Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Nesfatin – 1: Role as Possible New Anti Obesity Treatment
| Carmine Finelli1*, Rocco Rossano2, Maria Carmela Padula2, Nicolina La Sala1, Luigi Sommella3 and Giuseppe Martelli2 | |
| 1Center of Obesity and Eating Disorders, Stella Maris Mediterraneum Foundation, Chiaromonte, Potenza, Italy | |
| 2Department of Science, University of Basilicata, Potenza, Viale dell’Ateneo Lucano, 10, 85100, Italy | |
| 3Unit of Surgery, S. Giovanni Hospital - Lagonegro, Potenza, Italy | |
| Corresponding Author : | Carmine Finelli Center of Obesity and Eating Disorders Stella Maris Mediterraneum Foundation Chiaromonte, Potenza, Italy Tel: 39-3498667338 E-mail: carminefinelli74@yahoo.it |
| Received March 29, 2014; Accepted September 15, 2014; Published September 20, 2014 | |
| Citation: Finelli C, Rossano R, Padula MC, Sala NL, Sommella L, et al. (2014) Nesfatin – 1: Role as Possible New Anti Obesity Treatment. J Obes Weight Loss Ther 4:228. doi:10.4172/2165-7904.1000228 | |
| Copyright: © 2014 Finelli C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
In this article, we review on the current concepts about Nesfatin-1 as a new anti-obesity treatment and evaluate the existing issues in the context of this knowledge and the available literature. The intent is to enable clinicians to know Nesfatin-1 as a new anti obesity treatment and make rational decisions based on this perspective as possible clinical application. Future research should seek to clarify whether Nesfatin-1 would be beneficial in the management of obesity.
Abstract
In this article, we review on the current concepts about Nesfatin-1 as a new anti-obesity treatment and evaluate the existing issues in the context of this knowledge and the available literature. The intent is to enable clinicians to know Nesfatin-1 as a new anti obesity treatment and make rational decisions based on this perspective as possible clinical application. Future research should seek to clarify whether Nesfatin-1 would be beneficial in the management of obesity.
Keywords
Nesfatin-1; Obesity; Drug treatment
Abbreviations
NUCB2: Nucleobindin 2; PVN: Paraventricular Nucleus; NTS: Nucleus of the Solitary Tract; MCH: Melanin-Concentrating Hormone; THA: Tuberal Hypothalamic Area; PS: Paradoxical Sleep; SWS: Slow Wave Sleep; ARC: Arcuate Nucleus; Mtor: Mammalian Target of Rapamycin; CART: Cocaine and Amphetamine Regulated Transcript; pmTOR: Phospho-Mtor; NPY: Neuropeptide Y; POMC: Pro-opiomelanocortin; α-MSH: α-Melanocyte Stimulating Hormone; CSF: Cerebro Spinal Fluid
Introduction
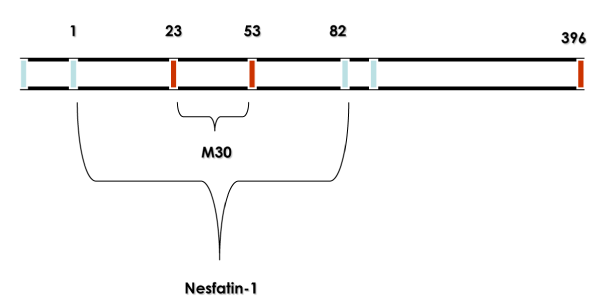
Nesfatin-1 is an 82-amino-acid peptide originated from post translational processing of the N-terminal fragment of Nucleobindin 2 (NUCB2), a 396-amino-acid protein exceptionally conserved across mammalian species [1]. Post translational cleavage of NUCB-2 appears in the expression of nesfatin-2 fragment (85–163) and nesfatin-3 fragment (166–396) in addition to nesfatin-1 [1]. Oh-I et al. [1] suggested that nesfatin-1 (named as acronym for NEFA/nucleobindin2-encoded satiety- and fat-influencing protein) may have physiological importance in regulating food intake (Figure 1).
In fact, nesfatin-1 injected into the third brain ventricle reduces food consumption occurring during the dark phase, whereas nesfatin-2 or nesfatin-3 had no effect [1]. In the same way, continuous infusion of nesfatin-1 (5 pmol/day for 10 days into the third brain ventricle) decreases significantly food intake and body weight gain in rats [1]. Conversely, third ventricle infusion of a NUCB-2 antisense oligonucleotide increases food intake and body weight gain compared with a missense NUCB-2 oligonucleotide [1]. Additionally, a 24-h fast decreased the expression of NUCB-2 in the Paraventricular Nucleus (PVN) [1].
