Research Article Open Access
Personality, Anxiety, and Individual Variation in Psychophysiological Habituation and Sensitization to Painful Stimuli
| Yoshio Nakamura, Gary W Donaldson and Akiko Okifuji* | |
| Utah Center for Exploring Mind-Body Interactions (UCEMBI), Pain Research Center, Department of Anesthesiology, University of Utah School of Medicine, USA | |
| Corresponding Author : | Akiko Okifuji Pain Research Center Department of Anesthesiology University of Utah School of Medicine 615 Arapeen Drive, Suite 200 Salt Lake City, UT 84108 Tel: (801) 585-7690 E-mail: akiko.okifuji@hsc.utah.edu |
| Received August 22, 2014; Accepted September 15, 2014; Published September 17, 2014 | |
| Citation: Yoshio Nakamura, Gary W Donaldson, Akiko Okifuji (2014) Personality, Anxiety, and Individual Variation in Psychophysiological Habituation and Sensitization to Painful Stimuli. J Pain Relief 3:156. doi: 10.4172/2167-0846.1000156 | |
| Copyright:2014 Nakamura Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Habituation and sensitization are two fundamental consequences in response to repeated painful stimulation. However, individual differences as to who systematically habituate versus sensitize are currently not well understood. Investigating changes in response modulation (habituation and sensitization) in laboratory subjects undergoing pain testing, we evaluated the hypotheses: 1) significant individual differences exist in patterns of habituation and sensitization, and 2) variations in response modulation are systematically associated with subjects’ characteristic personality styles. We explored personality and state predictors of individual differences in response modulation consisting of habituation and sensitization in pain-free, healthy subjects. We recruited 81 healthy subjects who underwent 36 trials of painful stimulations to their fingertips. Subjective pain reports and psychophysiological responses - evoked potential (EP) and skin conductance response (SCR) - served as measurement indicators for habituation and sensitization, and to evaluate how these different measures manifested in the group averages as well as in correlations across individuals. Results showed that although subjective pain report tended to indicate sensitization on average, both EP and SCR showed habituation. However, further analyses revealed that there was a positive convergence among the three channels across different subjects, suggesting that individuals tend to have consistent response styles in responding to repeated pain stimulations. Our analyses allowed us to make inferences about the relative proportions of individuals in the population who systematically habituate versus sensitize. Variations in the habituation-sensitization continuum are highly robust and systematic, and preexisting individual characteristics (personality and current psychological states such as state anxiety) in part accounted for these patterns.
| Keywords |
| Habituation, Sensitization, Laboratory pain, Personality, Anxiety, Mixed effects model |
| Introduction |
| Pain perception involves a complex, dynamic process. Research over the past several decades has indicated numerous factors that influence the modulation of pain perception in humans. Research also suggests that pain perception presents an intricate balance between a warning sign to protect the biological integrity of an organism and an adaptive process not to overtax the system. One such example is how our response to pain changes over time. |
| In typical pain experiments, repeated exposure to noxious stimulations may lead to amplified pain response (sensitization) or diminished pain response (habituation). In an experimental setting, sensitization has been demonstrated with a localized tissue injury, which subsequently produced acute sensitization and heat hyperalgesia [1]. Such sensitization is thought to help protect the injured area. However, in human pain experiments, noxious stimulation typically produces transient pain without actual tissue damage; habituation appears to be the dominant response to such repeated noxious stimulations [2-4]. |
| Habituation seems to involve both peripheral and central processes. Greffrath et al. [5] have demonstrated rapid habituation to noxious heat stimuli in a fixed stimulus location relative to non-stimulated sites, suggesting the presence of peripheral neural fatigue of nociceptors. Habituation also occurred when the stimulus location varied, albeit to a lesser extent. Lesser habituation is also observed in un-stimulated limbs [6], suggesting the involvement of central mechanisms. Furthermore, recent neuroimaging studies [7,8] suggest that habituation is an active process involving the central anti-nociceptive system. |
| Habituation does not seem to be a universal phenomenon. In some clinical pain conditions - including fibromyalgia, low back pain, and migraine - the ability to habituate to noxious stimuli is often attenuated [9-11]. Given evidence suggesting that central sensitization mechanisms underlie many chronic pain syndromes [12-15], a study on how people habituate vs. get sensitized to painful stimuli may lead to further understanding of these complex phenomena [16]. Habituation is certainly not universal, and it also may depend on various psychosocial contexts and personal traits. For example, recent research suggests that higher cognitive processes influence the extent of habituation to noxious stimulations. Habituation in healthy adults can be attenuated when they are informed that noxious stimulus levels may intensify over time [17], whereas expectation of attenuated stimulus levels over time seems to potentiate habituation [18]. |
| Research examining the role of invoked emotion suggests however, that an experimentally induced emotional valence may not impact one’s ability to habituate [19]. The same study also found that various “output” channels exhibiting habituation (typically, verbal pain report, skin conductance response, evoked potential and nociceptive flexion reflex) do not necessarily converge with one another. Thus, within a single individual, it is possible that pain report may show an amplified response (sensitization) while evoked potential may show an attenuated response (habituation). |
