Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Phosphorus storage in Microorganisms: Diversity and Evolutionary Insight
| Tatiana Kulakovskaya* | |
| Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Russia | |
| *Corresponding Author : | Tatiana Kulakovskaya Skryabin Institute of Biochemistry and Physiology of Microorganisms Russian Academy of Sciences, pr. Nauki 5 Pushchino, Moscow region, 142290 Russia E-mail: alla@ibpm.pushchino.ru |
| Received January 16, 2015; Accepted January 16, 2015; Published January 23, 2015 | |
| Citation: Kulakovskaya T (2015) Phosphorus storage in Microorganisms: Diversity and Evolutionary Insight. Biochem Physiol 4:e130. doi:10.4172/2168-9652.1000e130 | |
| Copyright: © 2015 Kulakovskaya T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
| Introduction |
| Phosphorus, a vital cell element, was undoubtedly involved into the earliest stages of life origin on the Earth [1-6]. |
| Inorganic polyphosphate was proposed to be an ancient molecule performing energy functions in primary living cells [4,6]. The opinion that polyphosphate formed as a result of geothermal activity could be used by primitive kinases for the ancient transphosphorylation processes was supported by several researchers [1-7]. Modern hypothesis modeling life origin suggests the participation inorganic polyphosphate and pyrophosphate in energy transduction and membrane transport in progenote metabolic pathways [8,9]. |
| Phosphorus deficiency suppresses the growth and development of microorganisms, while their excess has a negative effect on regulation of phosphate metabolism. The intracellular content of Pi is strictly regulated. Microorganisms living in the varying environment have mechanisms of adaptation to phosphate deficiency and excess. One of such mechanisms is the Pi transport systems with different affinity and mechanism of action. An another pathway of microbial adaptation to the changes in phosphorus accessibility in the environment is the formation of reserve phosphorus compounds, which are accumulated or utilized under excess or deficiency of phosphorus sources in the medium, respectively. These compounds are of diverse chemical nature and not only play the role of relatively inert phosphorus reserves in a microbial cell but also perform structural and regulatory functions. |
| Phosphorus Storage Compounds in Microorganisms |
| The simplest reserve of phosphorous compounds of microorganisms is low-soluble phosphates: MgPO4OH·4H2O formed in the halophilicarchaea, Halobacterium salinarium and Halorubrum distributum [10,11] and NH4MgPO4·6H2O formed in bacteria of the genus Brevibacterium [11] and Acetobacter xylinum [12]. |
| The archaea H. salinarium and H. distributum concentrate phosphate (Pi) from aqueous solutions during their growth and at Pi, excess its considerable part accumulates in the biomass [10,11]. The Pi content in the biomass of both archaea at Pi, excess is nearly 10-folds higher than that of inorganic polyphosphate. The massive accumulation of Pi leads to the changes in the cell morphology and cellular lysis [10,11]. However, after reinoculation into Pi-deficient medium, the cells pregrown under Pi excess give more biomass yieldthan the Pi–starved cells [10,11]. This fact confirms the hypothesis that both intracellular and extracellular Pi as a low-soluble salt performs the function of phosphate reserve for the cell population. |
| Reservation of phosphate as low-soluble salts was also revealed in several species of Brevibacteria, which during their growth almost completely consumed Pi from the medium at its concentration of about 11 Mm [11,12]. In contrast to the archaea, Brevibacteria demonstrated intracellular accumulation of Pi. The reserve phosphorous compoundwas extracted from the B. antiquum cells by high-pressure extrusion and identified as NH4MgPO4·6H2O [11]. |
| The accumulation of Pi in Brevibacteria cells was accompanied by cell shape changes, the appearance of electron-dense zones in cytoplasm and cell wall, and cell wall thickening [11]. It seems that cell wall thickening allows these bacteria, in contrast to halophilic archaea, to remain intact in spite of the high degree of mineralization. |
