Research Article Open Access
Physical Activity and Oxidative Stress Biomarkers in Generally Healthy Women
Shuman Yang1, Majken K Jensen2,3, Palash Mallick1, Eric B Rimm2,3, Walter C Willett2,3 and Tianying Wu1,*1Department of Environmental Health, Division of Epidemiology and Biostatistics, University of Cincinnati Medical Center, Cincinnati, Ohio, USA
2Departments of Nutrition and Epidemiology, Harvard T.H. Chan, School of Public Health, USA
3The Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
- *Corresponding Author:
- Tianying Wu
Division of Epidemiology and Biostatistics
Department of Environmental Health
University of Cincinnati Medical Center
Kettering Complex, 3223 Eden Ave, Cincinnati, Ohio, USA, 45267-0056
Tel: 15135566229
Fax: 15135580925
E-mail: tianying.wu@uc.edu
Received date: October 12, 2015 Accepted date: October 23, 2015 Published date: October 30, 2015
Citation: Yang S, Jensen MK, Mallick P, Rimm EB, Willett WC, et al. (2015) Physical Activity and Oxidative Stress Biomarkers in Generally Healthy Women. J Community Med Health Educ 5:377. doi:10.4172/2161-0711.1000377
Copyright: © 2015 Yang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Objectives: The associations between physical activity and oxidative stress biomarkers are still controversial, and few large human studies have comprehensively investigated the relationship between physical activity and oxidative stress biomarkers. The purpose of this study was to examine the association between physical activity and oxidative stress biomarkers in a large sample of women by measuring biomarkers of both oxidation and antioxidant defense.
Design and Methods: We conducted a cross-sectional study among 1,144 generally healthy women ages 43-70 years, who were included in a prospective nested case-control study of coronary heart disease in the Nurses’ Health Study. Fluorescent oxidation products (FlOPs) are oxidation markers reflecting global oxidation burden. Antioxidant defense was quantified by the activities of three major antioxidant enzymes in erythrocyte (superoxide dismutase [SOD], glutathione peroxidase [GPx] and catalase [CAT]). Self-reported physical activity was estimated in metabolic equivalents per week.
Results: Physical activity was not associated with FlOP levels, or GPx and CAT activities after adjusting for covariates (all Ptrend>0.15). Higher levels of physical activity were associated with decreased SOD activity (Ptrend<0.01). We then conducted subgroup analysis of participants with and without any vigorous physical activity. Greater levels of physical activity were associated with lower SOD activity among participants with any vigorous physical activity (Ptrend=0.02).
Conclusions: Greater physical activity was associated with lower SOD activity, but not with higher plasma FlOPs in generally healthy women. Our findings may be important for women to maintain a low level of oxidative stress during exercise because high oxidative stress is related to the development of many chronic diseases
Keywords:
Physical activity; Superoxide dismutase; Antioxidant enzymes; Fluorescent oxidation products
Abbreviations:
CAT catalase, CVs coefficient of variations, Flops fluorescent oxidation products, GPx glutathione peroxidase, IQR inter quartile range, MDA malondialdehyde, METs metabolic equivalents, NHS Nurses’ Health Study, ROS-reactive oxygen species, SOD superoxide dismutase
Introduction
Oxidative stress occurs when the reactive oxygen species (ROS) overwhelm the capacity of antioxidant defense system. The excessive ROS can cause oxidative damage to the components of protein, lipids and DNA. Numerous studies have suggested that high level of oxidative stress is an important etiological risk factor for coronary heart disease, cancer and neurodegenerative diseases [1-6].
To comprehensively assess the potential burden of oxidative stress, biomarkers of both oxidation and antioxidant defense should be integrated. Previously, we [4-7] and others [8,9] have reported that the level of oxidation assessed by the fluorescent oxidation products (FlOPs) can be measured at three pairs of excitation/emission wavelengths (360/420 nm for FlOP_360, 320/420 nm for FlOP_320, 400/475 nm for FlOP_400). FlOPs are considered a global measure of oxidation stress because they are generated from many different oxidation pathways (lipid, protein and DNA) [4,6,10]. Moreover, it has been suggested that FlOP assay is 10-100 times more sensitive than measurement of malondialdehyde (MDA) via colorimetric thiobarbituric acid assay [9]. To reduce ROS, superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) are three primary antioxidant enzymes in human cells. Assessment of the levels of FlOPs and the activities of SOD, GPx and CAT in blood samples are a noninvasive and reliable approach to measure the circulating oxidative stress biomarkers [11].