| The primary objectives of the present exploratory study were to: 1) document individual differences in habituation or sensitization to noxious stimuli and 2) determine how much of this variability could be explained by personality traits. First, little evidence exists on the extent to which it varies across people, as the relative proportions of individuals in the population who systematically habituate versus sensitize are unknown. Second, to evaluate how different response measures cohered with one another in group averages as well as in correlations across individuals, we used three response channels to assess dynamic response modulation: verbal pain report, skin conductance, and evoked potential. |
| Methods |
| The research protocol was approved by the Institutional Review Board at the University of Utah. All subjects provided written consent prior to entering the study. |
| Participants |
| A total of 81 subjects, 49 male and 32 female healthy volunteers (ranging from 18 to 43 in age) participated in the study. Subjects received $15/h for participation, but they did not otherwise benefit from participating in the study. The recruitment was done through study flyers posted on campus and locally. Each prospectus participant was telephone screened by a study coordinator to determine the eligibility. The inclusion criteria specified that each person must be 1) at least 18 years old, and 2) in good health with no ongoing pain problems. A person was excluded if he/she 1) was taking psychotropic or hypertension medications, 2) reported to be pregnant, 3) reported to have the following conditions: Sleep disorders, hypertension, seizure disorder, serious skin allergy/sensitivity, multiple sclerosis, diabetes, and HIV positive. |
| Procedures |
| Upon arrival, subjects underwent a consenting process, completed two self-report inventories to assess trait/ personality and received pain testing sessions. |
| Self-report inventories |
| NEO-PI-R: This is a comprehensive psychological personality inventory, a 240-item measure of the Five Factor Model (FFM): Extraversion (E), Agreeableness (A), Conscientiousness (C), Neuroticism (N), and Openness to Experience (O). Additionally, the test measures six subordinate dimensions (known as “facets”) of each of the FFM personality factors [20]. The test was developed for use with adult (age 17+) men and women without overt psychopathology. NEO-PI-R was selected for the study because this inventory provides a comprehensive picture of how different individuals vary on the five “fundamental” personality factors or styles. The internal consistency of the NEO for each scale was found to be high, at: N= .92, E= .89, O= .87, A= .86, C= .90. |
| State-Trait Anxiety Inventory (STAI): The STAI is a 40 item self-report inventory that assesses current (STAI-State) and enduring anxiety as a characteristic of the person (STAI-Trait). Its psychometric properties have been well validated in patient and healthy populations [21,22]. Both types of anxiety were assessed in the present study in part because an earlier study [23] linked reduction of state anxiety to habituation in evoked potentials and in our prior work [24], anxiety turned out be a moderator in constraining pain modulation. |
| Pain testing |
| Each subject was tested individually in a sound-controlled experimental room. The experimental room was adjacent to the control room where all experimental procedures were controlled. A one-way mirror ensured that the subject was in full view of the experimenter at all times. The team operated from a control room that contained all data processing equipment except essential data capture hardware. A Mac G5 running a custom-designed LabView program coordinated and controlled experiments. LabView software controlled events such as warning signals, stimulus delivery and signal sampling. It captured, scored and stored data. The program summarized the data and formatted the record, displaying the data to human observers on command. Our laboratory hardware included a Grass-44 Stimulator with a stimulus isolation unit and National Instruments isolated amplifiers for skin conductance response and respiration recording. |
| After the adaptation period in which the subject sat quietly in the experimental room for 5-10 minutes, the experimenter fitted the subject with fingertip electrodes on the index, middle and ring fingers (the three fingers were used for the experimental objectives associated with later blocks of trials reported in another study [25]; In this present paper, we used the data from the first block of trials). She then placed electrodes for skin conductance on the dominant hand and attached the EEG recording electrodes to the scalp. They were instructed to refrain from blinking during data acquisition in order to protect evoked potential measurement from blink contamination. Both skin conductance and evoked potential responses were continuously recorded, and each trial epoch was defined as between 3 seconds prior to the stimulus and 5 seconds after the stimulus. |
| Apparatus |
| Noxious stimuli were electrically delivered to subjects’ fingertips. The apparatus was a modified version of the widely-used Bromm and Meier stimulation technology [26]. We employed a conventional Bayer Prick Lancetter (item # 8375ZA), designed for allergenic extract skin testing, as the cathode. The lancetter consisted of a flat 3 cm×5 mm steel shaft; at one end a triangular blade with a base of .33 mm extends 1 mm to a sharp point. We fitted the shaft into a specially designed holder to permit electrical contact. After cleaning the fingertip, the experimenter held the lancetter shaft at an oblique angle and inserted the blade into the stratum cornea of the second, third, or fourth digit on the non-dominant hand. The return electrode was a 6 cm×14 cm flat steel plate fitted with 0.5% saline conductive paste in a neutral base and taped to the volar surface of the ipsilateral forearm. The experimenter took care to assure that the blade, once placed, caused no pain. This approach assured a low impedance of stimulating-electrode for all subjects [27]. The stimuli consisted of square pulses of 5-millisecond duration, delivered by a Grass S-44 stimulator and a stimulus isolation unit connected in series. |
