Review Article Open Access
Physiological Role of Endocannabinoid-Hydrolyzing Enzymes in Brain Development and Neurodegeneration
| Zen Kouchi* | |
| Department of Pathology, Institute for Developmental Research, Aichi Human Service Center, Japan | |
| Corresponding Author : | Zen Kouchi Department of Pathology, Institute for Developmental Research Aichi Human Service Center, 713-8 Kamiya-cho Kasugai-city, Aichi 480-0392, Japan Tel: +81 568 88 0811 Fax: +81 568 88 0829 E-mail: zkouchi@inst-hsc.jp |
| Received September 17, 2015; Accepted October 06, 2015; Published October 13, 2015 | |
| Citation: Kouchi Z (2015) Physiological Role of Endocannabinoid-Hydrolyzing Enzymes in Brain Development and Neurodegeneration. Biochem Physiol 4:180. doi: 10.4172/2168-9652.1000180 | |
| Copyright: © 2015 Kouchi Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Endocannabinoids (eCBs) such as 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamide (AEA) are important lipophilic mediators for transducing in signals organizing neuronal wiring in brain development, tuning retrograde signaling during synaptic transmission, or regulating neuroinflammation. e-CB hydrolyzing enzymes such as monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH) are key enzymes that integrate with the eCB receptors (CBR) in a distinct or cooperative manner for signaling in these diverse processes. Recently, MAGL and α/β hydrolase domain-containing protein 6 (ABHD6) have been highlighted as the primary brain 2-AG hydrolases, categorized as Ser hydrolase with a unique α/β-hydrolase fold, although FAAH preferentially hydrolyzes AEA. Brain MAGL was originally noticed as an enzyme involved in the modulation of synaptic retrograde signaling through termination of eCBR signaling by 2-AG hydrolysis, but recent elegant studies have revealed new aspects of its function in the generation of arachidonoic acid (AA) or other molecular signals that induce neuroinflammation. ABHD6 is important for 2-AG homeostasis and controls synaptic plasticity by downregulating 2-AG accumulation and efficacy at CBRs or the GABAA receptor. ABHD12 has recently been reported to hydrolyze lysophosphatidylserine in vivo, and ABHD12 knockout mice exhibit a neurologic phenotype similar to that of patients possessing inborn mutation in ABHD12, leading to the neurodegenerative disease PHARC (polyneuropathy, hearing loss, ataxia, retinosis pigmentosa, and cataract). Here we review the recent progress in understanding the mechanisms underlying CBR signaling and e-CB hydrolyzing enzymes from a physiological aspect, with emergence of attractive avenues as therapeutic targets for several neurodegenerative diseases.
| Keywords |
| Endocannabinoid hydrolyzing enzyme; Brain development; Neurodegeneration |
| Introduction |
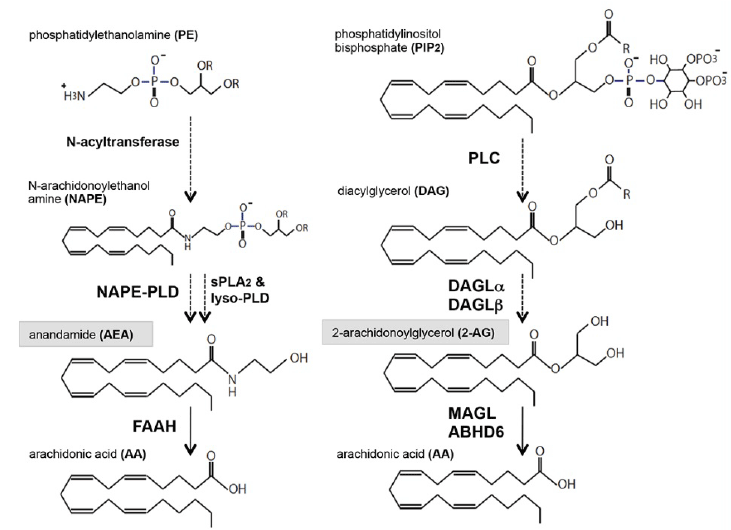
| Endocannabinoids (eCBs) are important lipophilic signaling mediators produced by neurons, astrocyte, and other glia; the primary bioactive eCBs in the brain are 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamide (AEA) [1]. Both eCBs are generated from two sequential catalytic reactions from membrane phospholipids. AEA is produced from N-arachidonoyl phosphatidylethanolamine (NAPE) hydrolyzed by phospholipase D (NAPE-PLD), with the precursor NAPE being generated from phosphatidylethanolamine (PE), although the AEA production also seems to be mediated by other multiple catalytic reactions [2]. 2-AG, the most abundant eCB, is mainly generated by either of the two sn-1-specific diacylglycerol lipases (DAGLα and DAGLβ) from diacylglycerol (DAG); DAG is obtained from phosphoinositol lipids such as sn-2 arachidnonoyl phosphatidylinositol 4,5-bisphosphate (PIP2), a reaction catalyzed by phospholipase C (PLC) (Figure 1) [3,4]. In the case of 2-AG homeostasis, depolarizationor ATP-induced Ca2+ influx and activation of metabotropic receptors by glutamate are known to trigger the sequential synthetic cascades for 2-AG via PLC and DAGL activation in neuron and microglia. Although the 2-AG and AEA generated activate CB receptors (CBRs), AEA works as a