior-posterior (AP) +0.7 and -0.4; mediolateral (ML) 2.8 and 3.4; dorsoventral (DV) -5.0 and -5.0; according to the atlas of the rat brain, edited by Paxinos) (Paxinos and Watson 1998)]. Injection speed was 0.5 μl/min, and the cannula was left in place for an additional 2 min. One hour before surgery, all rats received desimipramine (20 mg/kg i.p.) to prevent any effect of 6-OHDA on noradrenergic neurons. Sham-operated rats (Sham) received vehicle injections (0.9% saline and 0.2% ascorbic acid). Rats underwent electrode implantation in the same session. Two burr holes were made in the skull immediately above the STN (coordinates from Bregma: AP -3.8, ML 2.5, and DV -8.0) to allow the insertion of electrodes [27]. Two concentric bipolar stimulating electrodes were employed in this experiment inner electrode projection 1 mm, inner insulated electrode diameter 0.15 mm, outer electrode gauge 26 (Plastics One, Roanoke, VA) [33]. Three additional holes were drilled into the skull, and were used for surgical bone screws (Small Parts, Inc., Miramar, FL). Acrylic dental adhesive (Major Dental, Moncalieri, Italy) was applied as slurry around the bone screws to cover the skull and used to firmly secure the electrodes. The skin was sutured and an antibiotic ointment was applied on the wound. Rats were left for 2 weeks in order to recover from surgery. |
| Deep brain stimulation |
| Electrodes were connected to a stimulator (Hugo Sachs Elektronic Stimulator II Type 215/II, Hugo Sachs Elektronik – Harvard Apparatus GmbH March-Hugstetten, Germany) via a stimulus isolator. Stimulations were bipolar (inner electrode negative, outer electrode positive, 60 μs pulse width), and parameters were verified on-line by using a digital oscilloscope. During a period of 1 month, each rat was stimulated in a freely moving condition 1h per day. A pulse width of 60 μs, stimulation frequency of 130 Hz and amplitude of 30 μA were applied. The selection of these parameters was based on previous studies to evoke electrophysiological responses in animal models [27,32]. Parallel groups of age-matched non-surgical controls were maintained under the same conditions. These animals (Sham+EL and 6-OHDA+EL) were connected to the generators but were left unstimulated as control groups. |
| Metabolic parameters |
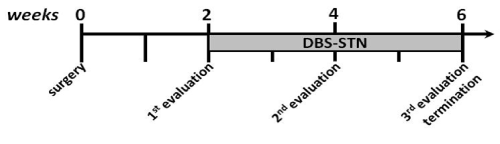
| For control of nutritional parameters rats were placed in metabolic cages before surgery and 2, 4 and 6 weeks after surgery (Figure 1). The following parameters were monitored 24 h: liquid intake, solid intake, urine volume and fecal weight. Animals were fed throughout the study ad libitum with standard chow (Letica, Barcelona, Spain; Na+, K+ and protein contents, respectively, 0.1%, 0.75% and 17%). |
| Histological processing |
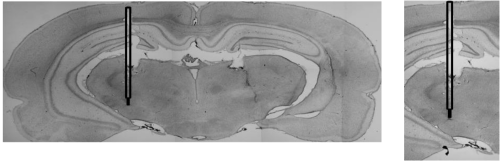
| At the end of the stimulation period the group of animals with electrode implantation and subjected to deep brain stimulation were deeply anaesthetized with an overdose of cloral hydrate (7.2 mg/kg) and were perfused transcardially with tyrode (0.1 M) and a fixative solution containing 4% paraformaldehyde, 15% picric acid, 0.05% glutaraldehyde in 0.1 M phosphate buffer (pH 7.6). Brains were removed and post-fixed for 2 h followed by overnight immersion in 15% sucrose at 4ºC. Brain tissue was then quickly frozen with CO2, stored (-80°C) and frontal sections (30 μm) were cut serially using a cryostat. A series of sections were stained with standard hematoxylin-eosin according to sections of rat brain atlas [34]. |
| Biochemical assessment of monoamine depletion |