| Stimulus calibration |
| Stimulus levels were determined for each individual subject based upon individual pain threshold and tolerance. Each subject received test stimuli of gradually increasing intensities. We set the threshold level to the intensity at which the subject first reported pain and set the tolerance level to the intensity at which the subject indicated that he or she had reached the maximum he or she was willing to tolerate during the study session. Our software then calculated 2 stimulus levels to use in each test condition. We computed stimulus levels as proportions of the intensity range SRange between threshold and tolerance where SRange = STol – SThresh. For the baseline block, we computed S1Test = SRange×0.75 + SThresh and S2Test = SRange×0.55 + SThresh. Baseline condition presented stimuli between 0.55 and 0.75. |
| We were aware that the stimulus parameters do influence the habituation process [28]; previous studies of habituation typically employed a single stimulus intensity level and the use of the same stimulus level typically showed more rapid and potent habituation results. However, we felt that habituation to varying levels of stimuli sampled within a small range, as we employed, would reflect more realistic stimulus environments (more comparable to stimuli people typically encounter in everyday life). Given this, it should be noted that the statistical analysis correctly matches the exact stimulus delivered within and across finger conditions. |
| Presentation of painful phasic stimulation |
| Each trial involved a 5-millisecond fingertip stimulation delivered through a small electrode imbedded in the stratum corneum of a fingertip. Stimulus level and location varied randomly within the block. Inter-trial intervals for the shocks varied randomly from 10 -14 sec. Each subject experienced 32 trials of noxious stimulation during the test session. |
| Dependent variables |
| There were three dependent variables used in the study. |
| Subjective pain report: For each of the three fingers stimulated, we asked subjects to rate the pain they felt in response to the most recent stimulus. The response vehicle was an 11-point scale anchored by: “No Pain at All” at one end and “Strongest Level of Pain” (determined by each subject) at the other. |
| Evoked potential EEG: Change in continuous electroencephalographic (EEG) and pupil diameter signals synchronized with stimulus onsets provided indicators of psychophysiological responses to noxious events. EEG data were collected from a single high impedance electrode placed at vertex (Cz) using an ActiveTwo high resolution biopotential EEG acquisition system (BioSemi Instrumentation, Amsterdam, Netherlands). This system provides 24 bit analog-to-digital conversion per channel with exceptional signal to noise ratio and linearity. The conditioned signal was band pass filtered at 60 Hz, and single trial signals 100ms before and 500ms after a stimulus event were identified. For each subject, a grand average of single trials was inspected to locate characteristic latencies for identifying negative (N150) and positive (P250) EEG peaks. Applying the identified grand average latencies, we manually inspected each single trial to identify the local minimum negative and maximum positive amplitude peaks corresponding to the single trial N150 and P250 amplitudes, respectively. The peak-to-peak amplitudes provided single trial stimulus evoked potential (SEP) values. |
| Skin conductance response (SCR): We collected continuous skin conductance measures using two silver/silver chloride recording electrodes placed at the proximal thenar and hypothenar eminences of the dominant (non-stimulated) hand, with an ActiveTwo high resolution biopotential acquisition system (BioSemi Instrumentation, Amsterdam, Netherlands). The SCR is the tonic level of the electrical conductivity of the skin. In addition, we measured respiration cycle with a belt and electrocardiography (ECG) using electrodes placed over the left and right supraclavicular spaces. The conditioned and amplified signal was acquired and sampled at 256 hz. For each subject, single trials, identified as the segment beginning 500 ms before and continuing 4 seconds after a stimulus event, were averaged and manually inspected to identify pilot baseline and peak latencies (usually around 3.5 seconds post-stimulus). Using the pilot latencies as a guide, an in-house software routine identified local baselines and maximum peaks for single trials. The calculated difference in baseline and peak amplitudes provided the single trial SCR values for subsequent analysis. |
| Data Analysis |
| Step one |