partial agonist to CBRs, whereas 2-AG acts as a full agonist [5]. There are two CBRs in the brain: CB1Rs is the most abundant G-protein coupled receptor (GPCR) in the brain and is important for retrograde signaling to modulate neurotransmitter release in excitatory or inhibitory neurons, called depolarization-induced suppression of excitation (DSE) or inhibition (DSI) mediated by the AEA and 2-AG. CB2R is normally undetectable in the brain and is expressed more in astrocytes and microglia when there is severe neuroinflammation [6,7]. Since CB1 agonists have detrimental effects on cognition and emotion, their pharmaceutical utilization has been limited. CB2R resides in immune cells, and clinical targeting by CB2 agonists is an attractive strategy for decreasing inflammatory factors in inflammation in several neurodegenerative diseases such as Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS) [8,9]. |
| In addition to e-CB synthesis, its degradation is spatiotemporally controlled by several e-CB hydrolases in the brain. Monoacylglycerol lipase (MAGL) is ubiquitously distributed in most of tissues [10]. Cytosolic MAGL catalyzes the most of the 2-AG hydrolysis in the brain, and the hydrolysis of the residual 2-AG pool is catalyzed by integral membrane hydrolases such as ABHD6 or FAAH [11]. Neuronal MAGL localizes at the presynaptic compartments and suppresses the 2-AG mediated CB1R signals through 2-AG degradation, affecting synaptic plasticity [12,13]. In contrast, neuronal ABHD6 localizes at the postsynaptic region juxtaposed with CB1R and its pharmacological attenuation induces activity-dependent 2-AG accumulation [14,15]. FAAH has broad substrate specificity in vitro but preferentially hydrolyzes AEA as an integral membrane protein at the postsynaptic compartment [16]. FAAH possesses nucleophilic serine for the catalysis with an amidase structural signature, which enables the hydrolysis of both amide and esters with the same efficiency [17]. Thus, the enzymatic property differs from that of MAGL and ABHD6 or ABHD12, which possess a catalytic triad containing histidine with a α/β hydrolase fold [18]. ABHD12 was originally considered to hydrolyze 2-AG and this putative enzymatic property might contribute to the development of PHARC, however, recent metabolic profiling analysis revealed ABHD12 as a lysophospholipase that regulates the progression of PHARC [11,19]. |
| Recent knockout mice studies have shown that MAGL is involved in synaptic dysfunction, microglial activation and production of proinflammatory cytokines along with the generation of arachidonic acid (AA) and prostaglandins (PGs) in several neurodegeneration models [20-22]. The novel finding that substantial enzymatic contribution to prostaglandin generation in the brain is dominated by MAGL but not by cytosolic phospholipase A2 (cPLA2) was initially reported by Dr. Cravatt’s group whose study involved liquid chromatography-mass spectrometry (LC-MS) analysis of lipopolysaccharide (LPS)- or 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP)-administered neuroinflammation models [20]. Similar dysregulated endocannabinoid-eicosanoid metabolism has been found to deteriorate synaptic function and Aβ clearance as seen in amyloid precursor protein (APP) pathogenesis in several APP mouse models [21,22]. However, recent progress in the understanding of the molecular mechanisms involved in neurodegeneration models reveals that a certain type of cell-specific MAGL activity plays a central role in regulating multiple proinflammatory pathways as a signaling hub to connect or dissect the CBR activation pathway [23,24]. In this review, endocannabinoid-degrading enzymes and their functions in brain development and neuropathology have been discussed with reference to several disease models. |
| eCB Metabolism in the Neurodegeneration Model |
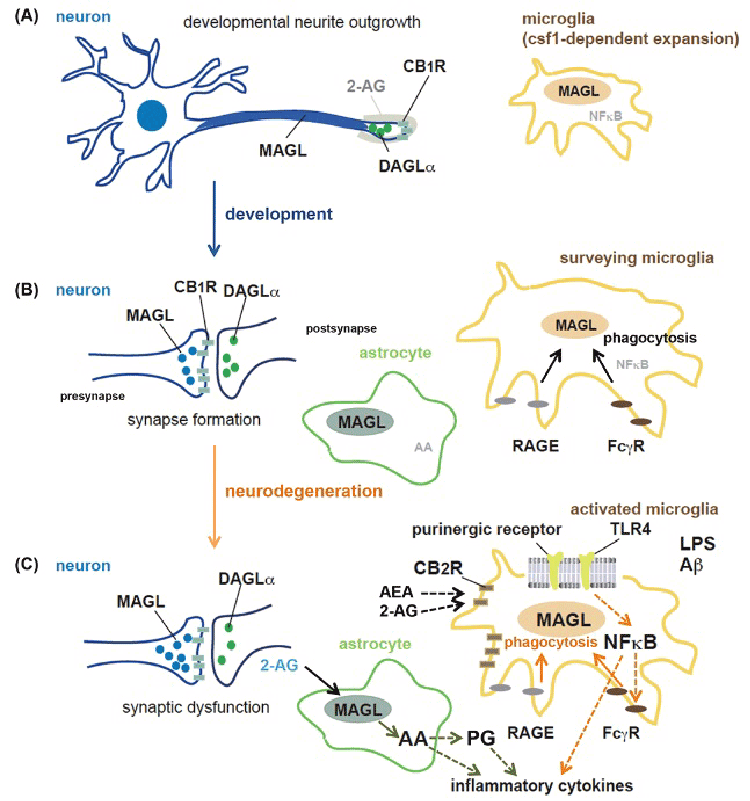