Some studies [1–4] showed high expression level of nesfatin-1/NUCB-2 expression in relevant hypothalamic and medullary sites implicated in feeding regulation in rats. The arcuate nucleus, PVN, and the Nucleus of the Solitary Tract (NTS), further supporting the evidence that nesfatin-1 is involved in food intake regulation.
Nesfatin-1 is co-expressed with Melanin-concentrating Hormone (MCH) in neurons from the Tuberal Hypothalamic Area (THA) which are recruited during sleep states, especially Paradoxical Sleep (PS) [5]. To help decipher the contribution of this contingent of THA neurons to sleep regulatory mechanisms, Jego et al. thus investigated in rats whether the co-factor Nesfatin-1 is also endowed with sleep-modulating properties [5]. It was found that the disruption of the brain Nesfatin-1 signaling achieved by icv administration of Nesfatin-1 antiserum or antisense against the Nucleobindin2 (NUCB2) prohormone suppressed PS with little, if any alteration of Slow Wave Sleep (SWS) [5]. Additionally, the infusion of Nesfatin-1 antiserum after a selective PS deprivation, designed for elevating PS needs, severely prevented the ensuing expected PS recovery [5]. Strengthening these pharmacological data, Jego et al. finally demonstrated by using c-Fos as an index of neuronal activation that the recruitment of Nesfatin-1-immunoreactive neurons within THA is positively correlated to PS but not to SWS amounts experienced by rats prior to sacrifice [5]. In conclusion, this work supports a functional contribution of the Nesfatin-1 signaling, operated by THA neurons, to PS regulatory mechanisms [5]. Jego et al. proposed that these neurons, likely releasing MCH as a synergistic factor, constitute an appropriate lever by which the hypothalamus may integrate endogenous signals to adapt the ultradian rhythm and maintenance of PS in a manner dictated by homeostatic needs [5]. This could be done through the inhibition of downstream targets comprised primarily of the local hypothalamic wake-active orexin- and histamine-containing neurons [5].
Activation of brain CRF signaling pathways by CRF acting on CRF1 and CRF2 receptors and by selective endogenous CRF2 agonists urocortin 2 or 3 [6] inhibits food intake [7]. Nesfatin-1 injected intracerebroventricularly significantly decreased gastric emptying [8]. Goebel-Stengel et al. showed that NUCB2/nesfatin-1 immunoreactivity is distributed in mouse brain areas involved in the regulation of stress response and visceral functions activated by an acute psychological stressor suggesting that nesfatin-1 might play a role in the efferent component of the stress response [8].
In fact, nesfatin-1 injected into the third brain ventricle reduces food consumption occurring during the dark phase, whereas nesfatin-2 or nesfatin-3 had no effect [1]. In the same way, continuous infusion of nesfatin-1 (5 pmol/day for 10 days into the third brain ventricle) decreases significantly food intake and body weight gain in rats [1]. Conversely, third ventricle infusion of a NUCB-2 antisense oligonucleotide increases food intake and body weight gain compared with a missense NUCB-2 oligonucleotide [1]. Additionally, a 24-h fast decreased the expression of NUCB-2 in the Paraventricular Nucleus (PVN) [1].
Some studies [1–4] showed high expression level of nesfatin-1/NUCB-2 expression in relevant hypothalamic and medullary sites implicated in feeding regulation in rats. The arcuate nucleus, PVN, and the Nucleus of the Solitary Tract (NTS), further supporting the evidence that nesfatin-1 is involved in food intake regulation.