| To investigate the degree of habituation or sensitization, we employed mixed effects models that allowed for linear and quadratic relationships with trial number, conditional on applied stimulus level (adjusted for personal threshold and tolerance). Finger was also represented as a categorical factor, even though the experimental procedure should eliminate this source of variation. Under this model, the linear and quadratic regression coefficients for trial convey the degree and sign of habituation/sensitization. The trial regression coefficients, adjusted in this manner, are proxy measures for more fundamental psychophysiological and biological mechanisms that cannot be directly observed. Of necessity, the regression coefficients for “trial” vary from positive to negative along a single mathematical dimension. For linguistic convenience, we refer to this mathematical continuum as the “H-S” (for Habituation-Sensitization) dimension, even though the biological mechanisms of habituation and sensitization are almost certainly distinct. Positive linear coefficients, for example, indicate sensitization (greater response over time for matched stimulus level) while negative linear coefficients indicate habituation (lesser response over time for matched stimulus level). To allow for individual variation, we treated the linear and quadratic coefficients as random effects in the model, providing a unique term for each individual. It is possible, therefore, for some individuals to experience sensitization even when the population average reveals habituation, and viceversa. The models also incorporate random intercept terms, allowing for lack of statistical independence in repeated measures of the same individuals. |
| To document individual H-S variability around population averages, we first fit, for each measure, an unconditional model with no explanatory covariates. The fixed effects coefficients for trial from these models describe the population average H-S trend. The variances (and standard deviations) of the random effects quantify the systematic (i.e., distinct from random error) individual variability about these average trends. The standard deviation of the individual variability represents the typical deviation of an individual’s trend from the population average trend. We also report the correlations among the individual trend lines for the three outcome measures. For each measure, we present the proportions of subjects habituating (negative adjusted rate-of-change) and sensitizing (positive adjusted rate-of-change). The rates-of-change are slopes in units of expected change in the dependent variable per unit change in trial. To provide a more realistic scaling for H-S interpretation, we convert the slope measures to expected change over the course of the entire block by multiplying the coefficient (and its standard deviation) by 32, the number of trials in the block. This conversion is a linear transformation that has no effect on statistical association or inference, but merely renders the coefficients more interpretable. |
| Step two |
| Next we include personality and anxiety covariates in the regression models to evaluate how much of the individual H-S variability they can explain, singly and collectively. Using a backwards elimination strategy, we first test whether the models require the complexity of the quadratic trial coefficients. Then, keeping all random effect specifications fixed, we removed at each step the covariate having the highest (least significant) trial-by-covariate p-value, continuing until all remaining trial-by-covariate interactions are significant. Next we similarly removed the covariate main effects in order, until all remaining covariate main effects were significant (with the exception that a non-significant main effect is retained if its trial-by-covariate effect is significant). The resultant conditional model is the best-fitting explanation of the population and individual H-S effects. |
| Of greatest substantive interest are the covariate-by-time interaction effects, our proxy measures of H-S. Mixed effects models provide estimates of both population average (fixed) and individual (random) effects. The fixed effects covariate-by-time, or slope, coefficient estimates the expected population change in the response per unit change in time adjusted for calibrated stimulus intensity. This coefficient is therefore the population average H-S. For each significant covariate-by-time interaction, we report a corresponding R2 statistic [29] that captures the degree of association between the predictor effect and the set of repeated measures of the dependent variable. The covariates may explain some of the individual variability quantified in Step One. The percentage reduction in individual (random effects) variability accounted for by the covariates is known as the “pseudo-R2” statistic [30], which we also report for each significant covariate-bytime interaction. Taken together, these interpretations help to provide an understanding of the practical importance of the covariates in explaining and predicting experimental habituation and sensitization. |
| Multivariate models provide another level of interpretation, that of the correlations among the random effects across individuals. Each random effect slope coefficient estimates a point for one individual on the unique H-S continuum, or factor, for one response measure. The H-S factors may themselves correlate in the population of individuals. A high correlation indicates that an individual having a relatively high (low) value on the H-S factor for one measure is also likely to have a relatively high (low) value on the H-S factor of the other measure. Note that relative position is defined with respect to the population average, so it is possible for individuals to have relatively similar positions even when the numeric signs of the mean population H-S differ (one showing mean habituation and the other showing mean sensitization). Means and covariances (correlations) are independent under the multivariate normal distribution assumption of mixed effect linear models. |
| All analyses are conducted within a mixed-effects linear model framework, using SPSS 18.1 (IBM, Armonk, NY, USA) and SAS 9.2 (SAS Institute, Cary, NC, USA), under full maximum-likelihood estimation with conservative Satterthwaite degrees of freedom for statistical tests. |
| Results |
| Descriptive results |
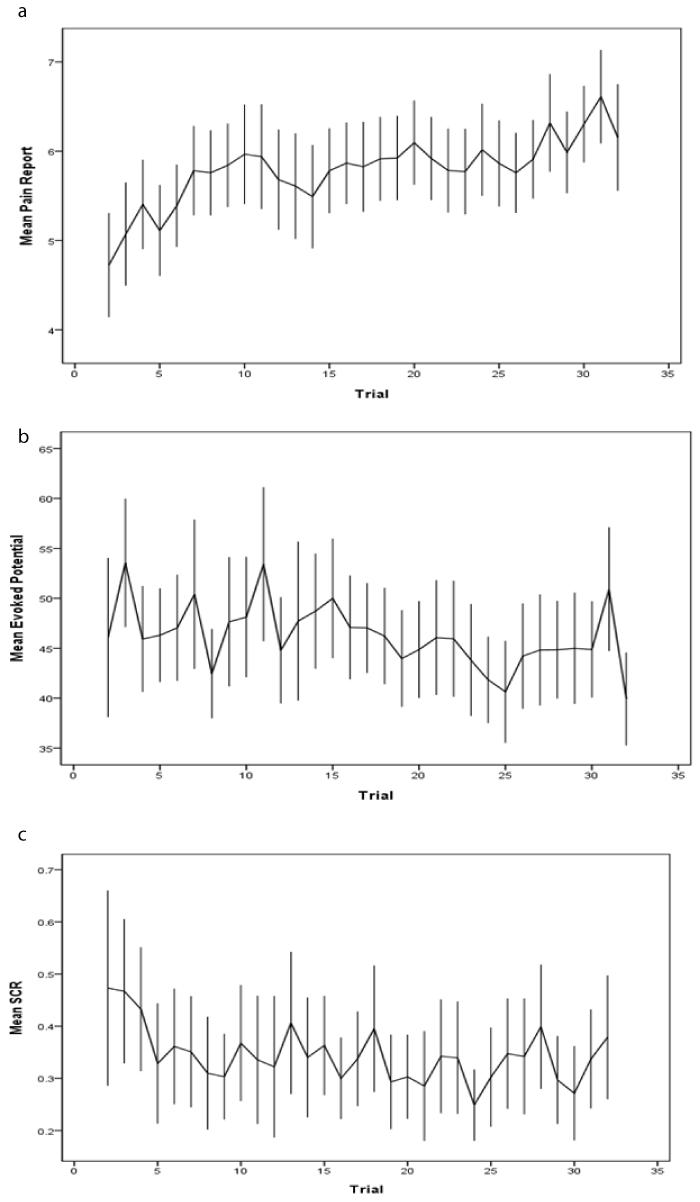
| Figure 1a-c show the mean change and 95% confidence intervals with trial for each of the three response measures. Note that the average trend for pain report indicates moderately strong sensitization, while evoked potential and SCR reveal weak average habituation. (Similar plots adjusted for stimulus intensity appear very similar, and are not shown.) |