| To evaluate the extent and selectivity of the monoamine depletion procedure, right and left portions of the anterior striatum were dissected from the sham and 6-OHDA groups of animals and placed in 500 μL of perchloric acid 0.2 M and stored at -4°C until their use in monoamine assays. Tissue dosages of L-DOPA and monoamines were performed by high performance liquid chromatography (HPLC) coupled with electrochemical detection, as previously described [35]. In brief, aliquots of 250 μL of the perchloric acid extract of tissues or 100 μL of the enzyme assay samples were placed in 5 ml conical base glass vials containing 50 mg of alumina, and the samples pH were adjusted to 8.6 by addition of Tris buffer. 3, 4-Dihydroxybenzylamine hydrobromide was used as internal standard. The adsorbed catecholamines were then eluted from the alumina with 200 μl of 0.2 M perchloric acid on Costar Spin-X microfilter tubes; 50 μl of the eluate was injected into an HPLCED system (Gilson Model 141, Gilson Medical Electronics, Villiers, Le Bel, France). The lower limit of detection of catecholamines and L-DOPA ranged from 350 to 1000 fmol. The assays for homovanillic acid and 3-methoxytyramine were performed by means of highpressure liquid chromatography, as previously described [36]. In brief, aliquots of 50 μL of the filtered perchloric acid extract of tissues were injected into an HPLC-ED system (Gilson Model 141, Gilson Medical Electronics, Villiers, Le Bel, France). The lower limit of detection of 3-methoxytyramine and homovanillic acid ranged from 350 to 1000 fmol. |
| Statistical analysis |
| Mean and standard error of the mean were calculated for the results of each group of animals. Comparisons between groups were performed using a two-way ANOVA with Newman-Keuls post-hoc multiple comparisons tests. A p value < 0.05 was considered to be significant. |
| Results |
| Surgical procedure and staining |
| The surgical procedure was well tolerated without negative incident in all animal groups. Histological evaluation of brain sections stained with hematoxylin-eosin confirmed that the electrode tips were implanted bilaterally in the STN and were placed symmetrically (interelectrode variation of <0.1 mm). Repeated stimulation with the present settings did not cause any tissue damage observable with routine HE staining (Figures 2A and 2B). Only data obtained from rats with correctly implanted probes (Figure 2) were included in the results (2 animals from the Sham+EL+DBS group and 2 animals from the 6-OHDA+EL+DBS group were excluded due to electrode misplacement). |
| Monoamine depletion |
| Selected animals for final analysis went through an additional validation step regarding the extent of monoamines depletion and to confirm 6-OHDA-lesioned rat model of PD. Tissue levels of L-DOPA, dopamine and the dopamine metabolites (DOPAC, 3-MT and HVA) in the striatum of 6-OHDA lesioned animals and Sham animals are presented in Table 1. Tissue levels of dopamine were reduced by over 70% in 6-OHDA lesioned animals compared to Sham. However the ratio of dopamine to the metabolites did not differ significantly between 6-OHDA lesioned animals and Sham operated rats. These data are in agreement with the dopamine depleting effect produced by the striatal 6-OHDA lesion used [37,38]. |
| Metabolic parameters |
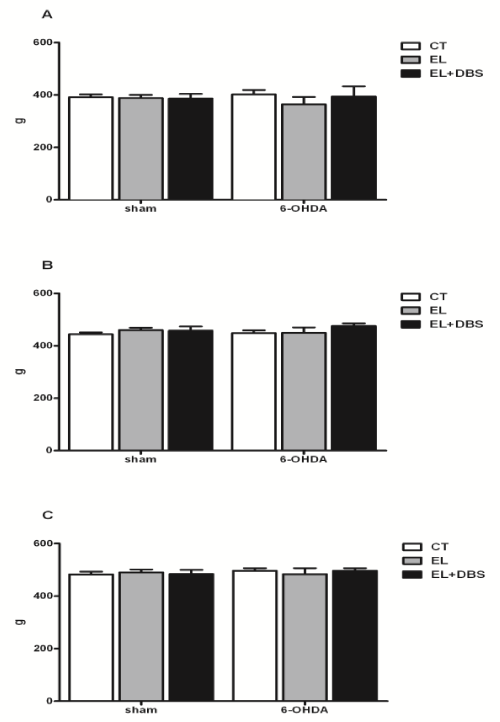
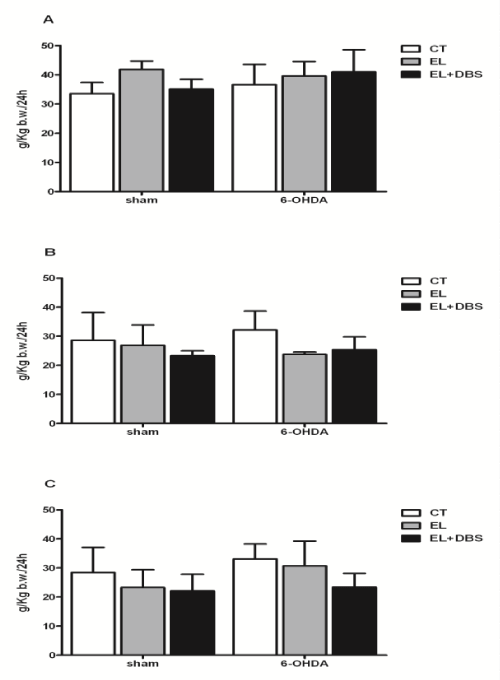
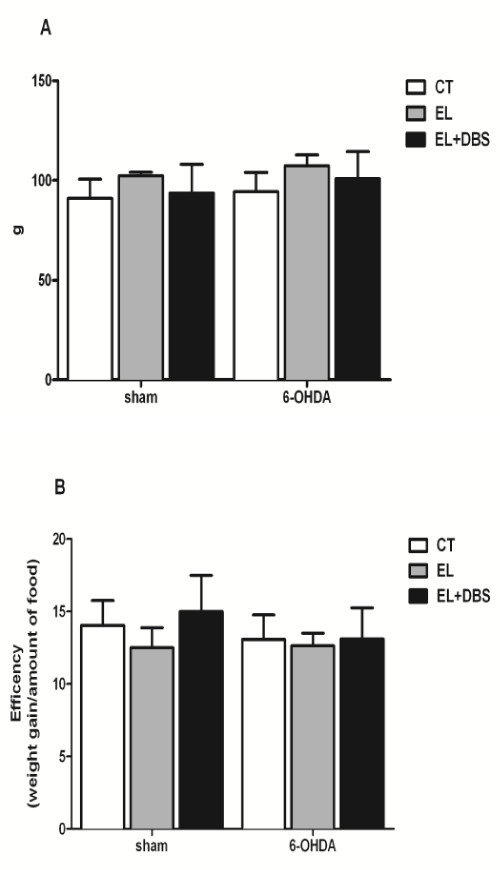
