Research Article Open Access
Perioperative Glucose Control in the Gastric Bypass Population: How Well Do We Do, How Well Do We Think We Do, and is it Predictable
| Mark J Perna*, Amy Wahlquist, Katherine A Morgan, Karl Byrne T and Megan Baker | |
| Department of Surgery, Medical University of South Carolina, Charleston, South Carolina 29425, USA | |
| Corresponding Author : | Mark J Perna Department of Surgery Medical University of South Carolina Charleston, South Carolina 29425, USA Tel: 573-882-6135 E-mail: pernam@health.missouri.edu |
| Received January 23, 2013; Accepted February 28, 2013; Published March 02, 2013 | |
| Citation: Perna MJ, Wahlquist A, Morgan KA, Byrne TK, Baker M (2013) Perioperative Glucose Control in the Gastric Bypass Population: How Well Do We Do, How Well Do We Think We Do, and is it Predictable. J Obes Wt Loss Ther 3:162. doi:10.4172/2165-7904.1000162 | |
| Copyright: © 2013 Perna MJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: Bariatric patients are prone to insulin resistance and Postoperative Hyperglycemia (PH), which adversely affects postoperative care. Clinicians may underestimate PH on surgical wards. We aimed to characterize inpatient Blood Glucose (BG) control and identify predictors of PH after RYGB.
Methods: From a single University-based center, a retrospective review of 431 patients undergoing RYGB was performed. Postoperative inpatient BG control and diabetic therapy were characterized. Attending bariatric surgeons and surgical house staff were surveyed regarding inpatient BG management. BG management was compared, and predictors of PH were identified.
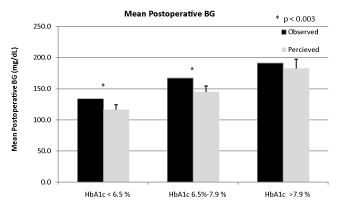
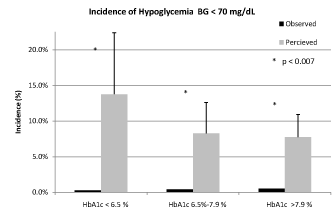
Results: PH (BG>180 mg/dL) was common particularly in patients with HbA1C>6.5%. From the observed sample, the mean postoperative BG was 133.5 ± 2.6 mg/dL, 167.0 ± 6.0 mg/dL, and 190.9 ± 9.2 mg/dL for each increasing HbA1c class, while physician perceived mean postoperative BG was 116.5 ± 7.9 mg/dL (p<0.002), 145.0 ± 9.3 mg/ dL (p<0.003), and 182.8 ± 14.5 mg/dL (p=ns) respectively. However, physicians overestimated the incidence of PH. Postoperative hypoglycemia was rare and also overestimated by clinicians. Four independent predictors of PH were identified, including preoperative HbA1c, preoperative nonfasting BG, a laparoscopic procedure, and preoperative diabetes. PH (mean BG>180 mg/dL) was predicted with a sensitivity of 42%, a specificity of 95%, a PPV of 60%, NPV of 90% and an overall accuracy of 87%.
Conclusions: The incidence of PH is common after RYGB and may be overestimated, while mean postoperative BG may be underestimated. Postoperative hypoglycemia is rare and overestimated. Preoperative HbA1c and nonfasting BG help identify patients at greatest risk PH.