| The neuroprotective role of eCB has been indicated in neurodegeneration models such as model of Alzheimer’s disease (AD) and Parkinson’s disease (PD) by several studies. CB1R is ubiquitously localized in neurons of the CNS, CB1R has anti-inflammatory properties and suppresses the neurotoxic effects of microglia-mediated oxidative stress induced by MPTP injection on nigrostriatal dopamine neurons in a PD model, by using selective agonists or antagonists [25-27]. A lesion model injected with 6-hydroxydopamine (6-OHDA) showed increased striatal levels of AEA and decrease in FAAH activity; treatment with an FAAH inhibitor reduced spontaneous glutamatergic activity in the lesioned animals [25]. Furthermore, treatment of an MPTP mouse model of PD with a CB1R agonist such as WIN55, 212- 2 or HU210 increased survival of nigrostriatal dopamine neurons and improved motor function; the CB1R antagonist rimonabant was effective against levodopa-induced dyskinesia, and treatment-induced involuntary movements seen in the MPTP-lesioned model [26,27]. Besides application of direct neurotoxins such as MPTP or 6-OHDA, chronic infusion of low concentration of LPS into the brain is also known to induce microglial activation and selective degeneration of dopaminergic neurons [28,29]. LPS is widely used as pathogenassociated molecular patterns (PAMP) to induce the initial activation of microglia, which express many sensing receptors, including Tolllike receptors (TLRs), in several neurodegeneration models (Figure 2) [30]. 2-AG, the most abundant endogenous e-CB, is known to prevent neurodegeneration in response to LPS through CB1R signaling and reduce the expression levels of interleukin-6 (IL-6), interleukin-1 (IL- 1), and tumor necrosis factor (TNFα) in microglia [31,32]. However, neither CB1R nor CB2R antagonist inhibited these effects, suggesting that other unknown e-CB-responsive receptors or eCB- metabolizing enzymes are implicated in the cytokine regulation. Notably MAGL has been shown to possess proinflammatory roles in microglial activation and generation of several cytokines through AA and PG generation in LPS- and MPTP-administrated mouse neuroinflammation models [20]. The authors suppose that COX-1 has a prominent contribution to the catalysis of PG generation, which has been supported by the report that COX-1 inactivation by knockout or a selective inhibitor, SC-560, attenuates LPS-induced inflammatory response and neuronal injury [33]. In contrast, COX-2 is also shown to induce neurotoxicity through the formation of reactive oxygen species and is involved in NF-κB–induced microglial activation in neuroinflammation, although the therapeutic potential of its inhibitor is context-dependent [34,35]. The cell-intrinsic effects of MAGL on microglial activation and cytokine production have been analyzed: inactivation of microglial MAGL by lentiviral shRNA knockdown or its highly specific inhibitor JZL-184 did not affect the cytokine induction, and exogenous expression of MAGL in BV-2 microglial cell lines, which lacks endogenous MAGL expression, did not promote cytokine induction, although its 2-AG hydrolyzing activity was upregulated by MAGL expression [23]. A recent LC-MS analysis of neuron and glia type-specific MAGL conditional knockout mice indicated that astrocytic MAGL is mainly responsible for the generation of 2-AG and PGs in neuroinflammatory conditions [36], suggesting the complexity of the cytokine regulation by neuroglial intracellular communication in neuroinflammation. These studies indicated that the 2-AG homeostasis in inflammation may be maintained by neuronal or astrocytic MAGL, and that the AA generated might be involved in signaling in diverse pathways through microglial activation, leading to detrimental conditions for the brain homeostasis (Figure 2). |