| Analytic results |
| Unconditional Models Key results appear in (Table 1), organized by dependent variable. Quadratic coefficients were not significant and were removed from the analysis models. Unconditional models are those providing the best linear fits, under a maximum likelihood criterion, for each individual, adjusted for individually calibrated finger-specific sensitivities. |
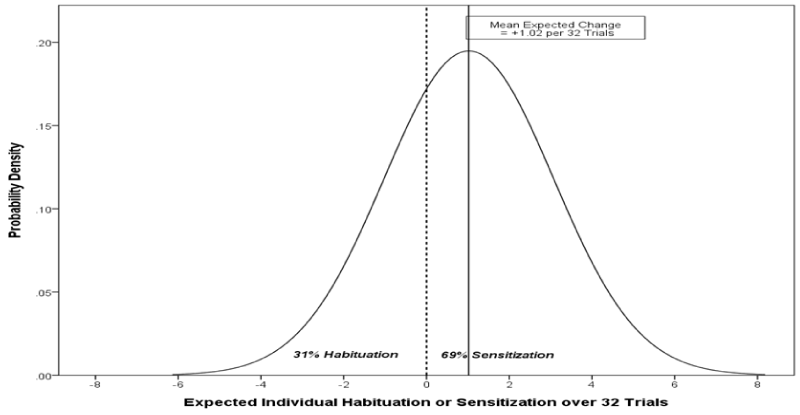
| For Pain Report, the mean linear trend is positive, indicating average sensitization of .032 pain report units per trial. Expressed as change over the entire 32-trial block, this is equivalent to an increase in average sensitivity of 32*.032 = 1.02 pain report units. But this effect is far from uniform, since individuals differ significantly in their adjusted rates of change. Scaling again to the 32-block trial, the standard deviation of change is 32*.064 = 2.05. Assuming normally distributed effects, 68% of subjects in the population would have H-S effects over the 32 trials ranging between 1.02 ± 2.05 pain units, or between changes of -1.03 and 3.07 on the 0-10 scale. Similarly, 95% of subjects would have H-S effects between 1.02 ± 4.10 pain units (between -3.08 and 5.12) over the course of the 32 trials, independent of stimulus intensity. Of note, the average rate of change, a sensitization of 1.02 pain report points, poorly represents the diversity of individual responses. In fact, 31% of subjects in the population would habituate, rather than sensitize, to repeated stimulation. Figure 2 represents the inferred population H-S distribution for Pain Report over the 32 trials. The strong central line marks the population average change of +1.02 pain points (sensitization), with the other vertical line dividing the distribution into sensitizers and habituators, at roughly 7:3 odds. |
| It is a common misconception that all individual variability about an average, such as the central mean trend in Fig 2, is in some sense “error.” This is not the case. Figure 2 is a theoretical population histogram, depicting the best estimation of systematic differences in individuals. Intercept and slope variances summarize diversity in systematic attributes of individuals in the inferred population. “Error” in mixed effects models of longitudinal change pertains instead to within-person deviations from the unique systematic trend characterizing each individual. The model assumes that random within-person error occurs on each trial, contributing to the measured observations for each person. The variance of this error, which includes error of estimation and measurement error in the dependent variable, appears in the first row of Table 1. All other rows in the Table pertain to systematic differences separate from the within-person error variance. |
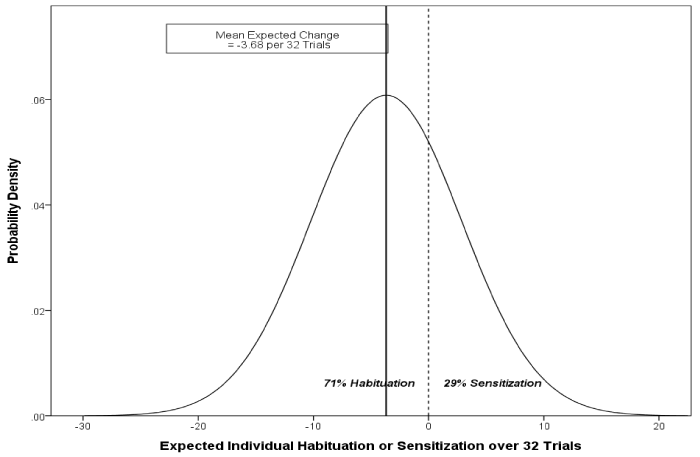
| For evoked potential (EP), (Table 1) indicates that the population H-S trends are negative, denoting mean habituation. The mean linear trend is negative, indicating average habituation of .115 EP units per trial. Expressed as change over the entire 32-trial block, this is equivalent to a decrease in average sensitivity of 32*.115 = 3.68 EP units. But once again, this effect is far from uniform, since individuals differ significantly in their adjusted rates of change. Scaling again to the 32-block trial, the standard deviation of change is 32*.205 = 6.56. Assuming normally distributed effects, 68% of subjects in the population would have H-S effects over the 32 trials ranging between -3.68 ± 6.56 EP units, or between changes of -10.24 and 2.88 on the EP units. Similarly, 95% of subjects would have H-S effects between -3.68 ± 13.12 EP units (between -16.80 and 9.44) over the course of the 32 trials, independent of stimulus intensity. Of note, the average rate of change, a habituation of 3.68 EP units, poorly represents the diversity of individual responses. In fact, 29% of subjects in the population would sensitize, rather than habituate, to repeated stimulation. Figure 3 represents the inferred population H-S distribution for EP over the 32 trials. The strong central line marks the population average change of -3.68 EP units (habituation), with the other vertical line dividing the distribution into habituators and sensitizers, at roughly 7:3 odds. |
| For SCR, (Table 1) indicates that the population H-S trends are negative, denoting mean habituation, suggesting that SCR changed in the same way as EP did. The mean linear trend is negative, indicating average habituation of .0035 SCR units per trial. Expressed as change over the entire 32-trial block, this is equivalent to a decrease in average sensitivity of 32*.0035 = .112 SCR units. But this effect is far from uniform, since individuals differ significantly in their adjusted rates of change. Scaling again to the 32-block trial, the standard deviation of change is 32*.011 = .352. Assuming normally distributed effects, 68% of subjects in the population would have H-S effects over the 32 trials ranging between -.112 ± .352 SCR units, or between changes of -.464 and .240 on the SCR scale. Similarly, 95% of subjects would have H-S effects between -.112 ± .704 SCR units (between -.816 and .592) over the course of the 32 trials, independent of stimulus intensity. Of note, once again, the average rate of change, a habituation of .112 SCR units, poorly represents the diversity of individual responses. In fact, 37% of subjects in the population would sensitize, rather than habituate, to repeated stimulation. The inferred population H-S distribution for SCR (not shown here) over the 32 trials looks similar to that for EP, with the population average change of -.112 SCR units (habituation). |
| Conditional models |
| The unconditional models detail the systematic individual variance in slopes and intercepts. Some of this variance may be predictable from a set of covariates. Table 1 Reports the particular covariates that emerged as significant predictors of H-S in the Step Two analyses. Non-significant correlations with covariates are not shown in Table 1. |
