Research Article Open Access
Laying Hen's Primary Immune Response to Staphylococcal Protein A (SpA)
| Angel Alberto Justiz Vaillant1*, Sehlule Vuma1, Wayne Mohammed1, and Norma McFarlane-Anderson2 | |
| 1Pathology and Microbiology Unit, Department of Para-Clinical Sciences, The University of the West Indies, St. Augustine, Trinidad and Tobago, West Indies | |
| 2Department of Basic Medical Sciences, The University of the West Indies, Mona campus, Jamaica, West Indies | |
| *Corresponding Author : | Angel Alberto Justiz Vaillant MD, PhD Department of Para-Clinical Sciences The University of the West Indies St. Augustine, Trinidad and Tobago Tel: +868-773-5914 E-mail: avail4883@gmail.com |
| Received November 05, 2014; Accepted November 19, 2014; Published November 26, 2014 | |
| Citation: Vaillant AAJ, Vuma S, Mohammed W, McFarlane-Anderson N (2014) Laying Hen’s Primary Immune Response to Staphylococcal Protein A (SpA) . Biochem Physiol 3:146. doi:10.4172/2168-9652.1000146 | |
| Copyright: © 2014 Vaillant AAJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
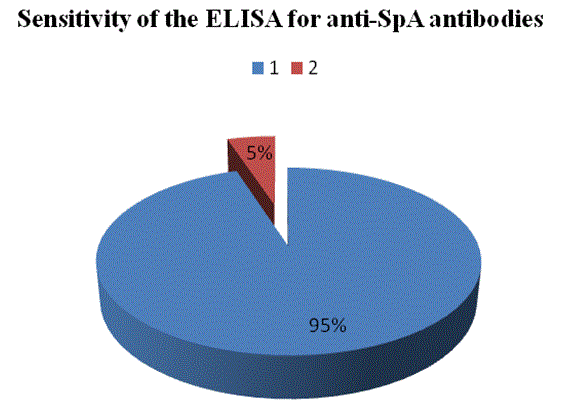
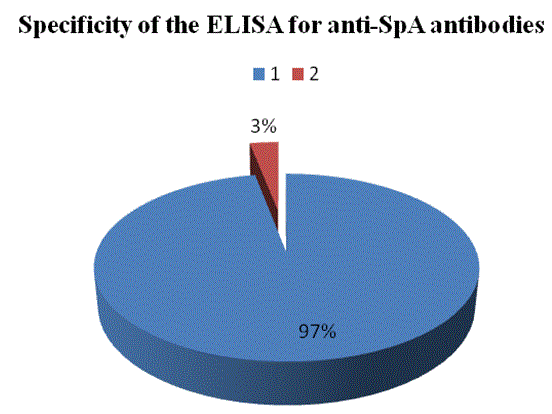
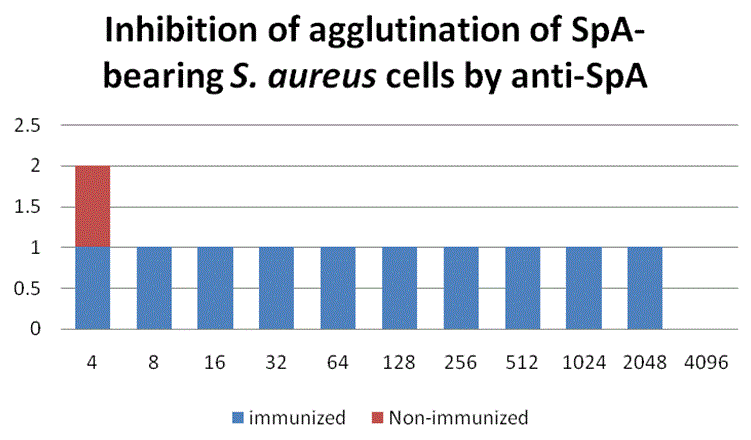
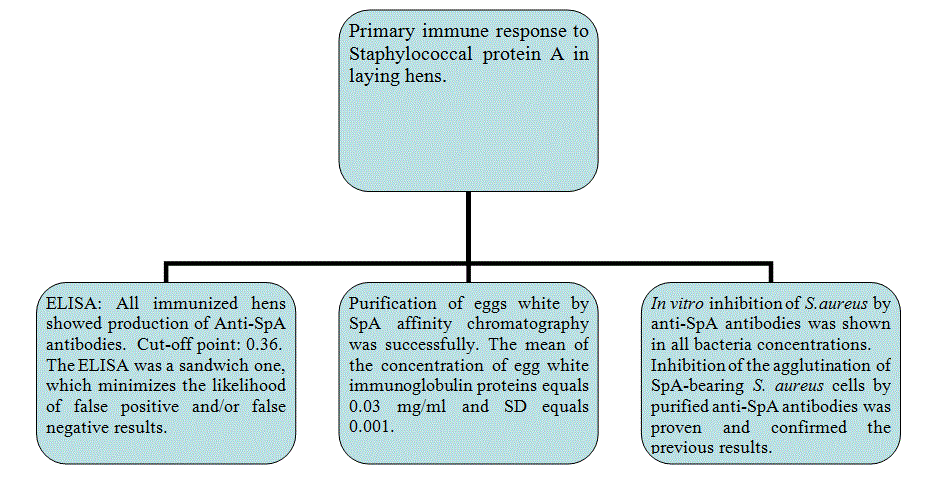
Our aim was to provide information about the production of Egg White Immunoglobulin (EWIg) with specificity to Staphylococcal protein-A, a surface antigen of Staphylococcus aureus and to study the inhibition of this bacterium growth in pre- and post-immunized hens. A sandwich Enzyme-Immunosorbent Assay (ELISA) showed a large concentration of anti-SpA antibodies in the eggs from hens immunized with protein A. The titer of these antibodies was at least 5 to 6-folds of that of the eggs from pre-immunized hens 10 days post-immunization. Inhibition of the growth of S. aureus by anti-SpA antibodies purified by SpA-affinity chromatography (PURE-1A) was observed in laying hens vaccinated. Growth of the bacteria in blood agar plates occurred in antibody samples from preimmunized laying hens only. Inhibition of the agglutination of SpA-bearing Staphylococcus aureus cells by purified anti-SpA antibodies was observed in vitro. The authors are not aware of previous studies of the primary immune system response developed in eggs from laying hens, so this research could set a precedent in the field of egg white immunoglobulin technology. The use of hyper-immune eggs as alternative to the use of antibiotics could be advantageous for the large amount of antibodies produced, low cost, the reduction of antigenic variation and very low toxicity.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14014
- [From(publication date):
December-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 9410
- PDF downloads : 4604
