Research Article Open Access
Nutritional Weight Loss Therapy with Cooked Bean Powders Regulates Serum Lipids and Biochemical Analytes in Overweight and Obese Dogs
| Genevieve M. Forster1, Cadie A. Ollila1, Jenna H. Burton1, Dale Hill2, John E. Bauer3, Ann M. Hess4, Elizabeth P. Ryan1* | |
| 1Department of Clinical Sciences, Animal Cancer Center, Colorado State University, Fort Collins 80523, USA | |
| 2ADM Alliance Nutrition, Inc. Quincy, IL 62301, USA | |
| 3Intercollegiate Faculty of Nutrition, Department of Small Animal Clinical Sciences, Texas A&M University, College Station 77843, USA | |
| 4Department of Statistics, Colorado State University, Fort Collins 80523, USA | |
| Corresponding Author : | Elizabeth P. Ryan Department of Clinical Sciences, Animal Cancer Center Colorado State University, Fort Collins 80523, USA Tel: 970-297-5301 Fax: 970-297-1254 E-mail: e.p.ryan@colostate.edu |
| Received August 30, 2012; Accepted September 23, 2012; Published September 25, 2012 | |
| Citation: Forster GM, Ollila CA, Burton JH, Hill D, Bauer JE, et al. (2012) Nutritional Weight Loss Therapy with Cooked Bean Powders Regulates Serum Lipids and Biochemical Analytes in Overweight and Obese Dogs. J Obes Wt Loss Ther 2:149 doi:10.4172/2165-7904.1000149 | |
| Copyright: © 2012 Forster GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: Emerging evidence supports that dry bean (Phaseolus vulgaris L.,) intake promotes weight loss and regulates blood lipids. Overweight and obese dogs represent a novel translational model for diet controlled evaluation of dry beans and for assessing the effects of bean intake on serum biomarkers of weight loss. Our objective was to evaluate changes in serum biomarkers associated with weight loss after four weeks of cooked navy bean or black bean powder intake (25% weight/weight) compared to an isocaloric, macro and micronutrient matched control diet in overweight/obese dogs.
Methods: Thirty client-owned, adult dogs of diverse breeds were randomized to 1 of 3 dietary study groups at the Colorado State University Veterinary Teaching Hospital. Body weights were measured weekly and a blood serum chemistry panel was performed at baseline and 2 and 4 weeks post intervention.
Results: Average percent weight lost after 4 weeks for dogs consuming the control diet was 4.20% (± 0.88), 5.22% (± 0.91) for dogs consuming the black bean diet, and 6.52% (± 0.95) for dogs consuming the navy bean diet. Serum cholesterol decreased by an average of 17 mg/ml (P<0.02) in the control group, 40 mg/dl (P<0.001) in black bean group, and 54 mg/dl (P<0.001) in the navy bean group. Triglycerides, high-density lipoprotein, and lowdensity lipoprotein were also changed in bean groups compared to control. Furthermore, serum blood urea nitrogen was decreased in the navy bean group, creatinine was increased in both bean groups, alkaline phosphatase was decreased in the black bean group, and total protein, aspartate aminotransferase, and total bilirubin were decreased in the control group at 4 weeks compared to baseline.
Conclusion: Overweight and obese canines represent an advanced translational model and dietary bean intake regulates lipid metabolism in overweight and obese dogs.
| Keywords |
| Dogs; Obesity; Weight loss; Beans; Biomarkers; Lipids; Cholesterol |
| Abbreviations |
| BCS: Body Condition Score; CSU VTH: Colorado State University Veterinary Teaching Hospital; BW: Body Weight; ME: Metabolizable Energy; BUN: Blood Urea Nitrogen; ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; GGT: Gamma-Glutamyl Transferase; LDL: Low Density Lipoprotein; HDL: High Density Lipoprotein; TG: Triglycerides |
| Introduction |
| Over 35% of American adults are obese [1] with the consequence that obesity has now surpassed smoking as the leading cause of preventable disease in the U.S. [2]. Companion animal obesity is estimated at 30 – 40% [3] and mirrors the obesity epidemic in humans. Similarities between dogs and humans with obesity associated comorbidities also exist for osteoarthritis, diabetes, nephropathy, cancer, and dyslipidemia [4-7]. Dogs represent an advanced translational model for cancer treatment [8,9] and other disease management therapies [10-12] and have been used extensively to evaluate surgical methods such as endoscopic pyloric suturing [13], reversible gastric restriction implants [14], and gastric bypass techniques [15]. Additionally, dogs have been shown to be successful models for evaluating protein intake, solving the nutritional mysteries behind pellagra and rickets [16] as well as evaluating effects of diet on brain physiology [17]. Pet dogs are especially relevant for evaluating nutritional weight loss therapies because they experience naturally occurring weight gain in the same home and living environment as humans. Companion dogs have similarities to human disease when compared to colony dogs possibly due to the role of environmental factors, including variable diets [18-20]. Furthermore, recent reports identified biomarkers of canine obesity with similarities to human biomarkers such as blood lipid values that change with weight loss [3,21,22]. While many nutritional intervention strategies exist for weight loss, epidemiological studies reveal that legume consumption promotes satiety and is more effective for weight loss than calorie restriction alone [23]. Beans have also been shown to improve nutrient intake levels and regulate body weights and waist circumferences in humans providing additional weight management benefits [24]. In addition to weight loss, beans have also shown to harbor chronic disease fighting properties for obesity related