| Imbalance of eCB synthesis and degradation systems in neuroinflammation with the progression of phenotypic conversion of microglia and neuronal toxicity has been reported by several studies involving neurodegenerative models [37]. Microglia in the resting condition express low level of MAGL due to their high susceptibility to proteolytic degradation, and LPS-induced microglial activation causes posttranslational accumulation of MAGL, which was found to be involved in the morphological changes by knockdown analysis [23]. In patients with AD, cumulative DAGLβ localization in activated microglia surrounding senile plaques was detected and IBA-1-positive microglia expressing ABHD6 seemed to be attracted to amyloid β (Aβ)- containing neuritic plaques [38]. The pathological symptoms of AD have been found to be highly correlated with MAGL expression levels, accompanied with a shift in its expression from damaged neurons to activated microglia and astrocytes in AD [38]. These changes in AG metabolism with AD progression might reflect the neuropathological changes from initial synaptic dysfunction to detrimental inflammatory conditions. Aβ peptide–induced amnesia is known to be prevented by treatment with SR141716A, a CB1R antagonist, whereas intracellular CB1R signaling supports lysosomal stabilization by interfering with Aβ-induced neurotoxicity as seen in the case of excess membrane permeability, suggesting that spatiotemporal CB1R activation may be important for neuroprotection at the onset of AD [39,40]. As neurological symptoms deteriorate with the inflammatory response, CB2R and FAAH are selectively upregulated in neuritic plaqueassociated microglia, indicating that CB2R signaling in immune cells plays an important role in the inflammatory process [41]. Treatment with selective CB1R or CB2R agonists such as WIN55, 212-2 or JWH- 133, respectively, shows attenuation of microglial activation and cognitive impairment in β-amyloid-peptide-administrated rats [42]. |
| Recently, several reports have shown that MAGL is a critical mediator for AD pathogenesis with dysregulated production of PGs being independent of CBR signaling in presenilin/APP transgenic mice harboring familial Alzheimer’s disease (FAD) mutations [21,22]. In these models, MAGL inactivation suppressed Aβ deposition and microglial activation with reduced cytokine production [21]. However, pharmacological MAGL inactivation also decreased BACE1 expression and Aβ production, suggesting that MAGL regulates specific proinflammatory cascades involved in β-secretion of APP [22]. Subsequent studies have shown that downregulation of noncoding small RNA miR-188-3 by MAGL in patients with AD or the APP transgenic model has an important effect on BACE expression, and MAGL inactivation and the resulting PPARγ activation restore the function of miR-188-3, leading to resolution of neuroinflmammation and prevention of unusual neurotransmission and spatial learning [24]. These finding indicate that the MAGL signaling axis activates diverse neuroinflammatory signaling in AD and that lipophilic 2-AG exerts neuroprotective effects, including nuclear receptor activation via MAGL inhibition [43]. |
| Endocannabinoid formation at the postsynaptic neuron is important for synaptic function, and selective impairment of α-subunits of Gq and G11 in mice leads to increased epileptic seizures in an age-dependent manner [44]. Interestingly, the amounts of 2-AG in Gαq/Gα11 knockout mice remain at significant levels, suggesting that other metabolic pathways are important for 2-AG biosynthesis. ABHD6 degrades 2-AG at the postsynaptic compartment, and its inhibition by the specific inhibitor WWL123 decreases pentylenetetrazol (PTZ)-induced seizure incidence and severity in a CB1R-independent manner in R6/2 mice, a genetic model of juvenile Huntington’s disease (HD) [45]. ABHD6 can modulate 2-AG tone at lower expression levels than MAGL, which hydrolyzes the bulk of 2-AG at the presynaptic compartment. Increase in CB2R expression is also detected in striatal microglia in R6/2 mice, and genetic ablation of CB2R in these mice promotes microglial activation and motor symptom with striatal neuronal loss, suggesting that CB2R signaling in parenchymal microglia is also important for therapeutic intervention in HD [46]. Moreover, CB2R stimulation in the peripheral immune cells suppresses neurodegeneration and CNS inflammation, resulting in improvement of motor function by selective application of the restricted CB2R antagonist SR14428 in a slowly progressing model of HD [47]. Thus, immune function through CB2R signaling has been shown as an attractive therapeutic target in neuroinflammation. |