| Two weeks after surgery, and before stimulation, body weight was similar between the 6-OHDA treated rats and the Sham rats (Figure 3A). Furthermore, electrode implantation did not cause significant changes in body weight in both groups (Figure 3A). Two weeks after the beginning of stimulation and 4 weeks after 6-OHDA lesion, animal weight remained similar between groups (Figure 3B). At the end of the study, 4 weeks after the beginning of stimulation and 6 weeks after 6-OHDA lesion, body weight remained unaltered between all animal groups (Figure 3C). In regard to the amount of food eaten, 2 weeks after surgery there were no significant differences in solid intake between Sham operated rats- and 6-OHDA groups or between groups with and without electrode implantation (Figure 4A). No additional differences between these groups were observed 4 and 6 weeks after 6-OHDA lesion (Figures 4B and 4C). DBS-STN did not alter solid intake in Sham operated rats or 6-OHDA lesion rats 2 or 4 weeks after the initiation of stimulation (Figures 4A-4C). Analysis of body weight gain throughout the study and the efficiency of food consumption (change in weight/amount of food eaten) are shown in (Figures 5A and 5B) respectively. As shown, there were no differences in body weight gain throughout the study between the different groups. Lesion of dopaminergic neurons with 6-OHDA and DBS-STN failed to alter the efficiency of the amount of food eaten, described as the change in weight per amount of food eaten. |
| In regard to the amount of fecal matter produced by these animals 2 weeks after surgery and before the initiation of DBS-STN, there were no differences between the different animal groups (Table 2). Two and 4 weeks after the beginning of stimulation, there were no additional effects of DBS-STN or of the lesion protocol on fecal weight (Table 2). The effects of DBS-STN on the amount of water consumed and urine volume were also monitored. Two weeks after surgery, animals exhibited similar liquid intake (Table 2) and similar urine volume (Table 2). Four weeks after surgery and after 2 weeks of DBS-STN animals presented with unaltered liquid intake and urine volume, a situation that was maintained after 4 weeks of DBS-STN and 6 weeks after 6-OHDA lessoning (Table 2). |
| Discussion |
| To investigate weight variation and nutritional state in PD we used the 6-OHDA rat model with bilateral lesion of the striatum that has been validated as an experimental model of PD [17,26,27]. In this study we also investigated the influence of DBS-STN on the nutritional state of parkinsonian rats. The data presented here shows that in the experimental group of parkinsonian animals with 6-OHDA striatal degeneration there were no significant changes in weight and in general metabolic parameters and that STN-DBS delivered 1h a day for a month also failed to alter these parameters. |
| The 6-OHDA rat is probably the most popular model of PD. Different 6-OHDA models of PD have been developed in which the toxin is injected into different parts of the nigrostriatal pathway to cause dopaminergic cell loss in the substantia nigra pars compacta (SNc) [29]. 6-OHDA can be injected directly into the SNc, the MFB or the striatum. The first two modes of injection are used to develop severe lesions of dopaminergic neurons corresponding to an advanced stage of the disease [23]. In the striatal model, injection of 6-OHDA produces a dose-dependent decrease in striatal dopamine levels [23,29,39]. The time course of degeneration produced by the striatal model allows evaluation of changes that occur in early (2 weeks) and latter stages of PD (4 and 6 weeks). Changes in feeding behavior (aphagia and adipsia) develop in rats with bilateral 6-OHDA lesion of the MFB [40,41]. This modified behavior is typically found in electrolytic lesions of the lateral hypothalamus. In PD patient’s weight loss is not secondary to a decrease in food intake; patients do not present changes in feeding behaviour besides the change in weight [5]. |