conditions such as heart disease [25,26], diabetes [27], cancer [28-30], and dyslipidemia [31-33]. While studies investigating the role of beans for weight loss in dogs have not been previously performed, we have established the safety and digestibility of beans as a novel ingredient for inclusion in healthy adult dog diets [34]. Changes in dietary patterns, such as a calorie restricted diets lead to weight reduction; however the role of diet composition during the weight loss process merits research attention [23]. Given that cooked beans are a novel food ingredient for dogs, this study was conducted to evaluate whether eating beans as a major staple diet ingredient will alter metabolic parameters compared to dogs not consuming beans. We hypothesized that a 25% weight/ weight bean based diet improves the metabolic status of dogs compared to control diets during comparable weight loss. |
| Materials and Methods |
| The Colorado State University Institutional Animal Care and Use Committee approved all clinical trial operations, animal care procedures, and collection of biological samples for analysis before beginning the study. |
| Study design |
| Thirty overweight or obese, clinically healthy, client owned adult dogs of different breeds were individually randomized based on their baseline Body Condition Score (BCS) into 1 of 3 diet groups (control, black bean, or navy bean). This prospective controlled dietary intervention trial screened a total of 40 dogs. Both the owner and clinician were blinded to which diet the dog was consuming. Dogs were transitioned onto their assigned study diet over a 4 day period; this was accomplished by mixing increasing proportions of the study diet with decreasing proportions for their normal diet over the 4 days period. Blood samples were collected at baseline and at 2 and 4 weeks post intervention. At the end of the 4 weeks dogs were transitioned over a 4 days period to their original diets. A signed consent form and medical history were required before enrolling in the study. The owner was required to bring the dog to the CSU VTH for weekly body weight (BW) checks and biweekly for physical exams, BCS monitoring, and blood collection. Owners were required to feed the dog only the study diets in amounts that were calculated to achieve weight loss, and to record daily food intake and fecal scores. Exercise was recorded; however no changes in exercise activity were required for the study. A medical history form was completed by the owner at weeks 2 and 4 of the study to assess changes in the dogs’ health and behavior, such as vomiting or diarrhea, flatulence, energy level, as well as to assess palatability of the study diet. |
| Inclusion/Exclusion criteria |
| Dogs between the ages of 2 to 7 years with a BW of at least 10 kg and a BCS of at least 6 on a 9 point scale [35] were eligible to participate in this clinical trial. Dogs were required to have normal biochemical and hematological values and normal thyroid function as determined by a screening total thyroxine (T4) test. If total thyroxine levels were below the lower limit of normal, a full canine thyroid panel, including thyroid stimulating hormone, thyroid globulin autoantibodies, and free thyroxine by dialysis, was performed to ensure adequate thyroid function. Dogs were excluded if they had hypothyroidism, dietary allergies, prior or current cancer history or other major medical illnesses, or had been administered antibiotics or analgesics within one month of starting the diet. Concomitant medications were not allowed while on study, with the exception of heartworm preventative. |
| Diet compositions |
| All diets were formulated to meet the Association of American Feed Control Officials nutrient and energy requirements for adult dogs [36]. Table 1 shows the nutrient profiles of the control and 25% weight/weight black and navy bean diets. Staple ingredients such as wheat, corn, and pork and bone meal were adjusted to account for the inclusion of bean powders (Vegefull; ADM Edible Bean Specialties, Decatur, IL), and to match nutrient and energy density. The control diet was manufactured and processed in the same location and under the same conditions as the bean diets (ADM Alliance Nutrition Feed Research Pilot Plant, Quincy, IL; Applied Food Biotechnology Plant in St. Charles, MO). Navy and black beans were selected for investigation given their widespread consumption by humans and availability as cooked powders. |
| Weight loss intervention |
| Total daily energy intake requirements for each dog were determined by the dog’s BW and BCS at baseline. The BCS was determined by the clinician and study nurse using a 9-point Body Condition Scale [35]. Using this scale, a score of less than 4 is underweight, a score of either 4 or 5 is considered ideal BW, a score of 6 or 7 is overweight, and a score of 8 or 9 is considered obese. Ideal BW was determined using BCS. For a BCS of 6, a dog was considered 10% over ideal BW, and for a BCS of 9, a dog is considered at least 40% over ideal BW [37]. The total required daily caloric intake to maintain ideal body weight was calculated using the following formula: daily Metabolizable Energy (ME) requirement (kcal) = 110×(ideal BW (kg) ^0.75). To achieve a weight loss rate of 2% BW/week, dogs consumed 60% of the energy calculated to maintain ideal BW. Dog owners were instructed to only feed the prescribed diet for the duration of the study according to the dog’s normal feeding schedule. Daily food amounts were provided to the owners in pre-measured packets and determined by dividing the daily energy requirements of each dog for weight loss by the energy density of the diet. Water was provided ad libitum. Each dog owner maintained daily records of all food consumed, including any nonstudy consumed food. Any leftover or uneaten food was collected and weighed in the laboratory. The total amount consumed was calculated by subtracting the weight of the leftover food from the original weight of the food prescribed for that day. If dogs failed to achieve at least 0.5% BW loss, caloric intake amounts were further decreased by 10% at 2 weeks. |