| Keywords |
| Perioperative glucose; Control bariatric surgery; Gastric bypass; Postoperative hyperglycemia |
| Introduction |
| Postoperative Hyperglycemia (PH) is deleterious in a wide range of surgical patients as it increases morbidity and possibly mortality. Wound infections and other infectious complications are often associated with PH [1-12]. Improving glucose control may decrease postoperative morbidity [13,14]. However, in critically ill patients intensive insulin regimens have been associated with hypoglycemic events, a potentially deadly consequence [15]. |
| Various organizations have recognized PH as an independent risk factor for surgical morbidity. Surgical Care Improvement Program (SCIP) and National Surgical Quality Improvement Program (NSQIP) have recommended that blood glucoses (BG) should under 200 mg/dL [16]. American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) have adopted more stringent recommendations, suggesting all inpatient BG should be below 180 mg/dL [17]. |
| As morbid obesity and the insulin resistance/diabetes continuum are tightly linked, bariatric patients are particularly prone to PH [18-21]. In our data, we have confirmed that PH is an independent risk factor for wound infections and Acute Renal Failure (ARF) in bariatric patients undergoing Roux-en-Y Gastric Bypass (RYGB) [22]. |
| According to recent estimates, approximately 220,000 bariatric surgery cases are performed yearly and are now some of the most common general surgical operations [23]. Also, the number of surgeons registered with the American Society for Metabolic and Bariatric Surgery (ASMBS) has doubled in a 6 year period [24]. With an increasing demand for weight loss surgery, more high volume surgical centers, and an expanding set of bariatric surgeons, inpatient bariatric services tend to be quite busy. It remains unclear if clinicians are entirely aware of the frequency and severity of PH in RYGB populations. |
| Therefore, we characterized inpatient glycemic control and postoperative diabetic therapy after RYGB. In addition, we compared these results with clinician perceptions of inpatient glycemic control and postoperative insulin therapy. Finally, we aimed to identify independent risk factors of postoperative glycemic control in RYGB patients. |
| Patient/Subject and Methods |
| From a single institution with ASMBS and Surgical Review Corporation (SRC) center of excellence accreditation, a retrospective chart review was performed of 431 patients undergoing RYGB procedures during 2006 - 2009. IRB approval was obtained. Patients were excluded for age <18 and BMI<40 kg/m2. In general, patients underwent a laparoscopic retrocolic, retrogastric RYGB. |
| Most patients were on at least an insulin sliding scale while Nil Per Os (NPO) with Finger Stick Blood Glucose (FSBG) analyses every four hours. Some patients however required insulin infusions, which prompted FSBS analyses every one hour. Postoperative inpatient BGs were recorded for each patient. According to AACE guidelines, patients were grouped based on preoperative Hemoglobin A1c (HbA1c) status. |
| A voluntary survey of attending bariatric surgeons, recent former chief residents, and advanced resident surgical house staff was performed with respect to inpatient BG control and diabetic management (Appendix). The retrospectively observed sample versus physician perceived survey of BG control and inpatient diabetic management strategies were compared. Continuous vs. continuous variables were compared via two-tailed student t-test. Continuous vs. dichotomous variables were compared via one sample t-test. Descriptive statistics were performed using Microsoft Excel (Microsoft, Redmond, WA) spreadsheets and GraphPad QuickCalc software (La Jolla, CA). Statistical significance was defined as p<0.05. SAS 9.2 (SAS Institute Inc., Durham, NC) was used for multivariate modeling. |
| Next, 381 of 431 (88.4%) patients were included into the predictive modeling for PH. Patients were excluded if records were incomplete. Univariate predictors were identified and then included into the multivariable modeling if they were at least borderline statistically significantly (p<0.15) related to postoperative BG. A stepwise backwards elimination approach was used by removing one variable at a time starting with the least significant (i.e. p-value closest to 1.0) until all remaining variables in the model were statistically significantly related to postoperative BG (p<0.05). |
| Results |
| Of the 431 patients reviewed, 278 (64.5%) had HbA1c<6.5% (group1), while 88 (20.4%) were between 6.5%-7.9% (group 2) and 66 (15.3%) were >7.9% (group 3). The preoperative HbA1c class and the observed mean postoperative glucose were positively correlated (data not shown). As defined by ACEE as BG>180 mg/dL the incidence of PH, was quite frequent, particularly in group 2 (27.7%) and group 3 (47.8%) (Table 1). The vast majority of patients were on “lower” dose insulin sliding scales, and there little was documented record regarding why treatment decisions were made. Also, neither insulin sliding scale (mostly low dose) or Diabetic Management Service (DMS) consult appeared to significantly reduce the incidence of PH, while insulin drips did (data not shown). |