Regular physical activity is an important lifestyle factor for maintaining general health, but the association between physical activity and oxidative stress biomarkers is still controversial [12-15]. On one hand, as oxygen consumption increases during exercise, the production of ROS is up-regulated. Several exercise intervention studies in humans found that MDA and lipid peroxidation were significantly elevated after high-intensity physical activity [14,15]. On the other hand, the MDA, protein carbonyl and other ROS have been shown to decrease among participants with repeated or low-intensity physical activity [12,13]. Similarly, the activities of antioxidant enzymes after physical activity have been suggested to increase in some [14,15], but not in other studies [13,16]. These controversies between studies are likely due to small sample size, different study designs and dynamic response of antioxidant defense against ROS.
Few large human studies have comprehensively investigated the relationship between physical activity and oxidative stress biomarkers. The purpose of this study was to examine the association of physical activity with the levels of FlOP_360, FlOP_320, FlOP_400 and the activities of SOD, GPx, CAT in a large sample of women.
Methods
Study setting and participants
The Nurses’ Health Study (NHS) initiated in 1976 is a longitudinal cohort of 121,701 female nurses investigating the factors that influence women’s health [17]. The NHS collected blood samples from 32,826 women between 1989 and 1991. The association of oxidative stress biomarkers with coronary heart disease has previously been evaluated in a prospective nested case-control study within the NHS blood cohort [4,18]. All participants of this prospective nested case-control study were free of cardiovascular diseases and cancers at the time of blood draw. The present cross-sectional study was conducted with a subset of the baseline data of this prospective nested case-control study, in which the levels of FlOP_360, FlOP_320, FlOP_400 and the activities of SOD, GPx, CAT and physical activity were available. One thousand one hundred and forty four women aged 43-70 years were included in the final analysis. This investigation was approved by the Institutional Review Board of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and the University of Cincinnati. All participants provided written informed consent to participate the NHS.
Demographic data collection
We ascertained age, anthropometric data (e.g. weight and height), life style data (e.g. tobacco smoking and alcohol intake), menopause status, family history of MI, history of diabetes and hypertension, and postmenopausal hormone therapy via 1990 questionnaire. Current smokers were defined as present tobacco use on the same questionnaire. History of diabetes and hypertension was reported by the participants based on their physicians’ diagnosis.
Physical activity assessment
Physical activity was assessed via structured questionnaire which has been previously described to be valid and reproducible [19]. By using the 1992 questionnaire (the time period closest to the blood draw), weekly physical activity during the previous year was estimated based on the time of sitting, standing and walking at home or work, jogging, running, bicycling, tennis playing, swimming, other aerobic exercise, low intensity exercise and flights of stairs’ climbing. Each woman was also asked her usual walking pace (<2 miles per hour for easy pace; 2-2.9 miles per hour for normal pace; >3 miles per hour for brisk pace). One MET is the energy expenditure for sitting quietly for one hour, which is approximately 3.5 ml of oxygen*kg body weight-1*min-1. As stated previously [20], physical activity expressed in metabolic equivalents (METs) per week was estimated in each study participant. Participants were classified into four groups according to METs per week: sedentary (<7.5 METs/week), light (7.5-14.9 METs/week), moderate (15-29.9 METs/week) and heavy activity lifestyle (30+METs/week) [21]. Vigorous activities were defined as requiring MET values ≥6, and included jogging (>10 minutes per mile), running (≤10 minutes per mile), bicycling, swimming, tennis, squash or racquetball, and other vigorous activities (i.e., lawn mowing).
Blood collection
Blood samples were collected in heparin anticoagulant tubes. The tubes were placed on ice packs, stored in Styrofoam containers and returned to central laboratory by overnight courier. The blood samples were centrifuged, and plasma, packed erythrocytes and buffy coats were divided into aliquots for storage in liquid-nitrogen freezers (-130°C or colder) within 36 hours. Time since last meal (hours) was collected by questionnaire during blood collection. Fast participants were defined if the length of fasting before blood draw was at least 8 hours. All the others were non-fasting participants.