Nesfatin-1 is co-expressed with Melanin-concentrating Hormone (MCH) in neurons from the Tuberal Hypothalamic Area (THA) which are recruited during sleep states, especially Paradoxical Sleep (PS) [5]. To help decipher the contribution of this contingent of THA neurons to sleep regulatory mechanisms, Jego et al. thus investigated in rats whether the co-factor Nesfatin-1 is also endowed with sleep-modulating properties [5]. It was found that the disruption of the brain Nesfatin-1 signaling achieved by icv administration of Nesfatin-1 antiserum or antisense against the Nucleobindin2 (NUCB2) prohormone suppressed PS with little, if any alteration of Slow Wave Sleep (SWS) [5]. Additionally, the infusion of Nesfatin-1 antiserum after a selective PS deprivation, designed for elevating PS needs, severely prevented the ensuing expected PS recovery [5]. Strengthening these pharmacological data, Jego et al. finally demonstrated by using c-Fos as an index of neuronal activation that the recruitment of Nesfatin-1-immunoreactive neurons within THA is positively correlated to PS but not to SWS amounts experienced by rats prior to sacrifice [5]. In conclusion, this work supports a functional contribution of the Nesfatin-1 signaling, operated by THA neurons, to PS regulatory mechanisms [5]. Jego et al. proposed that these neurons, likely releasing MCH as a synergistic factor, constitute an appropriate lever by which the hypothalamus may integrate endogenous signals to adapt the ultradian rhythm and maintenance of PS in a manner dictated by homeostatic needs [5]. This could be done through the inhibition of downstream targets comprised primarily of the local hypothalamic wake-active orexin- and histamine-containing neurons [5].
Activation of brain CRF signaling pathways by CRF acting on CRF1 and CRF2 receptors and by selective endogenous CRF2 agonists urocortin 2 or 3 [6] inhibits food intake [7]. Nesfatin-1 injected intracerebroventricularly significantly decreased gastric emptying [8]. Goebel-Stengel et al. showed that NUCB2/nesfatin-1 immunoreactivity is distributed in mouse brain areas involved in the regulation of stress response and visceral functions activated by an acute psychological stressor suggesting that nesfatin-1 might play a role in the efferent component of the stress response [8].
Nesfatin-1/NUCB-2 and Anorexigenic Effect
Peptides regulating food intake often act in concert or in series with other neurotransmitters to exert their actions [9]. Nesfatin-1/NUCB-2 is co-localized with a number of hypothalamic peptides regulating food intake [10–16]. Several interactions have been described to underlie the central anorexic effect of nesfatin-1 [17]. It has also been shown to play important roles in the control of cardiovascular function [18]. In situ hybridization and immunohistochemical researches have evidentiated the expression of nesfatin-1 throughout the brain and, particularly, in the medullary autonomic gateway known as the Nucleus of the Solitary Tract (NTS) [18]. Mimee et al. showed that provide critical insight into the circuitry and physiology involved in the profound effects of nesfatin-1 and highlight the NTS as a key structure mediating these autonomic actions [18].
Two proteins have been localized in the Arcuate Nucleus (ARC) and implicated in the regulation of food intake: the serine-threonine-kinase Mammalian Target of Rapamycin (mTOR) as part of the TOR Signaling Complex 1 (TORC1), and nesfatin-1 derived from the precursor protein nucleobindin2, as reported by Inhoff et al. [19]. In fact, nesfatin-1 is not only intracellularly co-localized with Cocaine and Amphetamine Regulated Transcript (CART) peptide as reported before, but also with Phospho-mTOR (pmTOR) and Neuropeptide Y (NPY) in ARC neurons [19]. This data could also confirm results from previous studies, showing that the majority of nesfatin-1 neurons are also positive for CART peptide, whereas most of the pmTOR is co-localized with NPY and only to a lesser extent with CART [19].
A study, described by Maejima et al. [12], provided a strong evidence for the involvement of oxytocin pathway in nesfatin-1’s inhibitory effect on food intake. First of all, oxytocin injected into the 3v reduces food intake via a leptin-independent mechanism [12]. At the same time nesfatin-1 injected into the 3v activates oxytocin-positive neurons in the magnocellular part of the PVN as assessed by double labelling for Fos/oxytocin immunoreactivity and in vitro it stimulates the release of oxytocin from PVN neurons [12]. In addition there is pharmacologic and anatomical support for oxytocinergic projections from the PVN to the nucleus of the solitary tract to be involved in the anorexigenic signalling of nesfatin-1 [12]. An oxytocin receptor antagonist injected into the hindbrain at the level of the 4v blocked the food intake reducing effect of nesfatin-1 injected into the PVN and tracing studies showed synaptic contacts between oxytocinergic nerve terminals and Pro-opiomelanocortin (POMC) neurons in the nucleus of the solitary tract. Yosten et al. [20] also showed that an oxytocin antagonist injected intracerebroventricular blocks the food intake suppressing effects of intracerebroventricular nesfatin-1 and α-Melanocyte Stimulating Hormone (α-MSH). Therefore, nesfatin-1 acts through a serial neuronal circuit, in which nesfatin-1 activates the central melanocortin system, which, in turn, acts through the central oxytocin system, leading to an inhibition of food and water intake and an increase in mean arterial pressure [20].