| In our study, in contrast to what is observed in humans, the striatal rat model of PD did not present changes in weight or differences in net weight gain at the end of the study. Furthermore in the different stages of the disease there were no changes in feeding behavior, both in respect to solid and liquid intake, in PD rats compared to control animals. In agreement with these observations, there were no changes in fecal weight and in urine volume of these animals compared to sham-operated rats. |
| To investigate the additional effect of DBS in the 6-OHDA striatal rat model of PD a model of electrode implantation and of bilateral high frequency stimulation at the level of the STN was used [17,32,42]. Insertion of the electrodes did not cause any significant changes in the metabolic parameters tested, therefore ruling out any effect on structures others than the ones being targeted by stimulation. DBS-STN failed to alter weight or metabolic parameters both in control animals and in the rat model of PD with dopaminergic neurodegeneration. Taken together these first observations of this model of striatal dopaminergic degeneration shows that there was no variation in the nutritional state in opposition to what has been observed in patient with PD before and after DBS-STN. |
| Eexperimental studies of behaviour motivation suggest that DBSSTN increases motivation for natural rewards, like the motivation for food, but this did not appear to elicit binge eating, ruling out the effect of weight gain in the stimulated subjects [43]. These results are in agreement with the data presented here, where parkinsonian rats with DBS-STN did not present significant changes in weight gain or efficiency of eating. DBS-STN was performed 1h per day over a period of 1 month. Although this amount of STN modulation may be sufficient to obtain beneficial effects on dopaminergic cell survival, in the clinical setting, DBS-STN is usually performed chronically [27]. This could be regarded as a limitation in respect to changes in eating behaviour. Nevertheless it should be mentioned that in the study performed shortterm DBS of the STN was sufficient to increase the motivation for food over 24 h periods, suggesting that in the long term this change in behavior could have resulted in weight variation [44]. Nevertheless, a study in which stimulation is performed continuously through long periods is required to further validate the idea presented in this study. |
| In this study the effect of medication namely of L-DOPA on weight loss and weight gain in the 6-OHDA striatal rat model, before and after surgery was not addressed. This owes to the fact that weight loss in PD is not caused by antiparkinson therapy. Adams and coworkers tried to establish a correlation between levodopa/benserazide medication and weight loss in PD, but concluded that levodopa/benserazide medication per se does not contribute to body fat loss in patients with PD [45]. Several clinical studies also failed to show a consistent correlation between parkinsonian motor symptoms and energy metabolism suggesting that an underlying metabolic deregulation may be present in the disease [45-47]. Recently, our group has discussed other possible mechanisms that may influence weight variation in PD [48]. We suggest that isolated dopaminergic neurodegeneration, such as in the striatal model of PD, may not be sufficient to induce weight variation in PD; we proposed that a complex interaction between dopaminergic nigrostriatal projections and noradrenergic system may modulate PD nutritional sate. |