| Dietary intake records and fecal scores |
| Owners recorded the total amount of food consumed each day and recorded a daily fecal score. A 5-point fecal scoring system was used: 1 = hard and dry, 2 = well formed, 3 = moist, 4 = no form, 5 = diarrhea. Space was also provided for any comments and owners were instructed to report any food intake outside of the prescribed diet. Study compliance was determined by total number of days that each dog consumed only the prescribed amount of food. |
| Blood sample collection and analysis |
| Blood was collected after a 12 hour fast via jugular venipuncture at baseline, 2, and 4 weeks post intervention. Two mL of whole blood was collected into an evacuated red top tube without anticoagulant for biochemistry panel analysis and 4-6 mL was collected into a plasma separation tube with EDTA and used to determine lipid profiles. |
| The CSU Clinical Pathology Laboratory performed all blood analyses as previously described [34]. Briefly, the biochemistry panel was analyzed using a clinical chemistry analyzer (Hitachi 917; Roche Diagnostics, Indianapolis, IN), analytes evaluated include cholesterol, Blood Urea Nitrogen (BUN), creatinine, total protein, albumin, globulin, Alkaline Phosphatase (ALP), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Gamma-glutamyl transferase (GGT), total bilirubin, glucose, calcium, chloride, magnesium, phosphorus, potassium, and sodium. Total thyroxine and endogenous thyroid stimulating hormone were analyzed using an immunology analyzer (Siemens Immulite 1000, Los Angeles, CA), thyroid globulin autoantibodies were analyzed by ELISA (Oxford Laboratories, MI) and free thyroxine was analyzed by equilibrium dialysis at the Endocrine Section, Animal Health Diagnostic Laboratory (Michigan State University) using previously reported methods [38]. The lipid analysis was performed by a Cobas c501 chemistry analyzer (Roche Healthcare Diagnostics, Indianapolis, IN) which measured total triglycerides (TG) and high-density lipoprotein (HDL). Low-density lipoprotein (LDL) levels were calculated to equal: total cholesterol – HDL – (TG / 5) [39]. Cholesterol values were obtained from the biochemistry panel, performed at the time of collection. |
| Statistical analysis |
| Statistical analysis was performed using SAS 9.2 (SAS Institute Inc. Cary, NC). A model was fit separately for each response variable (weight, lipids, etc) using proc mixed. The model included main effects for diet and week and a diet*week interaction term. Repeated measures on dogs were captured using a random dog effect (where dog is nested within diet). Comparisons of interest were estimated and tested using contrasts of the model. TG, ALP, ALT, and AST were log transformed to satisfy the assumption of normality. Data are reported as means ± SEM for all response variables. One dog in the black bean group had elevated levels of TG at 4 weeks, was deemed a statistical outlier as the dog may have gotten into food before the blood draw. This data point was removed from the 4 week TG analysis. Differences between groups in baseline, age, and weight were assessed using a one-way ANOVA (Graphpad V.5.2, La Jolla, CA). Differences between groups in sex and baseline BCS were analyzed using Fisher’s exact test (R Project software, R Foundation for Statistical Computing, Vienna, Austria). Results were considered significant when P≤0.05. |
| Results |
| Of the 40 dogs screened, 7 failed to meet inclusion criteria and three were dropped from the study between 1 and 2 weeks for food refusal, physical injury, and an owner’s schedule not permitting follow up visits. Thirty dogs completed the study and no differences were observed in age, weight, sex, or BCS at baseline between 30 dogs in the control, black bean and navy bean diet groups (Table 2). The average age (years) in the control group was 5.8 ± 0.4, 4.3 ± 0.5 in the black bean group, and 4.6 ± 0.6 in the navy bean group (P>0.12). In the control group, 7/10 dogs were female, 3/10 were male; In the black bean group 6/10 dogs were female and 4/10 were male; and in the navy bean group 4/10 dogs were female and 6/10 were male. All male dogs were castrated and all female dogs except one in the control group were spayed. Dogs with BCS of either 6 or 7 were considered overweight and dogs with BCS of either 8 or 9 were considered obese. In the control group 7/10 dogs were overweight while 3/10 were obese; in the black bean group, 4/10 dogs were overweight and 6/10 were obese; and in the navy bean group 7/10 dogs were overweight and 3/10 were obese. No gastrointestinal discomfort or changes in flatulence were reported by owners. All owners reported adherence to the study diet, with a few exceptions (such as eating food scraps dropped by children, receiving cookies from the groomer, etc.) that were evenly distributed across groups. Some owners expressed uncertainty of the total amount of extra food their dog consumed. All owners were reasonably sure that their dog was fasted at the time of blood draw. |
| Effect of caloric restricted diets on canine weight loss |
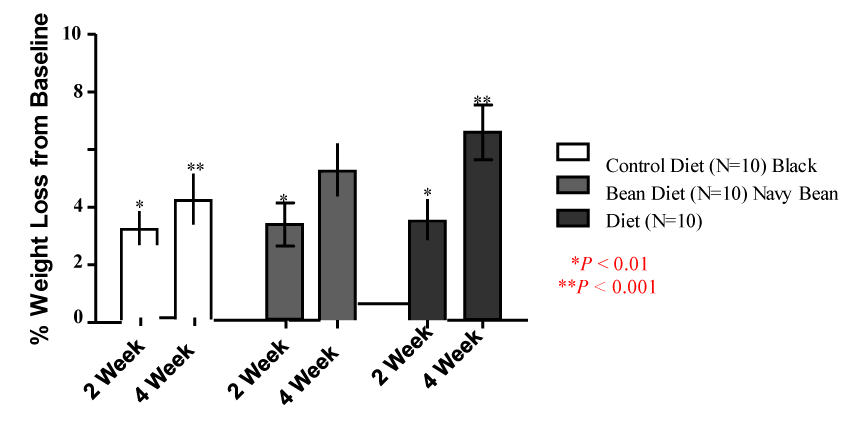