| eCBs in Development and Neuronal Homeostasis. |
| Commitment of progenitor cells to neuronal lineage and specification in the embryonic brain occurs in the subcortical ventricular zone (SVZ) and ventricular zone (VZ), and the radial migration of immature pyramidal cells is necessary for the formation of the cortical plate in which layer-specific neuronal circuits are developmentally acquired. Several phospholipid signaling pathways are involved in the neuronal migration and differentiation process, and eCBs are also known to control the neuronal fate determination and specification in the fetal brain (Figure 2) [48-51]. CB1R and FAAH are involved in neural progenitor proliferation and radial migration of pyramidal cell progenitors, and DAGLα and DAGLβ accumulation in the motile tip of the axon for neuronal pathfinding suggests that an intrinsic eCB tone in pyramidal cells itself is important for neuronal wiring [50]. In these processes, AEA induces the elongation of the leading axon of vesicular glutamate transporter-1 (VGLUT1)-positive glutamatergic neurons, whereas local 2-AG degradation by MAGL also contributes to spatiotemporal establishment of signaling competence of 2-AG in accordance with the function of CBR and DAGL in the corticofugal axon fasciculation [50,51]. Recent multiple high-resolution immunolabeling analysis have also implicated layer-specific CB1R signaling in the inhibitory control of pyramidal cells for intracortical or corticospinal processing [52]. For example, excessive 2-AG signaling by JZL-184 affects directional axonal pathfinding, resulting in the enlargement of the corpus callosum, and MAGLs regulate spatiotemporal axonal growth through CB1R-mediated Robo1 function in corticofugal axons and oligodendrogenesis regulated by CB2R-mediated slit2 secretion [53]. CB1R activation positively regulates Coup-TFI interacting protein 2 and determines the subcerebral and corticospinal neuronal projection fate in adulthood [54]. A similar mode of 2-AG/CBR signaling in the cholinergic nervous system is depicted as an altered cholinergic innervation of CA1 pyramidal cells of the hippocampus in DAGLα null mice [55]. Interestingly, MAGL functions as a rate-limiting factor for 2-AG availability in response to nerve growth factor (NGF), which is implicated in the morphogenesis and survival of cholinergic neurons [56]. These findings indicate that MAGL regulates the developmental neuronal wiring in a spatially and temporally coordinated manner through the CB1R signaling axis with DAGL activation. |
| MAGL plays a detrimental role in neuropathology in relation to developmental disorders and MAGL inhibitors are attractive as therapeutic candidates for the improvement of the neuropsychiatric aspects. Down syndrome (DS) is caused by the triplication of chromosome 21. A mouse model of DS, that is, Ts65Dn mice, possesses three copies of mouse chromosome 16 including the APP gene, exhibits deficient working memory, and shows synaptic abnormality similar to AD type pathology [57]. Interestingly, treatment with JZL-184 completely normalizes the locomotor activity and increases the longterm potentiation with reduction in the levels of Aβ species to normal levels in Ts56Dn mice [58]. Transgenic mice expressing Dyrk1a, which is genetically localized in the DS critical region in chromosome 21, have shown altered synaptic plasticity with the disappearance of eCBmediated long-term depression (LTD) and inactivation of MAGL. JZL- 184 treatment normalizes the LTD in Dyrk1a TG mice [59]. Abnormality similar to that in eCB-mediated LTD with neuronal excitability has been seen in fragile X mental retardation protein (FMRP)-deleted mice, a model of fragile X syndrome [60]. FMRP comprises the core translational machinery in dendrites and its ablation reduces DAGL activity in synaptoneurosomes; therefore, FMRP inactivation disrupts mGluR-dependent 2-AG signaling at the excitatory synapses. The identification of the eCB signaling component as a molecular substrate for fragile X syndrome may offer a unique therapeutic approach for clinical intervention. |
