Research Article Open Access
Measurement of Pancreatic Polypeptide and Its Peptide Variant in Human Serum and Plasma by Immunocapture-Liquid-Chromatography-Tandem Mass Spectrometry. Reference Intervals and Practical Assay Considerations.
| Hernando Escobar1, Mark M Kushnir1,2, Julie A Ray1, Miles A Merrell1, Gwen Gomez1, Rebecca Fietkau1, Alan L Rockwood1,2* and Wayne Meikle A1,2,3 | |
| 1ARUP Institute for Clinical and Experimental Pathology®, Salt Lake City, UT 84108, USA | |
| 2Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT 84112, USA | |
| 3Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA | |
| *Corresponding Author : | Alan L Rockwood, PhD ARUP Laboratories, 500 Chipeta Way Salt Lake City, Utah 84132, USA Tel: 801-213-2934 Fax: 801.584.5109 E-mail: alan.rockwood@aruplab.com |
| Received July 25, 2014; Accepted August 14, 2014; Published August 21, 2014 | |
| Citation: Escobar H, Kushnir MM, Ray JA, Merrell MA, Gomez G, et al. (2014) Measurement of Pancreatic Polypeptide and its Peptide Variant in Human Serum and Plasma by Immunocapture-Liquid-Chromatography-Tandem Mass Spectrometry. Reference Intervals and Practical Assay Considerations. Biochem Physiol 3:140. doi:10.4172/2168-9652.1000140 | |
| Copyright: © 2014 Escobar H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Pancreatic polypeptide (PP) is secreted by pancreatic islets after ingestion of food. Meal composition is associated with concentration of PP released in circulation. PP can be elevated in blood of patients with endocrine pancreatic tumors, supporting its use as a biomarker. We developed an Immunocapture-Liquid Chromatography- Tandem Mass Spectrometry (IC-LC-MS/MS) assay to quantify Pancreatic Polypeptide (PP) and its metabolite, PP3-36, in human serum or plasma. PP and PP3-36 were enriched from serum or plasma using immunopurification and the samples were analyzed using LC-MS/MS (AB SCIEX 5500) Total imprecision of the method was less than 20%; LOD and LOQ for PP and PP3-36 were 5 pg/mL and 10 pg/mL, respectively. Reference intervals in healthy subjects were <265 pg/mL (fasting); and 20 to 639 pg/mL (postprandial). Regression analysis for the comparison with a commercial RIA method showed poor agreement. Different types of meal increase the peptides levels differently; enhanced secretion of PP and PP3-36 was observed after meals rich in either protein and fat, or carbohydrates and fat, as compared with meals containing only carbohydrates. No relationship was observed between PP and body mass index or body fat. In summary, a direct LC-MS/MS method for determination of PP and PP3-36 in serum and plasma was developed and validated. The only endogenous truncated form of PP detected along with the intact PP in patient samples was, PP3-36, Our data suggest association of the meal type with secretion of PP; associations between concentrations of PP and body mass index or body fat percentage were not observed.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
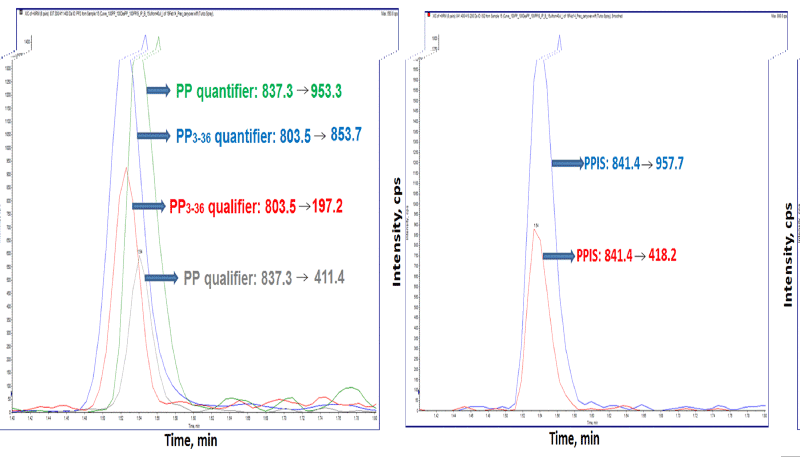 |
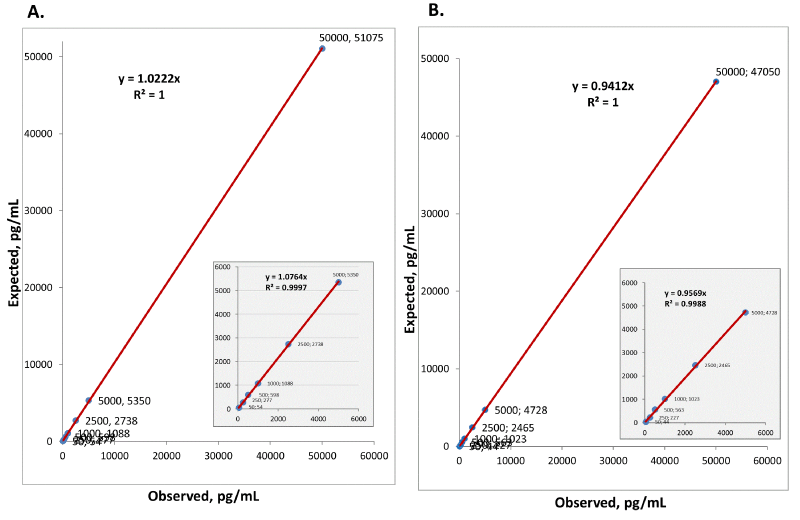 |
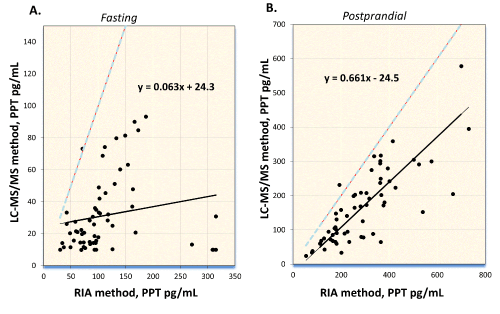 |
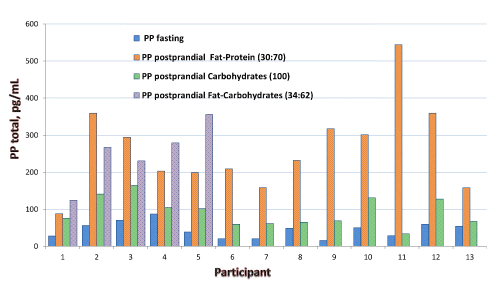 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15204
- [From(publication date):
December-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 10488
- PDF downloads : 4716