| In this animal model, focused on dopamine depletion with selective destruction of nigrostriatal dopaminergic pathway, the non-motor symptoms of PD, as weight variation, are not evident. One of the limitations of these animal models of PD is that the noradrenergic system is protected: rat models of the disease, generated by administration of the catecholaminergic neurotoxin 6-OHDA, incorporate the concomitant protection of noradrenergic neurons with the noradrenaline transporter blocker, desipramine [29,49]. If noradrenergic mechanisms participate in the metabolic state of PD, this role is not evaluated in this experimental model. The reasons that have led to ignore the role of noradrenergic neurons in the disease could be that the striatum has been the main target of the antiparkinsonian treatments. Nonetheless, noradrenergic mechanisms may also participate in the therapeutic outcome of L-DOPA and noradrenaline may act in brain regions that have a profound impact on the control of motor behaviors, including the STN and in the neurodegeneration process of PD [50-53]. |
| Noradrenaline depletion in the rodent by N-(2-chloroethyl) - N-ethyl-2-bromobenzylamine (DSP-4) is an approach commonly used to model human neuropsychiatric disorders in rodents [54]. Although there are yet no studies with DPS-4 and DBS, one study using only DSP- 4 shows that although administration of DSP-4 to rats does not result in any obvious behavioral changes, animals that received DSP-4 initially gained weight at a slower rate than control animals [19,43]. However, a comprehensive analysis of the effect of DSP-4 on the locus coeruleus noradrenergic system in the rat has shown that DSP-4 does not reduce locus coeruleus noradrenergic number or function, suggesting that DSP-4 is not an appropriate rat model to study the functional effect of noradrenergic neuronal loss in PD [54]. |
| Conclusion |
| In the rat model of PD with 6-OHDA induced lesion of the striatum, degeneration of dopaminergic neurons and protection of noradrenergic neurons failed to produce an effect on body weight gain and on food intake throughout the course of degeneration. Moreover DBS-STN delivered 1h a day for a month also failed to alter these parameters, both in PD and sham-operated animals. Additional degeneration of noradrenergic neurons, namely of the locus coeruleus, may be required to observe the side effects of PD unrelated to motor symptoms, as has been recently discussed by our group [19]. |
References
- Braak H, Del Tredici K (2008) Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 70: 1916-1925.
- Chaudhuri KR, Martinez-Martin P (2008) Quantitation of non-motor symptoms in Parkinson's disease. Eur J Neurol 15 Suppl 2: 2-7.
- Gaig C, Tolosa E (2009) When does Parkinson's disease begin? Mov Disord 24 Suppl 2: S656-664.
- Bachmann CG, Trenkwalder C (2006) Body weight in patients with Parkinson's disease. Mov Disord 21: 1824-1830.
- Barichella M, Cereda E, Pezzoli G (2009) Major nutritional issues in the management of Parkinson's disease. Mov Disord 24: 1881-1892.
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol 8: 67-81.
- Gironell A, Pascual-Sedano B, Otermin P, Kulisevsky J (2002) [Weight gain after functional surgery for Parkinsons disease]. Neurologia 17: 310-316.