Assay of FlOPs
Measurement of plasma FlOPs was performed with previously described procedures [10]. Briefly, plasma was extracted with ethanol/ether (3:1, v/v) and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was used to test fluorescence with a fluorescence spectro fluorometer. The level of fluorescence was measured at wavelengths of 360/420 nm (excitation/emission) for FlOP_360, 320/420 nm for FlOP_320 and 400/475 nm for FlOP_400, and expressed as relative fluorescent intensity units per milliter of plasma. FlOP_360 were generated from oxidized phospholipids or from lipid oxidation products reacting with proteins, DNA and carbohydrates in presence of phospholipids; FlOP_320 was formed when oxidation products such as lipid hydroperoxides, aldehydes, and ketones react with DNA in the presence of metals; and FlOP_400 reflected the interaction between MDA, proteins and phospholipids [9]. The average within-run coefficient of variations (CVs) for FlOP_360, FlOP_320 and FlOP_400 measurements were all <13%. The delay in processing blood samples up to 36 hours appeared to have minimal influence on the measurement of FlOP_360, FlOP_320 and FlOP_400. The overall intraclass correlation coefficients (ICCs) of FlOPs were all greater than 0.95 in the shorter- (0 to 24 hours) and longer-delayed processing (0 to 36 hours). A pilot study in 40 NHS participants showed that the between- and within-person ICC for repeated measurements over 1.4 year apart was 0.44 for FlOP_360, 0.55 for FlOP_320, and 0.70 for FlOP_400.
Assay of SOD, GPx and CAT activities
We measured erythrocyte SOD and GPx activities with previously described procedure [18]. Briefly, the erythrocytes were diluted with ice-cold high-performance liquid chromatography grade water and centrifuged at 10,000xg for 15 min at 4°C. Then, the samples were measured at 440-460 nm for SOD activity and 340 nm for GPx activity in contrast with the background and positive control wells. We measured erythrocyte CAT activity by using the method described by Beers et al. [22] and Aebi [23]. Erythrocyte supernatant was diluted with PO4 buffer. A standard solution was prepared using CAT from bovine liver (Cat#C1345, Sigma Corporation of America, Ronkonkoma, New York). The samples and standard were added with 20 mM H2O2. The wavelength absorbance for CAT samples and standard was 240 nm. SOD, GPx and CAT activities were expressed as U/mg of hemoglobin. The average within-run CVs for SOD, GPx and CAT measurements were 14%, 10% and 6%, respectively. The activities of the three antioxidant enzymes measured in the 48 hours delayed processing samples were not significantly different from those processed immediately (P=0.7, 0.3 and 0.5 for SOD, GPx and CAT, respectively). In the same pilot study as mentioned above (N=40), the between- and within-person ICCs for repeated measurements over an average of 1.4 years apart were 0.7, 0.8 and 0.9 for SOD, GPx and CAT, respectively.
Assay of other biomarkers
HDL cholesterol, LDL cholesterol and triglycerides were measured using reagents and standard methods designed by Roche Diagnostics and Genzyme (all CVs <6%) [24]. The level of C-reactive protein was quantified by the means of a highly sensitive immunoturbidimetric assay from Denka Seiken, and this assay has day-to-day variability between 1% and 2% [25].
Statistical Analysis
In descriptive analyses, continuous variables with normal and skewed distribution, and categorical variables were shown in means (standard deviation, SD), medians (inter-quartile range, IQR) and percentage, respectively. We further examined the distribution of the characteristics and level of biochemical variables of the study participants across quartiles of physical activity. The P for trend was estimated with linear regression models, in which physical activity was coded as a continuous variable (logarithmic transformed due to skewed distribution).
The associations between physical activity and the levels of FlOP_320, FlOP_360, FlOP_400, the activities of SOD, GPx, CAT were analyzed with linear regression models. Covariates included age (continuous), smoking (current smoker, past smoker and never smoked), month (in seasons) and time (am and pm) of blood sampling, body mass index (BMI; <25, ≥25 and <30, ≥30 and <35, and ≥35 kg/m2), alcohol intake (0, >0 and <5, ≥5 and <15, and ≥15 g/day), fasting status (<8 and 8+ hours), postmenopause (yes/no), family history of myocardial infarction (yes/no), history of diabetes (yes/no), history of hypertension (yes/no), postmenopausal hormone therapy (yes/no), LDL cholesterol (continuous), HDL cholesterol (continuous), triglycerides (continuous), C-reactive protein (continuous). We analyzed the associations of light, moderate and heavy physical activity with oxidative stress biomarkers compared to the reference group of sedentary lifestyle. Once we found a significant association between physical activity and oxidative stress biomarkers, we stratified our analysis by each covariate and intensity of the activities (with vigorous vs. without vigorous activities) to examine their influence on this relationship. Lastly, we also analyzed the association between physical activity and oxidative stress biomarkers in controls only (free of subsequent incident coronary heart disease) to rule out the potential influence of subclinical factors of coronary heart disease. All analyses were performed with Statistical Analysis System (Version 9, SAS Institute Inc., Cary, NC).