Future research should seek to clarify whether the hypothalamic/nesfatin-1-oxytocin-brainstem/POMC signalling is the predominant pathway or an intrahypothalamic nesfatin-1-POMC/oxytocin network exists.
Some studies showed that based on the observation of a delayed and long lasting anorexigenic effect, following intracerebroventricular injection of nesfatin-1 which mimics the characteristics of the food intake reducing effect of CRF2 receptor agonists, urocortins [6,21]. The possible involvement of the CRF2 receptors in the mediation of nesfatin-1’s effect was investigated. The CRF2 antagonist, astressin2-B [22], injected intracerebroventricular completely abolished the dark phase food intake reduction induced by intracerebroventricular nesfatin-1 [23]. By contrast, a control peptide of similar structure as astressin2-B but without affinity to the CRF2 receptor did not influence intracerebroventricular nesfatin-1’s action [23]. However as reported by Stengel et al. [23], astressin2-B injected intracerebroventricular did not modulate the rapid onset reduction of food intake observed after intracerebroventricular injection of nesfatin-1. In contrast to the effect on food intake, the CRF2 antagonist, astressin2-B injected intracerebroventricular did not alter the intracerebroventricular nesfatin-1 induced delayed gastric emptying [23] giving rise to different downstream signalling pathways mediating intracerebroventricular nesfatin-1’s inhibitory effects on food intake and gastric transit. Finally, as reported by Yosten et al. [20], the melanocortin 3/4 receptor antagonist, SHU9119 injected intracerebroventricular diminished, and into the 3v abolished [1], the anorexigenic effect of nesfatin-1. Nesfatin-1 probably act in series through the recruitment of the central melanocortin and CRF2's pathways to reduce food intake.
Another, intracerebroventricular administration of nesfatin-1 induced c-Fos expression in CRF neurons, and nesfatin-1 increased cytosolic Ca2+ concentrations in single CRF neurons in the PVN [24]. It is now well established that the brain CRF/CRF1 signaling system modulates pain responses [24]. These observations suggest that nesfatin-1 may be involved in the autonomic regulation of visceral sensation [24]. Jia et al. suggested that nesfatin-1 may be associated with the visceral hypersensitivity state of irritable bowel syndrome, and this may be mediated, at least in part, by brain CRF/CRF1 signaling pathways [24].
Two proteins have been localized in the Arcuate Nucleus (ARC) and implicated in the regulation of food intake: the serine-threonine-kinase Mammalian Target of Rapamycin (mTOR) as part of the TOR Signaling Complex 1 (TORC1), and nesfatin-1 derived from the precursor protein nucleobindin2, as reported by Inhoff et al. [19]. In fact, nesfatin-1 is not only intracellularly co-localized with Cocaine and Amphetamine Regulated Transcript (CART) peptide as reported before, but also with Phospho-mTOR (pmTOR) and Neuropeptide Y (NPY) in ARC neurons [19]. This data could also confirm results from previous studies, showing that the majority of nesfatin-1 neurons are also positive for CART peptide, whereas most of the pmTOR is co-localized with NPY and only to a lesser extent with CART [19].
A study, described by Maejima et al. [12], provided a strong evidence for the involvement of oxytocin pathway in nesfatin-1’s inhibitory effect on food intake. First of all, oxytocin injected into the 3v reduces food intake via a leptin-independent mechanism [12]. At the same time nesfatin-1 injected into the 3v activates oxytocin-positive neurons in the magnocellular part of the PVN as assessed by double labelling for Fos/oxytocin immunoreactivity and in vitro it stimulates the release of oxytocin from PVN neurons [12]. In addition there is pharmacologic and anatomical support for oxytocinergic projections from the PVN to the nucleus of the solitary tract to be involved in the anorexigenic signalling of nesfatin-1 [12]. An oxytocin receptor antagonist injected into the hindbrain at the level of the 4v blocked the food intake reducing effect of nesfatin-1 injected into the PVN and tracing studies showed synaptic contacts between oxytocinergic nerve terminals and Pro-opiomelanocortin (POMC) neurons in the nucleus of the solitary tract. Yosten et al. [20] also showed that an oxytocin antagonist injected intracerebroventricular blocks the food intake suppressing effects of intracerebroventricular nesfatin-1 and α-Melanocyte Stimulating Hormone (α-MSH). Therefore, nesfatin-1 acts through a serial neuronal circuit, in which nesfatin-1 activates the central melanocortin system, which, in turn, acts through the central oxytocin system, leading to an inhibition of food and water intake and an increase in mean arterial pressure [20].