| Daily caloric intake was calculated to achieve a loss of 0.5-2% BW per week. Across all groups, 26/30 dogs achieved loss within this range. Figure 1 shows the average percent weight lost at 2 and 4 weeks post intervention. In all groups, weight loss was significant between baseline and 4 weeks. In the control group, average weight lost at 2 weeks was 3.2% ± 0.6% and 4.2% ± 0.9% at 4 weeks (P<0.001). In the black bean group, average weight lost at 2 weeks was 3.3% ± 0.8% and 5.2% ± 0.9% at 4 weeks (P<0.001). In the navy bean group, average weight lost at 2 weeks was 3.5% ± 0.7% and 6.5% ± 1.0% at 4 weeks (P<0.001). No difference in percent weight loss was seen between groups (P>0.05). The greatest percent lost was seen in the navy bean group > black bean group > control group. |
| Navy and black beans differentially modulate blood lipids in dogs during weight loss |
| Total serum cholesterol was significantly decreased in overweight dogs after 2 and 4 weeks of calorie restriction in the control and bean diet groups while TG, HDL, and LDL were also significantly reduced in at least one of the bean diet groups (Table 3). In the control group, total serum cholesterol was reduced by an average of 15 mg/dl after 2 weeks (P<0.05), and showed a total average decrease of 17 mg/dl (P<0.02) after 4 weeks. In the black bean group, total serum cholesterol was reduced by an average of 38 mg/dl after 2 weeks (P<0.001), for a total average decrease of 40 mg/dl (P<0.001) after 4 weeks. In the navy bean group, total serum cholesterol was reduced by an average of 46 mg/dl after 2 weeks (P<0.001), and 54 mg/dl after 4 weeks (P<0.001). No significant differences in serum total cholesterol were observed between groups at 2 or 4 weeks. Total cholesterol was significantly higher in dogs consuming the black bean diet compared to control at baseline (P=0.05). |
| Total serum TG were significantly decreased in all groups at 2 weeks, however only navy bean fed dogs continued to show a significant decrease at 4 weeks (Table 3). TG levels were not normally distributed and statistics were performed on log transformed variables and are reported as means ± (SEM). In the control group, total serum TG decreased from 123 ± 21 mg/dl at baseline to 89 ± 29 mg/dl at 2 weeks (P<0.03) and 105 ± 25 mg/dl at 4 weeks, for a total decrease of 18 mg/dl (P=0.09). In the black bean group, total serum TG decreased from 127 mg/dl ± 22 mg/dl at baseline to 94 ± 12 mg/dl at 2 weeks (P=0.05) and 132 ± 29 mg/dl at 4 weeks for total increase of 5 mg/dl (P=0.22). In the navy bean group, total serum TG decreased from 140 ± 31 mg/dl to 96 ± 22 at 2 weeks (P<0.01) and 77 ± 11 mg/dl at 4 weeks for a total decrease of 63 mg/dl (P<0.001). |
| HDL and LDL were significantly reduced from baseline in both the black and navy bean diet at 2 and 4 weeks (Table 3). HDL of the dogs fed the control diet decreased by 5 mg/dl (P=0.45) after 4 weeks. In black bean fed dogs HDL decreased an average of 23 mg/dl (P<0.001), and in navy bean fed dogs HDL decreased an average of 27 mg/dl (P<0.001) after 4 weeks. In control fed dogs LDL decreased an average of 9 mg/dl after 4 weeks (P=0.08), in black bean fed dogs LDL decreased an average of 17 mg/dl after 4 weeks (P<0.001), and in the navy bean fed dogs LDL decreased an average of 15 mg/dl after 4 weeks (P<0.01). |
| Dietary navy or black bean effects on blood chemistry and electrolytes |
| Selected non-lipid serum analytes from the clinical biochemistry panel that have been associated with obesity and modulated by weight loss in both dogs and humans were assessed at baseline, 2, and 4 weeks post intervention in control, navy bean and black bean fed overweight and obese dogs (Tables 4 and 5). |
| After 4 weeks, total protein was decreased an average of 0.21 g/ dl (P=0.03), AST was decreased an average of 9 IU/L (P<0.01), and total bilirubin was decreased an average of 0.04 mg/dl (P=0.05) in the dogs fed with the control diet. In the black bean fed dogs, after 4 weeks creatinine was increased by 0.06 mg/dl (P=0.05) and ALP was decreased by 49 IU/L (P=0.01), yet all values remained within clinically normal ranges. In the navy bean fed dogs, after 4 weeks blood urea nitrogen (BUN) was decreased by 5 mg/dl (P<0.001) and creatinine was increased 0.11 mg/dl (P<0.001). At 4 weeks no changes were seen in albumin, ALT, GGT, globulin, or glucose within any group (Table 4). |
| Of the serum electrolytes measured, none were changed in the control group. In the black bean group, magnesium was increased 0.08 mg/dl (P=0.05) and phosphorus was increased 0.42 mg/dl (P=0.04). In the navy bean group, chloride was increased by 1 meq/L (P=0.03). No significant changes were observed in calcium, potassium, or sodium (Table 5). |
| Discussion |
| Laboratory, clinical, and epidemiological studies show that dry bean intake is associated with increased weight loss and lower body weights [24,33,40]. Weight loss was intentionally achieved in the control diet fed dogs as well as for the experimental bean diets in this study due to caloric restriction. The navy and black bean diets showed a trend towards enhanced effects on weight loss after one month compared to the control. While this finding was not statistically significant, we postulate that a larger sample size and longer weight loss time may reveal more robust differences as previously reported with bean intake in humans [33]. Three of the four dogs (1/10 black bean, 1/10 navy bean, 1/10 control) who failed to achieve weight loss may have been due to dietary non-compliance as the owners expressed uncertainty of the dog’s intake in spite of attempts to adhere to study diet. This