Results
Descriptive characteristics
The average age for 1,144 women was 60 years (range 43-70 years). The median physical activity per week was 11.8 METs (IQR: 4.8-25.2 METs). Approximately 46% of the study participants were overweight or obese (BMI ≥25 kg/m2). Approximately one quarter (24.3%) and one third (36.3%) of the study participants were current smokers and hypertensive, respectively. The levels of FlOPs and activities of antioxidant enzymes were in the comparable range as compared with our previous studies [4,18].
The characteristics and levels of biochemical variables stratified by quartiles of physical activity
Higher physical activity was associated with reduced BMI, triglycerides, C-reactive protein, FlOP_400, SOD activity, increased HDL cholesterol, greater proportion of postmenopausal hormone therapy and lower proportion of current smokers, history of diabetes and hypertension (Table 1). All the other factors were not significantly related to physical activity (Table 1).
| Variables | Quartile 1 <4.8 METs/week | Quartile 2 ≥4.8; <11.75 METs/week | Quartile 3 ≥11.75; <25.2 METs/week | Quartile 4 ≥25.2 METs/week | P for trend |
|---|---|---|---|---|---|
| N | 284 | 288 | 284 | 288 | --- |
| Age (years) | 59.8 (6.7) | 59.9 (6.5) | 60.2 (6.6) | 60.0 (6.3) | 0.82 |
| Body mass index (kg/m2) | 27.2 (5.4) | 24.9 (4.4) | 25.2 (4.3) | 24.8 (4.1) | <0.01 |
| Alcohol intake (g/day)a | 0.9 (0, 5.9) | 2.0 (0, 9.9) | 1.1 (0, 5.7) | 2.0 (0, 9.7) | 0.06 |
| Fasting hoursa | 11.0 (8.0, 12.0) | 11.0 (8.0, 13.0) | 11.0 (8.0, 12.0) | 12.0 (9.0, 13.0) | 0.23 |
| Current smokers (n, %) | 83 (29.2%) | 82 (28.5%) | 67 (23.6%) | 46 (16.0%) | <0.01 |
| Postmenopause (n, %) | 234 (82.4%) | 238 (82.6%) | 248 (87.3%) | 241 (83.7%) | 0.33 |
| Family history of MI (n, %) | 47 (16.6%) | 41 (14.2%) | 43 (15.1%) | 45 (15.6%) | 0.63 |
| History of Diabetes (n, %) | 47 (16.6%) | 20 (6.9%) | 21 (7.4%) | 15 (5.2%) | <0.01 |
| History of Hypertension (n, %) | 134 (47.2%) | 94 (32.6%) | 110 (38.7%) | 77 (26.7%) | <0.01 |
| PHT (n, %) Biomarkers | 94 (33.1%) | 113 (39.2%) | 113 (39.8%) | 111 (38.5%) | 0.047 |
| LDL cholesterol (mg/dL) | 137 (35) | 139 (38) | 138 (40) | 138 (36) | 0.54 |
| HDL cholesterol (mg/dL) | 52.5 (15.6) | 58.1 (16.8) | 57.1 (15.7) | 60.8 (16.9) | <0.01 |
| Triglycerides (mg/dL)a | 125 (88, 172) | 110 (77, 153) | 110 (81, 161) | 98 (70, 132) | <0.01 |
| C-reactive protein (mg/dL)a | 0.28 (0.13, 0.59) | 0.22 (0.10, 0.45) | 0.19 (0.09, 0.45) | 0.14 (0.07, 0.30) | <0.01 |
| FlOP_360 (FI/mL)a | 221 (176, 289) | 216 (178, 290) | 220 (177, 288) | 221 (171, 283) | 0.34 |
| FlOP_320 (FI/mL)a | 413 (316, 646) | 381 (296, 582) | 397 (301, 654) | 370 (288, 572) | 0.10 |
| FlOP_400 (FI/mL)a | 65.7 (49.4, 87.9) | 62.5 (49.5, 88.0) | 60.7 (49.0, 85.2) | 59.6 (47.2, 78.7) | <0.01 |
| SOD activity (U/mg of hemoglobin) | 9.05 (1.56) | 8.98 (1.61) | 8.83 (1.43) | 8.61 (1.62) | <0.01 |
| GPx activity (U/mg of hemoglobin) | 15.94 (3.83) | 16.34 (4.13) | 16.54 (4.20) | 16.18 (3.76) | 0.42 |
| CAT activity (U/mg of hemoglobin) | 235 (54) | 233 (52) | 233 (53) | 237 (54) | 0.58 |
Table 1: The characteristics and levels of biochemical variables according to quartiles of physical activity Values are means (standard deviation), unless otherwise specified. aValues for the variables that were not normally distributed are shown in medians (inter-quartile range). Abbreviations: METs: Metabolic Equivalents; PHT: Postmenopausal Hormone Therapy; Flop: Fluorescent Oxidation Products; CRP: C-Reactive Protein; GPx: Glutathione Peroxidase; CAT: Catalase; SOD: Superoxide Dismutase.