- Barichella M, Marczewska AM, Mariani C, Landi A, Vairo A, et al. (2003) Body weight gain rate in patients with Parkinson's disease and deep brain stimulation. Mov Disord 18: 1337-1340.
- Macia F, Perlemoine C, Coman I, Guehl D, Burbaud P, et al. (2004) Parkinson's disease patients with bilateral subthalamic deep brain stimulation gain weight. Mov Disord 19: 206-212.
- Tuite PJ, Maxwell RE, Ikramuddin S, Kotz CM, Billington CJ, et al. (2005) Weight and body mass index in Parkinson's disease patients after deep brain stimulation surgery. Parkinsonism Relat Disord 11: 247-252.
- Chen H, Zhang SM, Hernán MA, Willett WC, Ascherio A (2003) Weight loss in Parkinson's disease. Ann Neurol 53: 676-679.
- Perlemoine C, Macia F, Tison F, Coman I, Guehl D, et al. (2005) Effects of subthalamic nucleus deep brain stimulation and levodopa on energy production rate and substrate oxidation in Parkinson's disease. Br J Nutr 93: 191-198.
- Montaurier C, Morio B, Bannier S, Derost P, Arnaud P, et al. (2007) Mechanisms of body weight gain in patients with Parkinson's disease after subthalamic stimulation. Brain 130: 1808-1818.
- Aziz NA, van der Marck MA, Pijl H, Olde Rikkert MG, Bloem BR, et al. (2008) Weight loss in neurodegenerative disorders. J Neurol 255: 1872-1880.
- Guimarães J, Matos E, Rosas MJ, Vieira-Coelho A, Borges N, et al. (2009) Modulation of nutritional state in Parkinsonian patients with bilateral subthalamic nucleus stimulation. J Neurol 256: 2072-2078.
- Darbaky Y, Forni C, Amalric M, Baunez C (2003) High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur J Neurosci 18: 951-956.
- Temel Y, Visser-Vandewalle V, Aendekerk B, Rutten B, Tan S, et al. (2005) Acute and separate modulation of motor and cognitive performance in parkinsonian rats by bilateral stimulation of the subthalamic nucleus. Exp Neurol 193: 43-52.
- Baunez C, Christakou A, Chudasama Y, Forni C, Robbins TW (2007) Bilateral high-frequency stimulation of the subthalamic nucleus on attentional performance: transient deleterious effects and enhanced motivation in both intact and parkinsonian rats. Eur J Neurosci 25: 1187-1194.
- Baunez C, Dias C, Cador M, Amalric M (2005) The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nat Neurosci 8: 484-489.
- Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ (2007) Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson's disease. Neurosci Biobehav Rev 31: 643-657.
- Lacombe E, Carcenac C, Boulet S, Feuerstein C, Bertrand A, et al. (2007) High-frequency stimulation of the subthalamic nucleus prolongs the increase in striatal dopamine induced by acute l-3,4-dihydroxyphenylalanine in dopaminergic denervated rats. Eur J Neurosci 26: 1670-1680.
- Gubellini P, Salin P, Kerkerian-Le Goff L, Baunez C (2009) Deep brain stimulation in neurological diseases and experimental models: from molecule to complex behavior. Prog Neurobiol 89: 79-123.
- Bové J, Perier C (2012) Neurotoxin-based models of Parkinson's disease. Neuroscience 211: 51-76.
- Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res 162: 1-10.
- Grealish S, Mattsson B, Draxler P, Björklund A (2010) Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson's disease. Eur J Neurosci 31: 2266-2278.
- Vlamings R, Visser-Vandewalle V, Kozan R, Kaplan S, Steinbusch HW, et al. (2009) Bilateral high frequency stimulation of the subthalamic nucleus normalizes COX activity in the substantia nigra of Parkinsonian rats. Brain Res 1288: 143-148.
- Tan SKh, Vlamings R, Lim L, Sesia T, Janssen ML, et al. (2010) Experimental deep brain stimulation in animal models. Neurosurgery 67: 1073-1079.
- Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, et al. (2000) Methylxanthines reverse the adipsic and aphagic syndrome induced by bilateral 6-hydroxydopamine lesions of the nigrostriatal pathway in rats. Pharmacol Biochem Behav 66: 257-263.
- Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175: 303-317.
- Kirik D, Rosenblad C, Björklund A (1998) Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 152: 259-277.
- Spieles-Engemann AL, Collier TJ, Sortwell CE (2010) A functionally relevant and long-term model of deep brain stimulation of the rat subthalamic nucleus: advantages and considerations. Eur J Neurosci 32: 1092-1099.
- Vlamings R, Visser-Vandewalle V, Koopmans G, Joosten EA, Kozan R, et al. (2007) High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience 148: 815-823.
- Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, et al. (2010) Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis 39: 105-115.
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, (4thedn). Academic Press, San Diego.
- Vieira-Coelho MA, Serrão MP, Afonso J, Pinto CE, Moura E (2009) Catecholamine synthesis and metabolism in the central nervous system of mice lacking alpha-adrenoceptor subtypes. Br J Pharmacol 158: 726-737.
- Vieira-Coelho MA, Pestana M, Soares-da-Silva P (1996) High sodium intake increases the urinary excretion of L-3,4-dihydroxyphenylalanine but fails to alter the urinary excretion of dopamine and amine metabolites in Wistar rats. Gen Pharmacol 27: 1421-1427.
- Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, et al. (2006) Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res 1120: 100-105.
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, et al. (2007) Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A 104: 17087-17092.
- Meissner W, Harnack D, Paul G, Reum T, Morgenstern R, et al. (2002) Influence of Deep Brain Stimulation on Striatal Dopamine Release and Metabolism in the 6-OHDA-Model of Parkinson's Disease. Adv Behav Biol 52: 77-86.
- Ungerstedt U (1971) Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 367: 95-122.
- Konopacki J (1984) Motor activity and alimentary behavior after radio-frequency and 6-OHDA lesions of lateral hypothalamus and substantia nigra in cats. Acta Neurobiol Exp (Wars) 44: 17-28.
- Oueslati A, Sgambato-Faure V, Melon C, Kachidian P, Gubellini P, et al. (2007) High-frequency stimulation of the subthalamic nucleus potentiates L-DOPA-induced neurochemical changes in the striatum in a rat model of Parkinson's disease. J Neurosci 27: 2377-2386.
- Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, et al. (2010) Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A 107: 1196-1200.
- Baunez C, Amalric M, Robbins TW (2002) Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J Neurosci 22: 562-568.
- Adams F, Boschmann M, Lobsien E, Kupsch A, Lipp A, et al. (2008) Influences of levodopa on adipose tissue and skeletal muscle metabolism in patients with idiopathic Parkinson's disease. Eur J Clin Pharmacol 64: 863-870.
- Markus HS, Cox M, Tomkins AM (1992) Raised resting energy expenditure in Parkinson's disease and its relationship to muscle rigidity. Clin Sci (Lond) 83: 199-204.
- Delikanaki-Skaribas E, Trail M, Wong WW, Lai EC (2009) Daily energy expenditure, physical activity, and weight loss in Parkinson's disease patients. Mov Disord 24: 667-671.
- Guimarães J, Moura E, Vieira-Coelho MA, Garrett C (2012) Weight variation before and after surgery in Parkinson's disease: a noradrenergic modulation? Mov Disord 27: 1078-1082.
- Delaville C, Deurwaerdère PD, Benazzouz A (2011) Noradrenaline and Parkinson's disease. Front Syst Neurosci 5: 31.
- Olsson M, Nikkhah G, Bentlage C, Björklund A (1995) Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15: 3863-3875.
- Bertrand E, Lechowicz W, Szpak GM, Dymecki J (1997) Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson's disease. Folia Neuropathol 35: 80-86.
- Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A (2007) Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci 27: 9595-9606.
- Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60: 337-341.
- Szot P, Miguelez C, White SS, Franklin A, Sikkema C, et al. (2010) A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience 166: 279-291.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14101
- [From(publication date):
February-2014 - Sep 02, 2025] - Breakdown by view type
- HTML page views : 9504
- PDF downloads : 4597