Future research should seek to clarify whether the hypothalamic/nesfatin-1-oxytocin-brainstem/POMC signalling is the predominant pathway or an intrahypothalamic nesfatin-1-POMC/oxytocin network exists.
Some studies showed that based on the observation of a delayed and long lasting anorexigenic effect, following intracerebroventricular injection of nesfatin-1 which mimics the characteristics of the food intake reducing effect of CRF2 receptor agonists, urocortins [6,21]. The possible involvement of the CRF2 receptors in the mediation of nesfatin-1’s effect was investigated. The CRF2 antagonist, astressin2-B [22], injected intracerebroventricular completely abolished the dark phase food intake reduction induced by intracerebroventricular nesfatin-1 [23]. By contrast, a control peptide of similar structure as astressin2-B but without affinity to the CRF2 receptor did not influence intracerebroventricular nesfatin-1’s action [23]. However as reported by Stengel et al. [23], astressin2-B injected intracerebroventricular did not modulate the rapid onset reduction of food intake observed after intracerebroventricular injection of nesfatin-1. In contrast to the effect on food intake, the CRF2 antagonist, astressin2-B injected intracerebroventricular did not alter the intracerebroventricular nesfatin-1 induced delayed gastric emptying [23] giving rise to different downstream signalling pathways mediating intracerebroventricular nesfatin-1’s inhibitory effects on food intake and gastric transit. Finally, as reported by Yosten et al. [20], the melanocortin 3/4 receptor antagonist, SHU9119 injected intracerebroventricular diminished, and into the 3v abolished [1], the anorexigenic effect of nesfatin-1. Nesfatin-1 probably act in series through the recruitment of the central melanocortin and CRF2's pathways to reduce food intake.
Another, intracerebroventricular administration of nesfatin-1 induced c-Fos expression in CRF neurons, and nesfatin-1 increased cytosolic Ca2+ concentrations in single CRF neurons in the PVN [24]. It is now well established that the brain CRF/CRF1 signaling system modulates pain responses [24]. These observations suggest that nesfatin-1 may be involved in the autonomic regulation of visceral sensation [24]. Jia et al. suggested that nesfatin-1 may be associated with the visceral hypersensitivity state of irritable bowel syndrome, and this may be mediated, at least in part, by brain CRF/CRF1 signaling pathways [24].
Nesfatin-1 and Anti-obesity Treatment
It has also been postulated that nesfatin-1/NUCB-2 may be produced by the hypothalamus [16]; the relatively high Cerebro Spinal Fluid (CSF)/plasma nesfatin-1/NUCB-2 ratios suggest that a substantial amount of CSF nesfatin-1/NUCB-2 may originate from central neurons. The possible discrepancy in the production of nesfatin-1/NUCB-2, by these central neurons may account for the differences in CSF/plasma nesfatin-1/NUCB-2 ratio between obese and lean subjects observed by Tan et al. [14]. Furthermore, it is probable that nesfatin-1/NUCB-2 has protein binding, and that differences in protein binding in obese and lean subjects may also explain these findings. Finally, it is possible that the efficiency of nesfatin-1/NUCB-2 uptake into CSF is reduced in obese individuals, possibly due to saturation of transporters [14]. Further studies could be useful to elucidate this hypothesis. Some data demonstrated that nesfatin-1/NUCB-2 is a novel depot-specific adipokine preferentially expressed in subcutaneous adipose tissue/adipocytes. Adipose tissue nesfatin-1/NUCB-2 expression increases with obesity and is altered in states of feeding and malnutrition [25].