Association between physical activity and oxidative stress biomarkers
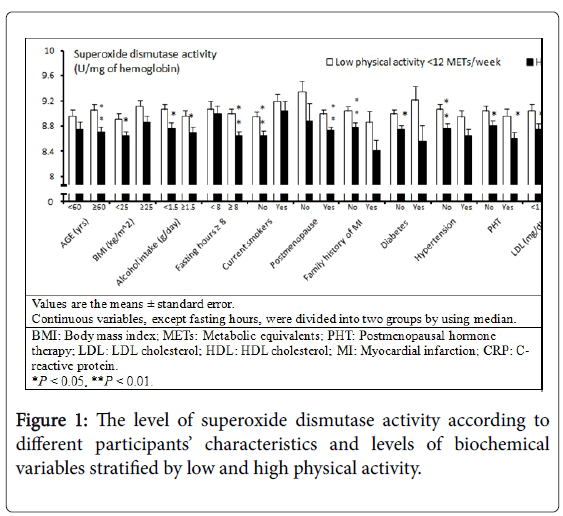
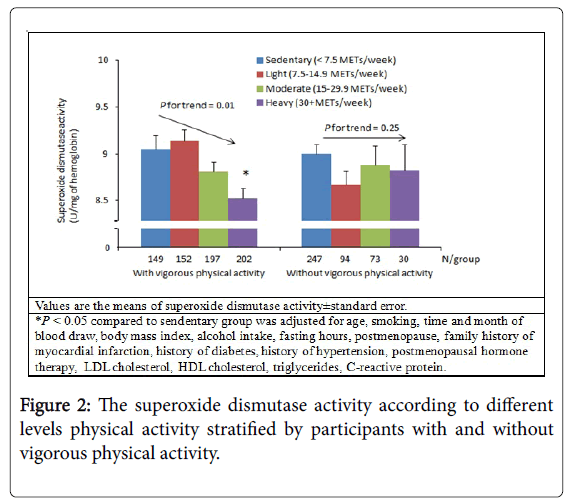
There were no associations between physical activity and FlOP_360, FlOP_320, FlOP_400, GPx, or CAT in multivariate models (Table 2). In the univariate model, higher levels of physical activity were associated with decreased SOD activity (Ptrend<0.01). In the multivariable model, the association between physical activity and SOD activity remained significant (Ptrend=0.01). Additional subgroup analysis stratified by each covariate suggested that the association between physical activity and SOD activity was likely not modified by other individual characteristics and levels of biomarkers (Figure 1). We then conducted subgroup analysis of participants with and without any vigorous physical activity. Greater levels of physical activity were associated with lower SOD activity among participants with any vigorous physical activity (Figure 2). Among multiple types of physical activity, longer time of walking or hiking outdoors, and standing or walking around home was associated with decreased SOD activity (all P values between extreme levels of PA were <0.05).