highlights the challenges faced by owners and clinicians when undertaking a weight loss program for a companion animal and underscores the importance of developing weight management therapies that account for these challenges. The fourth dog (black bean diet) failed to lose weight in the 4 weeks period most likely due to resting energy requirements outside of the calculated required energy estimates as the owner was reasonably sure the dog was only consuming prescribed food. Other weight loss related measurements modulated by dry bean intake include decreased waist circumference and inflammatory biomarker expression [24,25,33,41]. The macro and micronutrient composition of dry beans are thought to promote weight loss by providing low glycemic index sources of fiber, protein, minerals, and phytochemicals [40,42]. Increased protein intake has been shown to improve weight loss [43] with plant proteins having higher satiety ratings than animal proteins [44]. Dry bean fiber may promote weight loss by improving satiety with altered transit time in the intestines, increased fermentation by gut microflora that results in higher short chain fatty acid production that compete with protein and glucose for uptake and utilization [40], and enhanced release of cholecystokinin [45] which may have short term effects on energy intake and gastric emptying [46]. Bean phytochemicals such as phenolic compounds may interfere with glucose transport in the small intestine, phytic acid may delay glucose absorption leading to improved satiety and modulated energy uptake [40], and many phytosterols and saponins have been implicated in cholesterol reduction [47,48]. |
| Lipid metabolism is an important component to weight loss as dyslipidemia underlies many of the comorbidities associated with obesity [49]. Weight loss has repeatedly been shown to reduce serum cholesterol levels in both humans and dogs [50,51] and increased plant fiber intake has been shown to lower cholesterol levels after only 1 week with the same efficacy as first generation statins [52]. Our study shows serum cholesterol reduction after 2 weeks of weight loss and was sustained throughout the study (Table 3). While not statistically significant, dogs consuming beans showed the largest change at 2 weeks whereas the decrease in the control group was similar between 2 and 4 weeks. This finding demonstrated that serum cholesterol reduction may be an early biomarker for a metabolic response to bean intake and weight loss wherein previous reports in dogs showed decreased serum cholesterol after 60 days [53], 90 days [50], and after the full amount of time required to reach ideal weight [54,55]. Furthermore, TG, HDL and LDL were reduced in at least one of the bean diet groups, a finding consistent in human studies [32], with the exception of HDL which has been shown to increase in humans after bean consumption [56]. It should be noted, that LDL levels were determined indirectly using Friedewald’s equation [39] and are therefore only indicative of trends between groups, and may not be reflective of actual values due to variance with HDL and LDL metabolism in dogs compared to humans. |
| Modulation of lipid metabolism by dry bean consumption has several proposed mechanisms which may work in conjunction with weight loss. Epidemiological studies have revealed inverse relationships between dietary protein sources and cholesterol levels [57]. Dietary fiber is thought to correct dyslipidemia by decreasing fat intake and increasing bile acid and cholesterol losses in the small intestines [58]. A meta-analysis of non-legume soluble fiber also revealed a slight improvement in lipid profiles [59]. In contrast, dry bean fiber has been shown to alter blood lipids by preventing micelle formation and preventing absorption of cholesterol and fatty acids making these compounds available for fermentation in the large intestine, and increasing the rate of removal of LDL [60]. In vitro studies have suggested that dry beans have a higher capacity for binding to bile acids than do soy proteins or wheat gluten [61,62] and rat studies have shown higher bile acid synthesis and excretion with whole bean diets [63,64]. Human studies have further demonstrated the interruption of the enterohepatic circulation of bile acids and increased excretion of acidic fecal sterols after prolonged bean consumption [65]. Characterization of bean fractions associated with lipid modulation in rats revealed that the starch and fiber component had the greatest lipid lowering effects [64], as well as upregulation of hepatic LDL receptor and cholesterol 7 alpha-hydroxylase expressions [66]. These findings, taken together with the observed lipid metabolism changes in the bean groups suggest that there may be multiple compounds working in concert. Beans contain numerous bioactive components [67] and dietary intervention studies continue to show beneficial changes in blood lipids, regardless of underlying health conditions [23,31]. Non-lipid serum analytes previously reported to be associated with weight loss in both dogs and humans were also evaluated. BUN is decreased in overweight dogs compared to lean dogs [50], however changes in BUN after weight loss have been reported with inconsistent results, namely no changes [53,54], or increased levels of BUN [50,68]. In this study, all dogs had BUN values within normal ranges, and only dogs on the navy bean diet showed an average decrease of 5 mg/dl (P<0.001) during weight loss (Table 4). Creatinine levels have also been shown to be elevated in overweight dogs compared to lean dogs [50], however changes in serum creatinine levels