| For Pain Report, NEO-N and NEO-C had independent significant contributions. The positive sign of the coefficients indicates that higher values for these two personality factors predicted higher, or more sensitized, response patterns, with a one-unit increase in NEO-N and NEO-C predicting .000878 and .000820, respectively, higher rate-of-change in units of Pain Report change per trial. Even after multiplying by 32 to convert to a per-block basis, these units are exceedingly difficult to interpret, reflecting both the raw data scaling of Pain Report and the covariates. The associated R2β values, .061 and .057, for NEO-N and NEO-C, respectively, provide more familiar context, falling in the “small-to-medium” effect size range according to common rules of thumb [31]. The Pseudo-R2 statistic is the proportion, 8.2%, of systematic individual variability in H-S accounted for by both covariates together. The remaining 91.8% of individual variability constitute individual differences in H-S that are systematic, but as yet unexplained. |
| For EP, State Anxiety had large and significant contributions. The positive sign of the coefficient indicates that a higher value for this anxiety state measure predicted a higher, or more sensitized, rate-of response, with a one-unit increase in State Anxiety predicting .0194 higher rate-of-changes in units of EP change per trial. The associated R2β value of .087 again falls into the “small-to-medium” effect size range. The Pseudo-R2 statistic is the proportion, 32.2%, of systematic individual variability in H-S accounted for by this covariate. Knowing State Anxiety therefore produces a large proportionate reduction in the unexplained systematic variability in individual EP. The remaining 67.8% of individual variability constitute individual differences in H-S that are systematic, but as yet unexplained. |
| For SCR, NEO-O had significant contributions. The positive sign of the coefficients indicates that a higher NEO-O values predicted a higher, or more sensitized, response, with a one-unit increase in NEO-O predicting .000175 higher rate-of-change in units of SCR change per trial (fixed effects R2β = .058). The Pseudo-R2 statistic for SCR was quite small, however, (.009), indicating that almost all (99.1%) of the systematic individual differences in H-S trend in SCR remained unexplained. Taken together, the SCR results indicate that NEO-O is a reasonable predictor of population H-S trend, reduces the uncertainty in individual SCR levels (Pseudo-R2 for Intercept=.092), but fails to account for individual variation in H-S trend over time. |
| Multivariate analyses revealed that the three H-S factors were positively inter-correlated: +.18 between Pain Report and EP, +.13 between Pain Report and SCR, and +.94 between EP and SCR. An individual with high (low) relative H-S on EP would be very likely to have high (low) relative H-S on SCR. An individual with high (low) relative H-S on Pain Report would be likely to have slightly above- (below-) average H-S on EP and SCR. Given the discrepant mean H-S trends between Pain and the other two measures, it is quite possible for an individual with relatively high Pain H-S (an extreme sensitizer) to have slightly above-average H-S values that are somewhat above the EP and SCR mean H-S trend yet still negative in sign (slight habituation). |
| Discussion |
| The present study provides empirical support for the presence of diverse response styles to repeated noxious stimulations in healthy adults. One might expect that, in the absence of actual tissue damage, healthy people would habituate to noxious stimulations, as shown in some earlier studies [2,3,4]. However, our results show that, on average, people showed amplifications of pain report over trials, while both EP and SCR responses showed attenuation, suggesting habituation. The response patterns are similar to what Rhudy et al. found recently [19]. Furthermore, the incongruent patterns appear to exist within individuals. As Rhudy et al. discussed [19], it is possible that differential modulatory systems of these channels may contribute to the discrepancy in the response patterns. It is also possible that the process and expression of habituation is highly variable, possibly due to individual differences in how we process intraceptive and extraceptive stimuli. |
| Our study, however, took a step forward to investigate the relationships among different output channels within individual, which showed a more consistent pattern. That is, although the group means showed inconsistency between self-report pain pattern (sensitization) over trial and psychophysiological indices (habituation), within a person, the patterns typically were convergent. EP and SCR showed stronger positive correlation than they did with pain report, but the directionality was all positive among them. This suggests that overall tendency to either habituate or sensitize to repeat noxious stimulations are akin to a trait, specific to a person (i.e., an attribute of an individual). |
| Our results further suggest that different aspects of personality styles contribute to the different aspects of habituation/sensitization. Neuroticism and Conscientiousness traits both significantly contributed to the variance of the amplification of pain report, whereas it was State Anxiety for EP variance and Openness trait for SCR variance. Granted, the variances were small to modest (up to 32% for EP), but if we consider the complexity of habituation and sensitization processes, it seems quite impressive that personality traits may help explain the variance at those proportions. |