| Variables | Sedentary | Light | Moderate | Heavy | P for trend |
|---|---|---|---|---|---|
| METs/week | <7.5 | 7.5-14.9 | 15.0-29.9 | 30+ | --- |
| N | 396 | 246 | 270 | 232 | --- |
| FlOP_360 (FI/ml) in logarithmic scale | Ref | 0.04 (0.39) | 0.01 (0.86) | 0.06 (0.24) | 0.57 |
| FlOP_320 (FI/ml) in logarithmic scale | Ref | 0.03 (0.77) | 0.01 (0.91) | -0.09 (0.38) | 0.37 |
| FlOP_400 (FI/ml) in logarithmic scale | Ref | -0.03 (0.51) | -0.03 (0.39) | 0.00 (0.94) | 0.24 |
| SOD activity (U/mg of hemoglobin) | Ref | -0.03 (0.82) | -0.11 (0.38) | -0.32 (0.02) | 0.01 |
| GPx activity (U/mg of hemoglobin) | Ref | -0.26 (0.43) | 0.47 (0.15) | 0.01 (0.97) | 0.15 |
| CAT activity (U/mg of hemoglobin) | Ref | 2.21 (0.62) | 0.85 (0.85) | 5.78 (0.22) | 0.41 |
Table 2: The association between levels of fluorescent oxidation products, activities of antioxidant enzymes and physical activity: multivariable generalized linear regression analysis. Unless otherwise specified, values are β (P value) comparing sedentary lifestyle, and were adjusted for age, smoking status, time and month of blood draw, body mass index, alcohol intake, fasting hours, postmenopause, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, LDL cholesterol, HDL cholesterol, triglycerides, C-reactive protein. Abbreviations: METs: Metabolic Equivalents; FlOP: Fluorescent Oxidation Products; GPX: Glutathione Peroxidase; CAT: Catalase; SOD: Superoxide Dismutase.
When we analyzed the association between physical activity and oxidative stress biomarkers in controls only (free of subsequent incident coronary heart disease; N=760), the trend of the association between physical activity and SOD activity in this subgroup was similar to that in overall samples (β of heavy activity lifestyle vs. sedentary lifestyle=-0.18), but the association did not reach statistical significance (Ptrend=0.09), which was likely due to reduced power. Again, physical activity was not associated with other oxidative stress biomarkers in this subgroup of women (all Ptrend >0.45).
Discussion
In current study of a large sample of women, we have demonstrated that physical activity was not associated with the levels of FlOPs. Interestingly, greater physical activity was associated with decreased SOD activity. Furthermore, the inverse relationship between physical activity and SOD activity was more obvious in participants with vigorous activity than in those without vigorous activity.
The negative association between physical activity and SOD activity is in agreement with several previous studies [26-28], but not with other studies [29,30]. The specific reasons for this inconsistency are still unclear, but could be explained by dynamic response of antioxidants against ROS [31,32]. Several studies have suggested that oxidation level was either stable or decreased under repeated and relatively low-intensity types of physical activity [12,13]. A decreased SOD activity associated with increased physical activity may indicate that SOD has been used to reduce ROS generated from physical activity. This may be one of the reasons that we did not observe an elevation of FlOPs associated with higher physical activity. At the stage of mild to moderate physical activity, we hypothesize that high production of SOD may not be necessary because ROS generated from physical activity can be well controlled by existing antioxidants and antioxidant enzymes.
However, when the existing antioxidants and antioxidant enzymes are insufficient to control ROS, the production of SOD can be up-regulated, and an increased SOD activity in people with highly intensive and long-term physical activity may indicate a significantly elevated ROS due to heavy exercise. The reason that SOD is a more sensitive marker than other antioxidant enzymes in response to ROS is because SOD is the first-line antioxidant enzyme to reducing ROS [33-35]. Indeed, all the above studies with controversial results showed an elevated oxidative stress and antioxidant defense following long-term and highly intensive exercise [29,30]. In this scenario, although production of antioxidant enzymes was up-regulated, they are still insufficient to detoxify excessive ROS generated from this type of exercise. Thus, the risk of oxidative damage due to heavy exercise in these study participants is high. This is the major reason that antioxidant supplements are popular among athletes [36].
Although our study had some participants with heavy physical activity (≥30 METs/week), the physical activity level was still far less than the level reported by Tauler et al. [29]. Tualer et al. conducted an interventional study among nine male subjects, and every participant had a cycling exercise for 171.8 km within 283 minutes (it is relatively equivalent to ≥100 METs/week) before measuring the oxidative stress biomarkers. Besides the reason mentioned above, the inconsistent results of our study as compared with other studies were likely due to different study designs, in which our study was cross-sectional and the studies reported by Shin et al. [30] and Tauler et al. [29] were interventional studies. Unlike interventional studies, we did not control the physical activity level before blood draw in our study, which is a major limitation of our study. The above statements are only possible explanations for the results and should not be over interpreted. Further studies are warranted to investigate the role of SOD in the physical activity.
The major strength of this study is that a large number of female participants were included. Further, our study have measured markers of both oxidation and antioxidant defence in relation to physical activity. Thus, the associations of physical activity with oxidative stress biomarkers are comprehensive. Lastly, our study included most regular physical activities of healthy women. This is also an advantage of our study as compared to interventional studies in which types of physical activity intervention are limited.