have also been variably reported with increases and decreases after 60 and 90 days of weight loss [50,53,54]. Creatinine levels remained constant during the 4-week study in our control group and increased in both bean diet groups but remained within normal reference range. The precursor for creatinine, creatine, is generated from the urea cycle and has been touted as a supplement for stimulating muscle growth in athletes [69]. This candidate biomarker may have important implications in understanding the role of beans for maintaining muscle mass during weight loss. Total protein has been shown to be increased in overweight dogs compared to lean [50] and to decrease after 60 and 90 days of weight loss [50,53]. In this study, total protein decreased in the control group (P=0.03), however remained unchanged in the bean groups. Albumin has been demonstrated to be increased in overweight dogs [50] and reduced in dogs undergoing weight loss [50,53,54]. Serum albumin levels were not changed herein and may be reflective of the 4 weeks study time period of weight loss examined as previous studies have reported changes after 60-90 days. Hepatic enzymes ALP, ALT, AST, and GGT have all been positively associated with increased body fat in obese humans, with ALP dependent on gender [70]. ALP is elevated in overweight dogs and reduced in dogs undergoing weight loss [50,54]. ALP was significantly reduced in dogs consuming black beans (P=0.01) and had a decreasing trend in the navy bean group (P=0.08). AST levels have not previously been shown to change with weight loss in dogs [55], however was significantly decreased in the control group (P<0.01). Bilirubin, while not elevated in overweight dogs [50], has been shown to be reduced during weight loss with specific diets [53] as was observed in the control group. Glucose levels were not altered in any of the 3 diet groups and have not previously been shown to change with weight loss in dogs [50,53,55,71], although increased glucose levels in overweight dogs have been reported [50]. No analyte was changed outside of normal ranges. |
| Beans are an effective staple food ingredient and a quality protein source during weight loss and these results suggest that consuming a diet with high dry bean intake improves lipid profiles along with other metabolic biomarkers when compared to a non-bean caloric restricted diet alone. Furthermore, dry bean consumption has been associated with increased longevity [72], reduced tumor growth in colon [73,74], mammary tissue [75], upper digestive tract and stomach [30], and prostate [76], decreased risk for cardiovascular disease [56], and diabetes [77,78]. |
| This study has demonstrated the ability of dietary bean intake to modulate blood lipids beyond what is expected from weight loss alone. Furthermore, the changes in blood biochemical analytes suggest a role for beans in liver and kidney function in dogs undergoing weight loss. This overweight dog trial was a practical and relevant approach to advance our understanding of the effects of dry bean consumption for lipid modulation during weight loss, because it accounts for variations in non-controlled living environments. Moreover, the companion animal dogs in this study represent a realistic reflection of serum lipid and biochemical profile variations and population based responses. Taken together, these results provide rationale for further explorations of the effects of dry bean consumption for chronic disease prevention, and regulation of lipid metabolism with weight loss. |
| Acknowledgements |
| The authors would like the Dry Bean Health Research Program, the Shipley Foundation, and the Animal Cancer Center for support for this study. We would also like to acknowledge Dr. Susan Lana and Kim Arnett in the ACC Clinical Trials Core for providing expertise and technical assistance. |
References
- Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491-497.
- Jia H, Lubetkin EI (2010) Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med 38: 138-144.
- German AJ (2006) The growing problem of obesity in dogs and cats. J Nutr 136: 1940S-1946S.
- German AJ (2010) Obesity in companion animals. In Practice 32: 42-50.
- Kopelman PG (2000) Obesity as a medical problem. Nature 404: 635-643.
- Cignarelli M, Lamacchia O (2007) Obesity and kidney disease. NMCD 17: 757-762.
- Howard BV, Ruotolo G, Robbins DC (2003) Obesity and dyslipidemia. Endocrinol Metab Clin North Am 32: 855-867
- Thamm D, Dow S (2009) How companion animals contribute to the fight against cancer in humans. Vet Ital 45: 111-120.
- Pinho SS, Carvalho S, Cabral J, Reis CA, Gartner F (2012) Canine tumors: a spontaneous animal model of human carcinogenesis. Transl Res 159: 165-172.
- Baehr W, Frederick JM (2009) Naturally occurring animal models with outer retina phenotypes. Vision Res 49: 2636-2652.
- Schotanus BA, van den Ingh TS, Penning LC, Rothuizen J, Roskams TA, et al. (2009) Cross-species immunohistochemical investigation of the activation of the liver progenitor cell niche in different types of liver disease. Liver Int 29: 1241-1252.
- Murphy MP, Morales J, Beckett TL, Astarita G, Piomelli D, et al. (2010) Changes in cognition and amyloid-ß processing with long term cholesterol reduction using atorvastatin in aged dogs. J Alzheimers Dis 22: 135-150.
- Vegesna A, Korimilli A, Besetty R, Bright L, Milton A, et al. (2010) Endoscopic pyloric suturing to facilitate weight loss: a canine model. Gastrointest Endosc 72: 427-431.
- Guo X, Zheng H, Mattar SG, Lu X, Sandusky G, et al. (2011) Reversible gastric restriction implant: safety and efficacy in a canine model. Obes Surg 21: 1444-1450.