| We would like to emphasize, however, that our results do not indicate that personality traits explain clinical pain syndromes characterized by central sensitization. Our results are limited to relatively innocuous and safe laboratory pain experiments in healthy individuals, far less complex than chronic pain conditions. What our results indicate, however, is the importance of taking the individual psychosocial characteristics into consideration when trying to understand how humans respond to pain. In this regard, we may need to clarify how personality traits and cognitive styles can interact with mechanisms underlying central sensitization and attenuated habituation in chronic pain patients. |
| Inability to habituate to noxious stimulation seems to be common among people with chronic pain conditions. Given the available data, at this point we can only speculate about the meaning of this association. Habituation is considered to have an adaptive role for an organism by decreasing the amplitude of the response of the sensory cortex to repeated presentations of similar stimuli, to avoid over-stimulation [32]. Without this protective function, noxious events might wreak chaos within the central nervous system. |
| We were somewhat surprised that it was state anxiety, rather than trait anxiety, that emerged as a significant predictor of the EP-expressed H-S dimension. The results, however, may be consistent with previous research. State anxiety is known to increase anxiety, whereas the effect of trait anxiety has conflicting effects on pain [33,34]. State anxiety may also have specific effects on pain-related EP. In previous research, when anxiety was suppressed with a benzodiazepine, EP response to painful stimulation was attenuated while there was no change in pain report [23]. Thus, our results suggest that the anxiety state of a person may influence how EP response habituates or sensitizes when noxious stimuli are repeated. |
| The interesting question is whether and how the inability to habituate may interact with sensitization. From the behavioral response perspective, habituation and sensitization place themselves at the opposite ends of the same spectrum, with decreased response in one end and increased response in the other. However, the experimental literature generally supports the dual-process theory of habituation-sensitization in that the two processes, though not mutually exclusive, can co-occur in response to stimuli, possibly with a separate CNS pathway for each process, and the relative strengths of these parallel divergent processes determine the final response [35]. If we take this approach and assume that the observed responses to repeated stimulations reflect a balance between the two processes of sensitization and habituation, we would be led to ask what mechanisms are responsible for the dysregulation of this balance in chronic pain syndromes. Is it the lack of habituation that pushes the sensitization process to a pathologically escalated level? |
| Perhaps, in order to answer these questions, we may need to take intra-individual patterns of pain response into account. Our results strongly suggest that even healthy people without pain problems vary greatly in their responses to repeated noxious stimulations. It is possible that each person has a trait-like pattern of response profile which, under relatively normal circumstances, leads to varying degrees of habituation or sensitization to repeated noxious stimulations. When the response becomes overwhelmingly sensitizing and unable to habituate, the particular pattern may reflect pathological pain processing or may put a person at risk of developing chronic pain. |
| There are several obvious limitations of the present study that should be noted. First, the study was exploratory in the sense that only limited numbers of personality factors were examined in the study. Second, the pain protocol used in the study has been extensively used in prior psychophysiological research, but it may not have been most optimal to document some experimentally induced phenomena involving sensitization and temporal summation. Nonetheless, findings from the present study support the rationale for recognizing the importance of fundamental individual differences in basic pain processes. |
| In studying individual differences, we wish to advocate a two-step approach: first, research should document the extent of individual variability using a mixed effects model, and second, research should account for variations in terms of other explanatory variables (such as personality styles or other contextual factors) that are proactively included in data collection. Explaining why people react differently to comparable painful stimuli would be an indispensable contribution of the human laboratory research, as this could offer some meaningful insight for working clinicians who are forced to deal with unmistakable yet daunting differences exhibited by clinical pain patients. |
| The results from our study, we believe, provide a preliminary basis for arguing that personality and psychological traits of a person has a substantial role in determining how we process noxious stimuli. The present study may serve as the tiny first step toward considering the measurement of personality and psychological traits as a valuable part of ecologically meaningful, comprehensive clinical assessment of patients who suffer from pain. Personality should be seen as a context for modulating pain experience, and as such, a comprehensive evaluation of clinical pain patients should include ways to understand relevant personal styles and traits of an individual patient. |
| Acknowledgements |
| The study reported was supported by National Institutes of Health (NIH) Award R01 NS046230 (A Dynamic Constructivist Model of Placebo Analgesia) to the first author, from the National Institute of Neurological Disorders and Stroke (NINDS) and the National Center for Complementary and Alternative Medicine (NCCAM). NINDS and NCCAM had no role in the conduct of the study, collection, management, analysis and interpretation of the data, or preparation, review or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS or NCCAM. |
References
- Meyer RA, Cam pbell JN (1981) Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science 213: 1527-1529.