Besides the limitation mentioned above, our study has several other limitations. We only measured SOD activity for one time, which may not accurately reflect the average levels of the biomarker in a prolonged period of time. We have assessed the reproducibility of SOD measurements over approximately one-year period in a pilot study. The ICC was excellent, indicating that this marker is relatively stable over 1~2 year period and should represent the level of SOD during the time when physical activity was assessed. There is a concern about the stability of oxidative stress biomarkers measured in the blood samples that have been stored for more than 10 years in the -130 Celsius degrees. We cannot fully exclude this possibility in this study.
Our study is also limited as the data on the long-term stability of oxidative stress biomarkers stored in liquid-nitrogen freezers were not available. In addition, since only a very small number of participants reported to have extremely high level of physical activity in our study, our findings can only be generalized to the population with relatively low and moderate level of physical activity. The results of present study may not be able to generalize to men as such data are not available. Since we only measured the oxidative stress biomarkers in the erythrocytes, the oxidative stress biomarkers at extra-cellular sites or low intensity antioxidants may be more sensitive in relation to physical activity.
Regular physical activity reduces the risk of several diseases [37]. However, we are still unclear what specific oxidative stress biomarkers we should measure in order to avoid oxidative damage due to exercise on a regular basis. Physical activity has been suggested to be inversely associated with SOD activity in our study. We observed that higher physical activity was associated with a decreased SOD activity in generally healthy women, which may partly reflect a stable oxidative stress. Our findings may be important for women to maintain a low level of oxidative stress during exercise because high oxidative stress is related to the development of many chronic diseases [1-6].
In summary, the present study suggested that an increased physical activity is significantly associated with lower SOD activity, but not with plasma FlOPs in generally healthy women. These results suggest that SOD activity may be a more sensitive marker than oxidation markers in relation to higher physical activity in this population. Certainly, further research is necessary to investigate the clinical utilization SOD activity as a marker to optimize benefit of regular physical activity to maintain general health for women.
Acknowledgments
The current study was funded by Dr. Wu’s American Heart Association grant 0430202N, K07 award (CA138714), start-up funds and P30-ES006096 and by research grants including CA186107, HL34594, CA49449, CA131218 and CA87969 from the National Institute of Health, Bethesda, MD.
References
- Holvoet P (2004) Oxidized LDL and coronary heart disease.ActaCardiol 59: 479-484.
- Koenig W, Karakas M, Zierer A, Herder C, Baumert J, et al. (2011) Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study.ClinChem 57: 1196-1200.
- Southorn PA, Powis G (1988) Free radicals in medicine. II. Involvement in human disease.Mayo ClinProc 63: 390-408.
- Jensen MK, Wang Y, Rimm EB, Townsend MK, Willett W, et al. (2013) Fluorescent oxidation products and risk of coronary heart disease: a prospective study in women.J Am Heart Assoc 2: e000195.
- Fortner RT, Tworoger SS, Wu T, Eliassen AH (2013) Plasma florescent oxidation products and breast cancer risk: repeated measures in the Nurses' Health Study.Breast Cancer Res Treat 141: 307-316.
- Wu T, Rifai N, Willett WC, Rimm EB (2007) Plasma fluorescent oxidation products: independent predictors of coronary heart disease in men.Am J Epidemiol 166: 544-551.
- Rebholz CM, Wu T, Hamm LL, Arora R, Khan IE, et al. (2012) The association of plasma fluorescent oxidation products and chronic kidney disease: a case-control study.Am J Nephrol 36: 297-304.
- Liang JH, Lin CC (2000) Fluorescence kinetics of soybean flour oxidation. J Am Oil ChemSoc 77:709-13.
- Farankel EN (1998) Lipid oxidation. Dundee: The Oily Press.
- Wu T, Willett WC, Rifai N, Rimm EB (2007) Plasma fluorescent oxidation products as potential markers of oxidative stress for epidemiologic studies.Am J Epidemiol 166: 552-560.
- Andersen HR, Nielsen JB, Nielsen F, Grandjean P (1997) Antioxidative enzyme activities in human erythrocytes.ClinChem 43: 562-568.
- Lovlin R, Cottle W, Pyke I, Kavanagh M, Belcastro AN (1987) Are indices of free radical damage related to exercise intensity.Eur J ApplPhysiolOccupPhysiol 56: 313-316.