- Rao RS, Rao V, Kini S (2010) Animal models in bariatric surgery-a review of the surgical techniques and postsurgical physiology. Obes Surg 20: 1293-1305.
- Carpenter KJ (1991) Contribution of the dog to the science of nutrition. J Nutr 121: S1- S7.
- Snigdha S, Berchtold N, Astarita G, Saing T, Piomelli D, et al. (2011) Dietary and behavioral interventions protect against age related activation of caspase cascades in the canine brain. PLoS One 6:e24652.
- German AJ, Holden SL, Morris PJ, Biourge V (2012) Long-term follow-up after weight management in obese dogs: The role of diet in preventing regain. Vet J 192: 65-70.
- Schmidt PL (2009) Companion animals as sentinels for public health. Vet Clin North Am Small Anim Pract 39: 241-250.
- Reif JS (2011) Animal sentinels for environmental and public health. Public Health Rep 126: 50-57.
- Jeusette IC, Lhoest ET, Istasse LP, Diez MO (2005) Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 66: 81-86.
- Jericó MM, De Camargo Chiquito F, Kajihara K, Moreira MA, Gonzales R, et al. (2009) Chromatographic analysis of lipid fractions in healthy dogs and dogs with obesity or hyperadrenocorticism. J Vet Diagn Invest 21: 203-207.
- Abete I, Goyenechea E, Zulet MA, Martinez JA (2011) Obesity and metabolic syndrome: potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis 21: B1-B15.
- Papanikolaou Y, Fulgoni VL 3rd (2008) Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the National Health and Nutrition Examination Survey 1999-2002. J Am Coll Nutr 27: 569-576.
- Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, et al. (2001) Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med 161: 2573-2578.
- Winham DM, Hutchins AM, Johnston CS (2007) Pinto bean consumption reduces biomarkers for heart disease risk. J Am Coll Nutr 26: 243-249.
- Villegas R, Gao YT, Yang G, Li HL, Elasy TA, et al. (2008) Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 87: 162-167.
- Lanza E, Hartman TJ, Albert PS, Shields R, Slattery M, et al. (2006) High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the polyp prevention trial. J Nutr 136: 1896-1903.
- Thompson MD, Brick MA, McGinley JN, Thompson HJ (2009) Chemical composition and mammary cancer inhibitory activity of dry bean. Crop Sci 49: 179-186.
- Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, et al. (2009) Legume intake and the risk of cancer: a multisite case-control study in Uruguay. Cancer Causes Control 20: 1605-1615.
- Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM (2011) Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 21: 94-103.
- Finley JW, Burrell JB, Reeves PG (2007) Pinto bean consumption changes SCFA profiles in fecal fermentations, bacterial populations of the lower bowel, and lipid profiles in blood of humans. J Nutr 137: 2391-2398.
- Hermsdorff HH, Zulet MÁ, Abete I, Martínez JA (2011) A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr 50: 61-69.
- Forster GM, Hill D, Gregory G, Weishaar KM, Lana S, et al. (2012) Effects of cooked navy bean powder on apparent total tract nutrient digestibility and safety in healthy adult dogs. J Anim Sci 90: 2631-2638.
- Laflamme D (1997) Development and validation of a body condition score system for dogs. Canine Pract 22: 10-15.
- AAFCO (2010) Official Publication. Association of American Feed Control Officials Inc, Oxford, IN.
- German AJ, Holden SL, Bissot T, Hackett RM, Biourge V (2007) Dietary energy restriction and successful weight loss in obese client-owned dogs. J Vet Intern Med 21: 1174-1180.
- Daminet S, Paradis M, Refsal KR, Price C (1999) Short term influence of prednisone and phenobarbital on thyroid function in euthyroid dogs. Can Vet J 40: 411-415.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
- McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE (2010) Pulse consumption, satiety, and weight management. Adv Nutr 1: 17-30.
- McCrory MA, Lovejoy JC, Palmer PA, Eichelsdoerfer PE, Gehrke MM, et al. (2008) Effectiveness of legume consumption for facilitating weight loss: a randomized trial. FASEB J 22: 1084-1088.
- Mitsuhashi Y, Nagaoka D, Ishioka K, Bigley KE, Okawa M, et al. (2010) Postprandial lipid-related metabolites are altered in dogs fed dietary diacylglycerol and low glycemic index starch during weight loss. J Nutr 140: 1815-1823.
- Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, et al. (2005) A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82: 41-48.
- Keller U (2011) Dietary Proteins in Obesity and in Diabetes. Int J Vitam Nutr Res 81: 125-133.
- Bourdon I, Olson B, Backus R, Richter BD, Davis PA, et al. (2001) Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein b48 response to test meals in men. J Nutr 131: 1485-1490.
- Little TJ, Horowitz M, Feinle-Bisset C (2005) Role of cholecystokinin in appetite control and body weight regulation. Obes Rev 6: 297-306.
- Andersson SW, Skinner J, Ellegård L, Welch AA, Bingham S, et al. (2004) Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur J Clin Nutr 58: 1378-1385.
- Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, et al. (2004) Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food 7: 67-78.
- Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, et al. (2010) Inflammation, adiponectin, obesity and cardiovascular risk. Curr Med Chem 17: 4511-4520.
- Yamka RM, Friesen KG, Frantz NZ (2006) Identification of canine markers related to obesity and the effects of weight loss on the markers of interest. Int J Appl Res Vet Med 4: 282-292.