| The cyanobacterium Microcoleus chthonoplastes accumulated polyP in the cells up to 1.4% P/g dry biomass when Pi concentration was increased to 0.55 mM; its increase to 1.2 mM resulted in Pi precipitation on the mucous sheaths of the cells and their mineralization [13,14]. The mineral sheaths of cyanobacteria contain phosphorus and calcium [13]. The increase in Pi concentration to 2.5 mM resulted in trichomes mineralization and death. Degradation of the natural cyanobacterial mat is accompanied by destruction of these structures, and Pi released into the medium is sufficient for surviving cyanobacteria [14]. This process is generally similar to mineralization in the culture of halophilic archaea described above. |
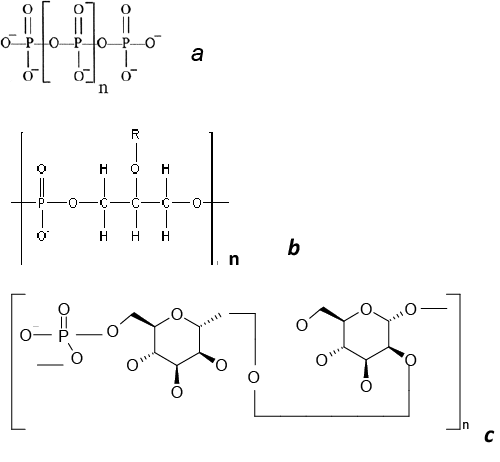
| In most microorganisms, the role of phosphate reserve is performed by inorganic polyphosphates (polyP), the linear polymers of orthophosphoric acid, containing 3 to several hundred phosphate residues (Figure 1a) [5]. Poly P, being polymers, have no effect on osmotic pressure and simultaneously are an energy reserve, because the energy of their phosphodiester bond is the same as in ATP molecule. The role of polyP as a phosphate reserve has been proved for many microorganisms belonging to different taxa, from archaea to fungi [15-17]. The amount of these polymers is lower under phosphate starvation and higher with sufficient phosphate content in the medium. PolyP is rapidly consumed under phosphate starvation even in E. coli characterized by low polyP reserve [18]. Some bacteria accumulate unusually high polyphosphate levels. Poly P was up to 30% of dry biomass in the bacterium A. johnsonii under Pi excess [19]. Corynebacterium glutamicum accumulates up to 600 mM Pi in the cytosol as polyP, and polyP granules may be up to 37% of the cell volume [20]. Bacteria belonging to the genera Mycobacteria and Corynebacteria accumulate a lot of polyP as cytoplasmic granules [4,20]. It seems that the high ability to accumulate polyP is associated energy function of these polymers. In addition to polyphosphate kinase, the key enzyme of polyP synthesis in prokaryotes [21], Mycobacteria and Corynebacteria possess the enzymes providing the direct consumption of polyP energy for substrate phosphorylation, such as polyphosphate glucokinase [22,23], NAD kinase [24,25], fructose and mannose kinases [26]. In most of the yeast species studied in this respect, the basic reserve phosphorous compound is inorganic polyphosphates [4]. In the typical case of cultivation in a complete medium with excess Pi (20 mM), the cells of S. cerevisiae accumulate little Pi (~ 94 μmole P/g dry biomass) and much polyP (~ 658 mole P/g dry biomass) [27]. PolyP with the chain lengths of 3–8 to 200–260 phosphate residues were obtained from yeasts [27]. PolyP has been found in yeasts in the most of cell compartments [28]. Pideficiency in the medium causes a decrease in the polyP level in S. cerevisiae cells [29]. However, even phosphate-starved cells maintain a low but quite reliable level of polyP [29]. The Pi-prestarved cells of S. cerevisiae transferred into a complete medium accumulate more polyP than the cells growing in the complete medium, i.e., there is a phenomenon of hypercompensation, or “phosphate overplus” [29], which is also known for bacteria [4]. Some yeast species accumulating considerable amounts of polyP were isolated from wastewaters containing excess phosphate: Candida humicola [30], Hansenula fabiani and Hansenula anomala [31]. The cells of many yeast species accumulate high polyp levels under nitrogen starvation [32]. |
| In some microorganisms organic phosphorus reserves were revealed. Teichoic acids (polymeric compounds of the cell walls of Gram-positive bacteria) consist of repeating polyol or glycosylpolyol residues linked by phosphodiester bonds (Figure 1). These polymers are involved in bacterial cell morphogenesis, regulate the activity of autolysins, and participate in the processes of adhesion and regulation of the ionic composition of the cell wall [33]. These polymers may contain up to 30% of total phosphorus of the cells and are consumed in a Pi- deficient medium [34]. The addition of teichoic acid into a phosphatelimited cultivation medium stimulated the growth of Bacillus subtilis [34]. Hence it was supposed that one of the functions of teichoic acids is phosphate reservation. It has been shown that B. subtilis strains with point mutations in the genes coding for the enzymes of teichoic acid biosynthesis are unviable under phosphate-limiting conditions [35]. |