- Condes-Lara M, Calvo JM, Fernandez-GuardiolaA (1981) Habituation to bearable experimental pain elicited by tooth pulp electrical stimulation. Pain 11: 185-200.
- GLASER EM, HALL MS, WHITTOW GC (1959) Habituation to heating and cooling of the same hand. J Physiol 146: 152-164.
- LeBlanc J, Potvin P (1966) Studies on habituation to cold pain. Can J PhysiolPharmacol 44: 287-293.
- Greffrath W, Baumgärtner U, Treede RD (2007) Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain 132: 301-311.
- Rennefeld C, Wiech K, Schoell ED, Lorenz J, Bingel U (2010) Habituation to pain: further support for a central component. Pain 148: 503-508.
- Bingel U, Schoell E, Herken W, Büchel C, May A (2007) Habituation to painful stimulation involves the antinociceptive system. Pain 131: 21-30.
- Bingel U, Herken W, Teutsch S, May A (2008) Habituation to painful stimulation involves the antinociceptive system--a 1-year follow-up of 10 participants. Pain 140: 393-394.
- de Tommaso M Federici A, Santostasi R, Calabrese R, Vecchio E, et al. (2011) Laser-evoked potentials habituation in fibromyalgia. J Pain 12: 116-124.
- Peters ML, Schmidt AJ, Van den Hout MA (1989) Chronic low back pain and the reaction to repeated acute pain stimulation. Pain 39: 69-76.
- Valeriani M, de Tommaso M, Restuccia D, Le Pera D, Guido M, et al. (2003) Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study. Pain 105: 57-64.
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, et al. (2004) Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 50: 613-623.
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, et al. (2009) Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 61: 1226-1234.
- Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, et al. (2009) widespreadhyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995-1001.
- Staud R, Nagel S, Robinson ME, Price DD (2009) Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 145: 96-104.
- Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, et al. (2008) Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain 140: 420-428.
- Rodriguez-Raecke R, Doganci B, Breimhorst M, Stankewitz A, Büchel C, et al. (2010) Insular cortex activity is associated with effects of negative expectation on nociceptive long-term habituation. J Neurosci 30: 11363-11368.
- Doganci B, Breimhorst M, Hondrich M, Rodriguez-Raecke R, May A, et al. (2011) Expectations modulate long-term heat pain habituation. Eur J Pain 15: 384-388.
- Rhudy JL, Bartley EJ, Williams AE (2010) Habituation, sensitization, and emotional valence modulation of pain responses. Pain 148: 320-327.
- Costa PTJ, McCare RR (1992) NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources, InC.
- Spielberger CD, Gorsuch RL, Lushene RE (1970) STAI Manual. Palo Alto, CA:Counsulting Psychologist Press.
- Stanley MA, Beck JG, Zebb BJ (1996) Psychometric properties of four anxiety measures in older adults. Behav Res Ther 34: 827-838.
- Zaslansky R, Sprecher E, Katz Y, Rozenberg B, Hemli JA, et al. (1996) Pain-evoked potentials: what do they really measure? ElectroencephalogrClinNeurophysiol 100: 384-391.
- Bradshaw DH, Donaldson GW, Jacobson RC, Nakamura Y, Chapman CR (2011) Individual differences in the effects of music engagement on responses to painful stimulation. J Pain 12: 1262-1273.
- Nakamura Y, Donaldson GW, Kuhn R, Bradshaw DH, Jacobson RC, et al. (2012) Investigating dose-dependent effects of placebo analgesia: a psychophysiological approach. Pain 153: 227-237.
- Bromm B, Meier W (1984) Theintracutaneous stimulus: a new pain model for algesimetric studies. Methods Find ExpClinPharmacol 6: 405-410.
- Chapman CR, Nakamura Y, Donaldson GW, Jacobson RC, Bradshaw DH, et al. (2001) Sensory and affective dimensions of phasic pain are indistinguishable in the self-report and psychophysiology of normal laboratory subjects. J Pain 2: 279-294.
- Groves PM, De Marco R, Thompson RF (1969) Habituation and sensitization of spinal interneuron activity in acute spinal cat. Brain Res 14: 521-525.
- Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O (2008) An R2 statistic for fixed effects in the linear mixed model. Stat Med 27: 6137-6157.
- Singer JD, Willett JB (2003) Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press. 672 p
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences (second ed.): Routledge Academic. 567 p.
- Thompson R, Spencer W (1966) Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73: 16-43.
- Arntz A, de Jong P (1993) Anxiety, attention and pain. J Psychosom Res 37: 423-431.
- Tang J, Gibson SJ (2005) A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain 6: 612-619.
- Groves PM, Thompson RF (1970) Habituation: a dual-process theory. Psychol Rev 77: 419-450.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 15237
- [From(publication date):
September-2014 - Jul 30, 2025] - Breakdown by view type
- HTML page views : 10601
- PDF downloads : 4636