- Nikolaidis MG, Paschalis V, Giakas G, Fatouros IG, Koutedakis Y, et al. (2007) Decreased blood oxidative stress after repeated muscle-damaging exercise.Med Sci Sports Exerc 39: 1080-1089.
- Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Valle DG (1997) Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. The Journal of sports medicine and physical fitness 37:235-9.
- Liu ML, Bergholm R, Mäkimattila S, Lahdenperä S, Valkonen M, et al. (1999) A marathon run increases the susceptibility of LDL to oxidation in vitro and modifies plasma antioxidants.Am J Physiol 276: E1083-1091.
- Sureda A, Tauler P, Aguilo A, Cases N, Fuentespina E, et al. (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radical Res 39:1317-24.
- Colditz GA, Manson JE, Hankinson SE (1997) The Nurses' Health Study: 20-year contribution to the understanding of health among women.J Womens Health 6: 49-62.
- Yang SM, Jensen MK, Rimm EB, Willett W, Wu TY (2014) Erythrocyte superoxide dismutase, glutathione peroxidase, and catalase activities and risk of coronary heart disease in generally healthy women: A prospective study. Am J Epidemiol 180:901-8.
- Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, et al. (1994) Reproducibility and validity of a self-administered physical activity questionnaire.Int J Epidemiol 23: 991-999.
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, et al. (1993) Compendium of physical activities: classification of energy costs of human physical activities.Med Sci Sports Exerc 25: 71-80.
- Shiroma EJ, Sesso HD,Moorthy MV, Buring JE, Lee IM (2014) Do moderate-intensity and vigorous-intensity physical activities reduce mortality rates to the same extent? Journal of the American Heart Association 3:e000802.
- Beers RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase.J BiolChem 195: 133-140.
- Aebi H (1984) Catalase in vitro.Methods Enzymol 105: 121-126.
- Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, et al. (2005) Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men.Circulation 112: 3375-3383.
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, et al. (2004) Inflammatory markers and the risk of coronary heart disease in men and women.N Engl J Med 351: 2599-2610.
- Sureda A, Ferrer MD, Tauler P, Maestre I, Aguilo A, et al. (2007) Intense physical activity enhances neutrophil antioxidant enzyme gene expression. Immunocytochemistry evidence for catalase secretion. Free Radical Res 41:874-83.
- Margaritis I, Palazzetti S, Rousseau AS, Richard MJ, Favier A (2003) Antioxidant supplementation and tapering exercise improve exercise-induced antioxidant response.J Am CollNutr 22: 147-156.
- Zembron-Lacny A, Ostapiuk J, Slowinska-Lisowska M, Witkowski K, Szyszka K (2008) Pro-antioxidant ratio in healthy men exposed to muscle-damaging resistance exercise.J PhysiolBiochem 64: 27-35.
- Tauler P, Sureda A, Cases N, Aguilo A, Rodriguez-Marroyo JA, et al. (2006) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J NutrBiochem17:665-71.
- Shin YA, Lee JH, Song W, Jun TW (2008) Exercise training improves the antioxidant enzyme activity with no changes of telomere length.Mech Ageing Dev 129: 254-260.
- Pietarinen-Runtti P, Lakari E, Raivio KO, Kinnula VL (2000) Expression of antioxidant enzymes in human inflammatory cells.Am J Physiol Cell Physiol 278: C118-125.
- Cases N, Sureda A, Maestre I, Tauler P, Aguiló A, et al. (2006) Response of antioxidant defences to oxidative stress induced by prolonged exercise: antioxidant enzyme gene expression in lymphocytes.Eur J ApplPhysiol 98: 263-269.
- Halliwell B (1978) Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase.Cell BiolInt Rep 2: 113-128.
- Matés JM, Pérez-Gómez C, Núñez de Castro I (1999) Antioxidant enzymes and human diseases.ClinBiochem 32: 595-603.
- Schacter LP, DelVillano BC, Gordon EM, Klein BL (1985) Red cell superoxide dismutase and sickle cell anemia symptom severity.Am J Hematol 19: 137-144.
- Powers S, Nelson WB, Larson-Meyer E (2011) Antioxidant and Vitamin D supplements for athletes: sense or nonsense?J Sports Sci 29 Suppl 1: S47-55.
- Warburton DE, Nicol CW, Bredin SS (2006) Health benefits of physical activity: the evidence.CMAJ 174: 801-809.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 15009
- [From(publication date):
October-2015 - Aug 28, 2025] - Breakdown by view type
- HTML page views : 10319
- PDF downloads : 4690