| 18/24 (75%) physician surveys were completed and analyzed. The range of responses varied significantly. Clinicians appeared to overestimate the incidence of PH across HbA1C classes. However, clinicians may underestimate mean postoperative BG, particularly in the group 1 and group 2 ((116.5 ± 7.9 mg/dL vs. 133.5 ± 2.6 mg/dL, p<0.002) and (145.0 ± 9.3 mg/dL vs. 167.0 ± 6.0 mg/dL, p<0.003)) (Table 2 and Figure 1). Clinicians underestimated the observed percentage of patients treated with insulin sliding scales and those requiring insulin drips postoperatively (Table 2). Rates of hypoglycemia (BG<70 mg/dL) were low throughout all HbA1c classes (Table 1), but clinicians highly overestimated the rates of hypoglycemia in all HbA1c groups (Figure 2). Finally, rates of severe hypoglycemia, as defined as BG<50 mg/dL, were exceptionally rare in this population (0.00%-0.04%) (Table 1). |
| There appeared to be many potential predictors of elevated mean PH in univariate modeling. Of note, age, ASA class and the other components of metabolic syndrome including hypertension and hyperlipidemia were associated with elevated mean PH, while BMI was not. However, only four of the predictors remained significant after multivariable modeling. These predictors included preoperative HbA1c, preoperative random glucose, preoperative DM, and surgery type (laparoscopic vs. open RYGB) (Table 3). An equation for estimating mean postoperative BG was constructed from the multivariable model: |
| Estimated mean postoperative BG (mg/dL)=81.3+7.7(Baseline HbA1c (%))+0.16(Preoperative BG (mg/dL))+9.6(Baseline DM)– 12.2(Laparoscopic Surgery) |
| In accordance with AACE/ADA guidelines which defined inpatient hyperglycemia as BG>180 mg/dL, a 2 × 2 table of observed sample versus model predicted mean postoperative BG was constructed for this model. The model demonstrated a sensitivity of 42.0% and a specificity of 95.0%. Also, the model had a positive predictive value (PPV) of 60.0% and a negative predictive value (NPV) of 90%. The overall incidence of mean postoperative BG > 180 mg/dL was approximately 17%, while the overall accuracy was 87.0% for the model (R2=50.5%). |
| Discussion |
| Clearly, PH affects surgical outcomes in a wide array of specialties, including general surgery. Despite frequent use of insulin sliding scales, insulin infusions, and DMS consults, PH was unfortunately still alarmingly common in the RYGB population. Also, it was potentially underestimated by clinicians on the busy surgical wards. Additionally, hypoglycemic events in this population were relatively uncommon and were far overestimated. These findings may be related to in the early postoperative period. A more standardized and aggressive treatment strategy may improve optimal postoperative BG homeostasis after RYGB. |
| Multiple univariate risk factors were associated with elevated mean PH, most of which were related to age, overall health (ASA class), and their diabetic parameters. Interestingly, BMI has no effect on PH, which suggests this issue is more related to insulin resistance and pancreatic reserve, and not necessarily their severity of obesity. Although not entirely unexpected, our multivariable findings demonstrated that elevated preoperative HbA1c, elevated preoperative BG, and baseline DM to be strong risk factors for PH after RYGB. Also, having a laparoscopic procedure was protective of PH, likely as a result of less physiologic stress and decreased postoperative pain. This simple model may help distinguish RYGB patients at highest risk of PH from those with the lowest risk of PH. Our data is similar to postoperative cardiac surgery patients in that HbA1c and DM are predictors of higher postoperative BG [25]. Other recent literature cardiovascular surgery identified predictors of postoperative PH after, but they presumptively excluded diabetics and HbA1c from the model [26]. The authors here found age, BMI, and male gender among others to be predictors of PH, while preoperative BG was not. More recently, Kwon et al. found multiple potential univariate predictors of PH in general surgery patients, including age, BMI, DM, bariatric surgery, and surgical approach (laparoscopic vs. open). However, the scope of the study was outcomes after surgery and not necessarily predictors of PH [12]. As BG control is a quality measure, there will need to be more literature predicting PH in surgical patients. |
| Our multivariable model has important limitations and is not all inclusive yet. First, it contains 381 patients, which is relatively small for predictive modeling. This predictive dataset lacks specific Insulin Resistance (IR) measurements, such as preoperative serum insulin levels and HOMA-IR calculations, C-peptide levels, and number of years with diagnosis of DM. Future studies will need to incorporate this data into predictive BG models after RYGB. Therefore, it will be important to augment and validate our current predictive model. Despite limitations, PH appears to be reasonably predictable preoperatively. |