- Fernandez ML, Metghalchi S, Vega-López S, Conde-Knape K, Lohman TG, et al. (2004) Beneficial effects of weight loss on plasma apolipoproteins in postmenopausal women. J Nutr Biochem 15: 717-721.
- Jenkins DJ, Kendall CW, Popovich DG, Vidgen E, Mehling CC, et al. (2001) Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 50: 494-503.
- Yamka RM, Frantz NZ, Friesen KG (2007) Effects of 3 canine weight loss foods on body composition and obesity markers. Int J Appl Res Vet Med 5: 125-132.
- German AJ, Hervera M, Hunter L, Holden SL, Morris PJ, et al. (2009) Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim Endocrinol 37: 214-226.
- Diez M, Michaux C, Jeusette I, Baldwin P, Istasse L, et al. (2004) Evolution of blood parameters during weight loss in experimental obese Beagle dogs. J Anim Physiol Anim Nutr 88: 166-171.
- Anderson JW, Major AW (2002) Pulses and lipaemia, short- and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr 88: S263-S271.
- Smit E, Nieto FJ, Crespo CJ (1999) Blood cholesterol and apolipoprotein B levels in relation to intakes of animal and plant proteins in US adults. Br J Nutr 82: 193-201
- Lairon D (2003) Dietary fibre, lipid metabolism and cardiovascular disease. Dietary fibre: bio-active carbohydrates for food and feed. Dietary Fibre 2003 Conference, The Netherlands.
- Brown L, Rosner B, Willett WW, Sacks FM (1999) Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 69: 30-42.
- Anderson JW, Gustafson NJ, Spencer DB, Tietyen J, Bryant CA (1990) Serum lipid response of hypercholesterolemic men to single and divided doses of canned beans. Am J Clin Nutr 51: 1013-1019.
- Kahlon TS, Smith GE, Shao Q (2005) In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaseolus aconitifolins). Food Chem 90: 241-246.
- Kahlon TS, Woodruff CL (2002) In vitro binding of bile acids by soy protein, pinto beans, black beans and wheat gluten. Food Chem 79: 425-429.
- Marzolo MP, Amigo L, Nervi F (1993) Hepatic production of very low density lipoprotein, catabolism of low density lipoprotein, biliary lipid secretion, and bile salt synthesis in rats fed a bean (Phaseolus vulgaris) diet. J Lipid Res 34: 807-814.
- Amigo L, Marzolo MP, Aguilera JM, Hohlberg A, Cortés M, et al. (1992) Influence of different dietary constituents of beans (Phaseolus vulgaris) on serum and biliary lipids in the rat. J Nutr Biochem 3: 486-490.
- Duane WC (1997) Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res 38: 1120-1128.
- Han KH, Iijuka M, Shimada K, Sekikawa M, Kuramochi K, et al. (2005) Adzuki resistant starch lowered serum cholesterol and hepatic 3-hydroxy-3-methylglutaryl-CoA mRNA levels and increased hepatic LDL-receptor and cholesterol 7 alpha-hydroxylase mRNA levels in rats fed a cholesterol diet. Br J Nutr 94: 902-908.
- Mensack MM, Fitzgerald VK, Lewis MR, Thompson HJ (2010) Characterization of low molecular weight chemical fractions of dry bean (Phaseolus vulgaris) for bioactivity using Caenorhabditis elegans longevity and metabolite fingerprinting. J Agric Food Chem 58: 6697-6705.
- Tvarijonaviciute A, Ceron JJ, Holden SL et al. (2012) Effects of weight loss in obese dogs on a range of renal biomarkers. J Vet Intern Med 26: 809-809.
- Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80: 1107-1213.
- Choi JW (2003) Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann Clin Lab Sci 33: 257-264.
- Peña C, Suárez L, Bautista I, Montoya JA, Juste MC (2008) Relationship between analytic values and canine obesity. J Anim Physiol Anim Nutr 92: 324-325.
- Darmadi-Blackberry I, Wahlqvist ML, Kouris-Blazos A, Steen B, Lukito W, et al. (2004) Legumes: the most important dietary predictor of survival in older people of different ethnicities. Asia Pac J Clin Nutr 13: 217-220.
- Hangen L, Bennink MR (2002) Consumption of black beans and, navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr Cancer 44: 60-65.
- Hughes JS, Ganthavorn C, Wilson-Sanders S (1997) Dry beans inhibit azoxymethane-induced colon carcinogenesis in F344 rats. J Nutr 127: 2328-2333.
- Thompson MD, Thompson HJ, Brick MA, McGinley JN, Jiang W, et al. (2008) Mechanisms associated with dose-dependent inhibition of rat mammary carcinogenesis by dry bean (Phaseolus vulgaris, L.). J Nutr 138: 2091-2097.
- Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT (1997) A case-control study of diet and prostate cancer. Br J Cancer 76: 678-687.
- Helmstädter A (2010) Beans and Diabetes: Phaseolus vulgaris preparations as antihyperglycemic agents. J Med Food 13: 251-254.
- Jiménez-Cruz A, Manuel Loustaunau-López V, Bacardi-Gascón M (2006) The use of low glycemic and high satiety index food dishes in Mexico: a low cost approach to prevent and control obesity and diabetes. Nutr Hosp 21: 353-356.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 7055
- [From(publication date):
September-2012 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 2395
- PDF downloads : 4660