| The yeast Kuraishia (Hansenula) capsulata on the medium with excess phosphate accumulates extracellular phosphomannan (Figure 1) [36]. Its amount decreases at lower Pi concentrations in the medium [37]. Further evidence of the reserve role of this polymer is the ability of this yeast to utilize phosphomannan from the medium under phosphate starvation [38]. Phosphate storage compounds in microorganisms are more often mineral compounds. Organic phosphorous reserve compounds occur rarely. |
| Microbial Phosphate Storage in Nature and Technogenic Environment |
| The concentration of Pi in the natural water reservoirs, including the ocean, is usually too low to provide the primary formation of calcium phosphates from solution (nucleation) [39]. Microorganisms are primarily responsible for assimilation and remineralization of phosphorus in the ocean [39-41]. Many marine microorganisms are able to concentrate Pi as intracellular polyP when oxygen is available (in surface water layers). It is followed by utilization of the polyP as an energy source under anaerobic conditions (in bottom sediments), release of Pi, increase in its local concentration, and precipitation of apatite in calcium-rich seawater [39-41]. Such release and hydrolysis of polyP may occur after cell death in the bottom sediments. Such processes are provided by marine bacteria belonging to the genera Pseudomonas and Acinetobacter [39], as well as the sulfide-oxidizing bacteria Beggitoa and Thiomargarita [42-44] that form bacterial mats. Beggitoa and Thiomargarita accumulated polyP in the presence of sulfur and nitrate [44]. At higher sulfide concentrations and under oxygen deficiency, polyP in the Beggitoa cells was depolymerizedand Pi was released into the medium [44].Diatoms are also capable of polyP accumulation [45]. PolyP granules found in the bottom sediments are similar in size to those found in diatoms. It is supposed that the accumulated polyP enters bottom sediments after the death of diatom cells and destruction of their silicate cell walls; then Pi is released by the alkaline phosphatase localized on the cell surface [45]. The novel genetic and bioinformation approaches made it possible to ascertain the broad distribution of the ppk1 and ppk2 genes coding for polyphosphate kinases and the ppx gene coding for polyphosphatase among marine oligotrophic microorganisms living under Pi deficiency [46]. These data are the evidence in favor of the global spreading of phosphorus concentration as polyP by microorganisms in the world ocean. |
| The wastewater treatment plants are technogenic econiches in which phosphate accumulation by microorganisms is a basic approach to the so-called Enhanced Biological Phosphorus Removal (EBPR) [47-50]. The role of polyP accumulation by sludge bacteria during wastewater purification from excessive phosphates was proved relatively long ago [51]. In the treatment facilities successively used in some countries, the content of Piin wastewaters is minimized due to activated sludge microorganisms. The microbiota of activated sludge consists of various species and phosphate absorption depends on many factors including the composition of microbial associations and wastewater composition. Wastewater purification from phosphate needs the alternation of anaerobic and aerobic conditions, which is achieved most often via the serial arrangement of anaerobic and aerobic zones in a series of flowthrough systems, with sludge returning into the cycle. At the anaerobic stage, the activated sludge bacteria take up the organic substrates of wastewaters. Intracellular polyP is used as an energy source, while Pi is released into the medium. Such conditions favor the accumulation of poly hydroxybutyrate (PHB) and other poly hydroxyalkanoates (PHA). It is considered that the bacteria accumulating large amounts of polyP have a selective advantage in the anaerobic zone. In the aerobic zone, PHA is hydrolyzed, ATP is synthesized, and sludge consumes more Pi than has been released at the previous aerobic stage. Pi scavenged from wastewaters accumulates in bacterial cells as a large amount of polyP. EBPR water treatment plants are a unique technogenic ecological niche, the peculiarities of which are determined just by the presence of anaerobic and aerobic zones, with different bacterial species or associations gaining selective advantage in each of them. |