| Chronic unpredictable mild stress is known to cause impairment of DSI in hippocampal CA1 pyramidal neurons, and the mouse model of stress captures the depression-like behavior. MAGL inhibition by JZL- 184 restores the decreased mTOR or ERK activity and the deficiency in retrograde synaptic depression in the hippocampus seen in stressed mice. CB1R mediated signaling is involved in the anti-depression-like behaviors as co-treatment with rimonabant, a selective CB1 antagonist, blocked the effects of JZL-184 [61]. Chronic MAGL inactivation by JZL-184 also ameliorates decreased neurogenesis and LTP induction in the dentate gyrus of the hippocampus [62]. Although the involvement of 2-AG mediated CBR signaling in the effects of MAGL inactivation on hippocampal neurogenesis remains unknown, MAGL inhibition may be effective as an anxiolytic drug target through enhanced synaptic plasticity of the hippocampus. |
| The Ser hydrolase ABHD12 has been originally identified to hydrolyze 2-AG in the brain membrane; however, untargeted liquid chromatography-mass spectrometry (LC-MS) analysis of ABHD12 knockout mice showed that it specifically possesses lipase activity toward lysophosphatidylserine in vivo [11,19]. Knockout of ABHD12 in mice precedes the development of microglial activation and an abnormal behavior phenotype resembling the PHARC phenotype, as seen in human patients [19]. ABHD12 promotes a metabolic cascade controlling the turnover of lysophosphatidylserine (lysoPS) with ABHD16a, a phosphatidylserine hydrolyzing enzyme [63]. Both ABHDs have been shown to regulate the cytokine release from macrophages with a relevance to the secreted lysoPS species as intermediate metabolites and signaling molecules. Inactivation of ABHD16a in macrophages by treatment with its specific inhibitor KC01 reduced the lyso-PS levels and TNFα production, whereas genetic ABHD12 inactivation caused lyso-PS accumulation and enhanced TNFα and IL-6 secretion. Recently triterpenoids were identified as specific inhibitors of ABHD12 from among the serine hydrolases by activity-based protein profiling of the brain membrane proteome [64]. |
| Conclusion |
| The CBR signaling system has been shown to be coordinated by several eCB-hydrolyzing enzymes such as MAGL, FAAH, and ABHD6 in distinct signaling pathways for the organization of developmental neural wiring or the maintenance of an interneuronal network. As synaptic dysfunction or mild inflammation proceeds in neurodegenerative conditions, their organized 2-AG regulation and signaling are disturbed, as seen by changes in the DAGL, MAGL, and ABHD6 activities and expression. When excitotoxic brain parenchymal lesions lead to the inflammatory cell-mediated secondary neuronal damage, such as in MS, AEA plays a neuroprotective role by regulating Erk pathway through the suppression of nitric oxide synthesis from microglial cells. Presumably, neuronal or glial damage may alter the subcellular localization of e-CB hydrolases. Disruption of eCB dynamics is detrimental to synaptic function and neuronal homeostasis regulated by microglia, and both eCB-mediated signaling and eCB hydrolysis function as molecular determinants for balance between promoting and resolving inflammatory condition during clinical onset. Recently, more specific chemical compounds for e-CB hydrolyzing enzymes and ABHD family proteins have been developed (Table 1). The use of a pharmacological strategy in addition to utilization of their conditional genetic inactivation models in neuron/glia co-culture systems and pathological mouse models will be useful for elucidating the unknown molecular mechanisms connecting eCB metabolism and biological function in order to determine promising therapeutic targets in neurodegenerative processes. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13632
- [From(publication date):
December-2015 - Aug 20, 2025] - Breakdown by view type
- HTML page views : 9005
- PDF downloads : 4627