Reduced leptin sensitivity or leptin resistance is a common phenomenon in obesity [25]. In the light of this and the pre-clinical findings that central and peripheral injection of nesfatin-1 exerts its food reducing effects via a leptin-independent mechanism [1,11], targeting nesfatin-1 may be a promising approach in the drug treatment of obesity and its complications. Ongoing pre-clinical data suggest the possible use of subcutaneous and intranasal routes of nesfatin-1 administration [26], which needs to be further explored. Another important aspect to be unraveled is the weight loss upon chronic administration of nesfatin-1 [27-30] and possible related changes in energy balance and/or basal metabolic rate [31-38] for which studies so far are limited. In fact, nesfatin-1 has a remarkably prolonged effect on food intake and body temperature [39]. Time course of nesfatin-1's effects may be varied depending on the time applied [39]. Many of the nesfatin-1/NUCB2 neurones are cold sensitive, and are positioned in key centres of thermoregulation [39]. Nesfatin-1 regulates energy expenditure a far more potent way than it was recognised before making it a preferable candidate anti-obesity drug [39].
Future research should seek to clarify whether nesfatin-1/NUCB-2 would be beneficial in the management of obesity.
Reduced leptin sensitivity or leptin resistance is a common phenomenon in obesity [25]. In the light of this and the pre-clinical findings that central and peripheral injection of nesfatin-1 exerts its food reducing effects via a leptin-independent mechanism [1,11], targeting nesfatin-1 may be a promising approach in the drug treatment of obesity and its complications. Ongoing pre-clinical data suggest the possible use of subcutaneous and intranasal routes of nesfatin-1 administration [26], which needs to be further explored. Another important aspect to be unraveled is the weight loss upon chronic administration of nesfatin-1 [27-30] and possible related changes in energy balance and/or basal metabolic rate [31-38] for which studies so far are limited. In fact, nesfatin-1 has a remarkably prolonged effect on food intake and body temperature [39]. Time course of nesfatin-1's effects may be varied depending on the time applied [39]. Many of the nesfatin-1/NUCB2 neurones are cold sensitive, and are positioned in key centres of thermoregulation [39]. Nesfatin-1 regulates energy expenditure a far more potent way than it was recognised before making it a preferable candidate anti-obesity drug [39].
Future research should seek to clarify whether nesfatin-1/NUCB-2 would be beneficial in the management of obesity.
Conclusive Remarks
The data obtained in basic experiments of nesfatin-1 should be useful for the development of a new anti-obesity treatment. However, the mechanisms of endocrine and metabolic effects of nesfatin-1 have not been known well by now, and the influences of nesfatin-1 administered peripherally should be much more clarified in vivo before starting controlled clinical trials in the future. In fact, the details of nesfatin-1 physiology ought to be clarified, and it may be considered suitable in the future, as a potential drug in the pharmacotherapy of obesity. On the other hand, some putative nesfatin-1 antagonists may improve eating disorders [34].
References
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, et al. (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709-712.
- Zheng H, Lenard NR, Shin AC, Berthoud HR (2009) Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 33 Suppl 2: S8-13.
- Ishida E, Hashimoto K, Shimizu H, Okada S, Satoh T, et al. (2012) Nesfatin-1 induces the phosphorylation levels of cAMP response element-binding protein for intracellular signaling in a neural cell line. PLoS One 7: e50918.
- Albayrak A, Demiryilmaz I2, Albayrak Y3, Aylu B3, Ozogul B3, et al. (2013) The role of diminishing appetite and serum nesfatin-1 level in patients with burn wound infection. Iran Red Crescent Med J 15: 389-392.
- Jego S, Salvert D, Renouard L, Mori M, Goutagny R, et al. (2012) Tuberal hypothalamic neurons secreting the satiety molecule Nesfatin-1 are critically involved in paradoxical (REM) sleep homeostasis. PLoS One 7: e52525.
- Chen P, Hover CV, Lindberg D, Li C (2013) Central urocortin 3 and type 2 corticotropin-releasing factor receptor in the regulation of energy homeostasis: critical involvement of the ventromedial hypothalamus. Front Endocrinol (Lausanne) 3:180.
- Doyon C, Moraru A, Richard D (2004) The corticotropin-releasing factor system as a potential target for antiobesity drugs. Drug News Perspect 17: 505-517.
- García-Galiano D, Pineda R, Ilhan T, Castellano JM, Ruiz-Pino F, et al. (2012) Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology 153: 1959-1971.
- Perry B, Wang Y (2012) Appetite regulation and weight control: the role of gut hormones. Nutr Diabetes 2: e26.
- Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, et al. (2008) The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 155: 174-181.
- Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, et al. (2009) Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology 150: 662-671.
- Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, et al. (2009) Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355-365.
- Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, et al. (2010) Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2: 775-784.
- Kerbel B, Unniappan S (2012) Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. J Neuroendocrinol 24: 366-377.
- Goebel-Stengel M, Wang L (2013) Central and peripheral expression and distribution of NUCB2/nesfatin-1. Curr Pharm Des 19: 6935-6940.
- Shimizu H, Ohsaki A, Oh-I S, Okada S, Mori M (2009) A new anorexigenic protein, nesfatin-1. Peptides 30: 995-998.
- Mimee A, Smith PM, Ferguson AV (2012) Nesfatin-1 influences the excitability of neurons in the nucleus of the solitary tract and regulates cardiovascular function. Am J Physiol Regul Integr Comp Physiol 302: R1297-1304.
- Inhoff T, Stengel A, Peter L, Goebel M, Taché Y, et al. (2010) Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides 31: 257-262.
- Yosten GL, Samson WK (2010) The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642-1647.
- Yakabi K, Noguchi M, Ohno S, Ro S, Onouchi T, et al. (2011) Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am J Physiol Endocrinol Metab 301: E72-82.
- Wang L, Stengel A, Goebel-Stengel M, Shaikh A, Yuan PQ, et al. (2013) Intravenous injection of urocortin 1 induces a CRF2 mediated increase in circulating ghrelin and glucose levels through distinct mechanisms in rats. Peptides 39: 164-170.
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, et al. (2009) Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology 150: 4911-4919.
- Jia FY, Li XL, Li TN, Wu J, Xie BY, et al. (2013) Role of nesfatin-1 in a rat model of visceral hypersensitivity. World J Gastroenterol 19: 3487-3493.
- Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS (2011) Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese humans: evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J Clin Endocrinol Metab 96: E669-673.
- Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, et al. (2010) Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology 151: 3169-3180.
- Shimizu H, Oh-I S, Okada S, Mori M (2009) Nesfatin-1: an overview and future clinical application. Endocr J 56: 537-543.
- Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY (2010) Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept 159: 72-77.
- Gonzalez R, Shepperd E, Thiruppugazh V, Lohan S, Grey CL, et al. (2012) Nesfatin-1 regulates the hypothalamo-pituitary-ovarian axis of fish. Biol Reprod 87: 84.
- Li Z, Gao L, Tang H, Yin Y, Xiang X, et al. (2013) Peripheral effects of nesfatin-1 on glucose homeostasis. PLoS One 8: e71513.
- Dong J, Xu H, Xu H, Wang PF, Cai GJ, et al. (2013) Nesfatin-1 stimulates fatty-acid oxidation by activating AMP-activated protein kinase in STZ-induced type 2 diabetic mice. PLoS One 8: e83397.
- Foo KS, Brismar H, Broberger C (2008) Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156: 563-579.
- García-Galiano D, Navarro VM, Roa J, Ruiz-Pino F, Sánchez-Garrido MA, et al. (2010) The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci 30: 7783-7792.
- Gonzalez R, Perry RL, Gao X, Gaidhu MP, Tsushima RG, et al. (2011) Nutrient responsive nesfatin-1 regulates energy balance and induces glucose-stimulated insulin secretion in rats. Endocrinology 152: 3628-3637.
- Pałasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, et al. (2012) Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides 46: 105-112.
- Kerbel B, Unniappan S (2012) Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. J Neuroendocrinol 24: 366-377.
- García-Galiano D, Pineda R, Ilhan T, Castellano JM, Ruiz-Pino F, et al. (2012) Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology 153: 1959-1971.
- Ghanbari Niaki A, Mohammadi Joojadeh F, Zare Kookandeh N, Najafi S, Chaichi MJ, et al. (2013) Liver and plasma nesfatin-1 responses to 6 weeks of treadmill running with or without zizyphus jujuba liquid extract in female rat. Int J Endocrinol Metab 11: 95-101.
- Vas S, Ádori C, Könczöl K, Kátai Z, Pap D, et al. (2013) Nesfatin-1/NUCB2 as a potential new element of sleep regulation in rats. PLoS One 8: e59809.
- Könczöl K, Pintér O, Ferenczi S, Varga J, Kovács K, et al. (2012) Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes (Lond) 36: 1514-1521.
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 13684
- [From(publication date):
September-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 9082
- PDF downloads : 4602