| Admittedly, there are several other limitations to this study. First, the sampled data was collected retrospectively from 2006-2009, while physicians were surveyed in 2011. Also, they were asked to reflect about their time on the bariatric wards, but were not necessarily on the bariatric service at time of survey. More optimally, the clinicians would have been surveyed simultaneously to the hospital course of these patients. However, at our institution these results have raised awareness of a potential discrepancy between perceived and observed glucose control. It has sparked prospective study designs and more aggressive treatment protocols for the RYGB patients. Also, our study is from a single high volume center of excellence, but may lack generalizability as BG management strategies may be different at other institutions. Finally, our data lacks any outpatient BG data in the first weeks postoperatively as their blood glucose metabolism is rapidly changing. |
| Conclusion |
| In conclusion, PH clearly increases morbidity and potentially mortality in various surgical patients including RYGB patients [1-11,22]. Improving glycemic control in RYGB patients is important, as it may improve postoperative morbidities such as wound infection and ARF22. This may have implications in reducing excess hospital cost and improving patient outcomes. As BG management is a recognized quality measure, there will be increasing surveillance and scrutiny of inpatient PH. Therefore, increased clinician awareness and predictive models are vital for improving outcomes in these patients on busy surgical wards. |
| Disclosures |
| The authors of this paper including Mark J Perna MD., Amy Wahlquist MS., Katherine A Morgan MD., T. Karl Byrne MD., and Megan Baker MD do not have any potential conflicts of interest to disclose. |
| Acknowledgements |
| The project described was supported by Award Number UL1RR029882 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. |
References
- Caddell KA, Komanapalli CB, Slater MS, Hagg D, Tibayan FA, et al. (2010) Patient-specific insulin-resistance-guided infusion improves glycemic control in cardiac surgery. Ann Thorac Surg 90: 1818-1823.
- Eshuis WJ, Hermanides J, van Dalen JW, van Samkar G, Busch OR, et al. (2011) Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg 253: 739-744.
- Mraovic B, Suh D, Jacovides C, Parvizi J (2011) Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol 5: 412-418.
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, Pomfret EA, Driscoll DF, et al. (1998) Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 22: 77-81.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, et al. (2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87: 978-982.
- Ata A, Lee J, Bestle SL, Desemone J, Stain SC (2010) Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 145: 858-864.
- Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, et al. (2010) Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology 112: 860-871.
- Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, et al. (2010) Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33: 1783-1788.
- Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS Jr (2001) The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol 22: 607-612.
- McConnell YJ, Johnson PM, Porter GA (2009) Surgical site infections following colorectal surgery in patients with diabetes: association with postoperative hyperglycemia. J Gastrointest Surg 13: 508-515.
- Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, et al. (2008) Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 248: 585-591.
- Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, et al. (2013) Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 257: 8-14.
- Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, et al. (1997) Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 63: 356-361.
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A (1999) Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 67: 352-360.
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, et al. (2009) Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 180: 821-827.
- Rosenberger LH, Politano AD, Sawyer RG (2011) The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt) 12: 163-168.
- Moghissi ES, Korytkowski MT, DiNardo M, Hellman R, Einhorn D, et al. (2009) American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 15: 353-369.
- Kral JG (2001) Morbidity of severe obesity. Surg Clin North Am 81: 1039-1061.
- Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, et al. (2004) Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 279: 27263-27271.
- Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, et al. (2007) Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 30: 2916-2921.
- Martyn JA, Kaneki M, Yasuhara S (2008) Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology 109: 137-148.
- Perna M, Romagnuolo J, Morgan K, Byrne TK, Baker M (2012) Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis 8: 685-690.
- Taylor K (2011) Metabolic and Bariatric Surgery: Fact Sheet 2011.
- Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, et al. (2011) Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg 213: 261-266.
- Masla M, Gottschalk A, Durieux ME, Groves DS (2011) HbA1c and diabetes predict perioperative hyperglycemia and glycemic variability in on-pump coronary artery bypass graft patients. J Cardiothorac Vasc Anesth 25: 799-803.
- Garg R, Grover A, McGurk S, Rawn JD (2012) Predictors of hyperglycemia after cardiac surgery in nondiabetic patients. J Thorac Cardiovasc Surg.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 13309
- [From(publication date):
March-2013 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 8757
- PDF downloads : 4552