| Some observations demonstrate that mycorrhiza contains a lot of Pi and polyP [52,53]. The studies in obligate mycorhhizal fungi have shown that polyP is accumulated in fungal cells and then locally hydrolyzed to supply phosphate to symbiotic plants [52]. The content of polyP in the fungus varies during mycorrhiza development and can be used as an activity indicator of the fungus as a phosphate supplier for the plant [52]. The obligate mycorrhizal fungi have recently been shown to have a polyP-synthetase activity in the presence of ATP [53]. Mycorrhizal fungi play a key role in phosphorus supply to symbiotic plants [54]. It is associated with the ability of fungal cells to concentrate Pi from soil, to dissolve low-soluble mineral phosphorous compounds due to organic acid excretion into the medium, and to accumulate polyP. |
| Polyphosphate and Apatite: An Evolutionary Insight |
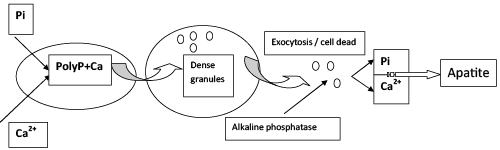
| Some pathways of phosphorus biomineralization have been maintained during the evolution from prokaryotes to the higher eukaryotes. Electron-dense granules (the so-called “dense granules”) with high Ca and P concentrations were found in rat liver mitochondria as early as in 1964 [40]. It was unclear why crystalline apatite was not formed in these granules. However, later on it was shown that such granules contained not Pi but polyP [55]. They were found in protozoa in special cell organelles (acidocalcisomes) [55] and in mammals: in the platelets [56] and mitochondria of bone and other tissues [57,58]. |
| To date, the ideas of the role of polyP in bone tissues are in brief as follows [57,59,60]. Mitochondria accumulate calcium and polyP in osteoclasts, forming dense granules. As a result of exocytosis, these granules are released into extracellular space in the place of bone growth or repair. Here, the granules are destroyed and the alkaline phosphatase hydrolyzes polyP and releases Pi. With the involvement of osteoblast-specific proteins, the structured bone apatite is formed from the released Pi and calcium. There are still a lot of unclear aspects in this process. It is unknown what enzymes are responsible for polyP synthesis in mitochondria, because the gene of the typical polyphosphate kinase responsible for polyP synthesis in bacteria has not been found in mammals. It is unknown what signals cause the release of polyP granules from osteoclasts either. |
| After destruction of platelets, polyP is released into blood, where it is involved in the coagulation cascade, being bound by factor XII and activating it, and then polyP and calcium ions enter the thrombus to increase its stability [61]. |
| There is an evolutionary analogy between phosphorus mineralization in microorganisms and the bone apatite formation and individual stages of clotting in mammals. For example, a similaritycanbe noted between the formation of sedimentary apatites by microorganisms and the formation of bone tissue apatite in mammals (Figure 2). Individual stages of these processes are characterized by predominance of either the uptake of phosphorous mineral compounds from the medium or their release from the cells (and/or release from the cells in case of death). Phosphate concentration from the medium is accompanied by local accumulation of inorganic polyphosphates in the cells. Under varying environmental conditions or cell death, polyP is released into extracellular medium and hydrolyzed by phosphatases; apatite is formed from the released Pi in the presence of calcium ions. The study of phosphate reserves in microorganisms, their structure, formation and destruction is of interest for understanding the phosphorus turnover in the biosphere and for modeling the normal and pathological processes in human organism associated with phosphate metabolism. |
References
|
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 17214
- [From(publication date):
March-2015 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 12441
- PDF downloads : 4773
