Research Article Open Access
Potential Role of TLR Ligand in Aethiopathogenesis of Tunisian Endemic Pemphigus Foliaceus
| Olfa Abida1*, Bouzid D1, Krichen-Makni S2, Kharrat N5, Masmoudi A3, Abdelmoula M4, Ben Ayed M1, Turki H3, Sellami-Boudawara T2 and Masmoudi H1 | |
| 1Immunology Department, Habib Bourguiba Hospital, University of Sfax, Tunisia | |
| 2Anatomy and Pathology Department, Habib Bourguiba Hospital, Sfax, Tunisia | |
| 3Dermatology Department, Hédi Chaker Hospital, Sfax, Tunisia | |
| 4Maxillofacial Surgery Department, Habib Bourguiba Hospital, Sfax, Tunisia | |
| 5Bioinformatics’ Unit, Biotechnology Center of Sfax, Sfax, Tunisia | |
| *Corresponding Author : | Olfa Abida Immunology Department Habib Bourguiba Hospital University of Sfax, PTT El Bustan BP 10 A, 3099 Sfax, Tunisia E-mail: olfaabida@yahoo.fr |
| Received September 17, 2013; Accepted October 16, 2013; Published October 21, 2013 | |
| Citation: Abida O, Bouzid D, Krichen-Makni S2, Kharrat N, Masmoudi A et al. (2013) Potential Role of TLR Ligand in Aethiopathogenesis of Tunisian Endemic Pemphigus Foliaceus. Biochem Physiol 2:117. doi:10.4172/2168-9652.1000117 | |
| Copyright: © 2013 Abida O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Pemphigus foliaceus (PF) is an autoimmune skin disease in which environmental factors are thought to participate. Recent studies suggest that microbial components use signaling molecules of the human Toll-like receptor (TLR) family to transduce signals in keratinocytes. The aim of our research was to investigate the expression of TLRs 2, 3 and 4 by keratinocytes of PF patients compared to normal keratinocytes, in order to characterise the nature of the microbial factor involved in the etiopathology of PF. Biopsies obtained from 43 PF patients and 20 healthy controls were assessed by immunohistochemical analysis using specific polyclonal antibodies. The TLR2, TLR3 and TLR4 expression was significantly upregulated in PF epidermis. The significant increase of those TLRs simultaneously may merely reflect the complicated environmental conditions of rural women in the southern rural regions of Tunisia. Interestingly, we have found that the TLR4 diffuse expression was associated with the production of anti-desmoglein 1 Abs (p=0.037). This could be in line with a potential role of TLR ligand in aethiopathogenesis of Tunisian endemic PF. TLR over-expression in pemphigus skin indicates that TLRs are involved in the pathogenesis of pemphigus through stimulation by infectious or endogenous ligands.
| Keywords |
| Pemphigus foliaceus; Toll-like receptor; Tunisia |
| Abbreviations |
| PAMPs: Pathogen-associated Molecular Patterns ; PRRs: Pattern Recognition Receptors; TLR(s): Toll-like receptor(s); PGN: Peptidoglycan; LPS: Lipopolysaccharide; PF: Pemphigus Foliaceus; auto-Abs: auto-antibodies; Dsg1: Desmoglein 1; KC(s): Keratinocyte(s); PBS: Phosphate Buffer Saline solution; HSP60: Heat Shock Protein 60; IFN-γ: Interferon gamma; IL-: Interleukin; NF-ĸB: Nuclear Factor kappa B; TNF-a: Tumor Necrosis Factor-alpha |
| Introduction |
| The pattern-recognition strategy is based on the detection of limited set of conserved molecular patterns that are unique to the microbial world and invariant among entire classes of pathogens. Pathogen-associated molecular patterns (PAMPs) are detected by pattern recognition receptors (PRRs) that signal to the host the presence of infection. Toll-like receptors (TLRs), originally identified in Drosophila [1], are the best characterized class of PRRs and comprise at least 12 proteins [2-4]. Among these, TLR2 interacts with peptidoglycan (PGN), a component of all bacterial cell walls, as well as additional constituents of Gram-positive bacteria, Mycobacteria and fungi [5]; TLR3 recognizes double-stranded RNA, a component of the life cycle of most viruses [6], and initiates immune responses to specific viral pathogens [7]. While the most important ligand of TLR4 is lipopolysaccharide (LPS), a major component of the Gram negative bacterial outer membrane [8], the TLR5 and TLR9 ligands, are bacterial flagellin [9] and bacterial CpG DNA [10], respectively. |
| Interaction of PAMPs with TLRs expressed by cells of the innate immune system or epithelia triggers a complex signaling pathway that leads to the activation of several transcription factors, such as nuclear factor kB, which then induces the activation of the inflammatory genes, such as TNF, IL-1, IL-6 and IL-8 [9-11]. Through these activation processes, TLRs also interact and modulate the adaptive immune system. In this regard, several experimental and clinical observations suggest the involvement of TLR in the pathogenesis of autoimmune diseases, like type 1 diabetes [12] and inflammatory disorders, such as ulcerative colitis and Crohn disease [13]. Understanding the ‘crosstalk’ between pathogens and epithelial cells will help to unveil the role of the environment in the triggering of autoimmune responses and the development of autoimmune diseases. |
| Endemic pemphigus foliaceus (PF), originally described as fogo selvagem in Brazil in 1903, is an organ-specific autoimmune disease characterized by the production of autoantibodies (auto-Abs) directed against desmoglein 1 (Dsg1), a protein of the specialized keratinocyte (KCs) adhesion structure called desmosome. These auto-Abs induce the loss of adhesion between KCs, leading to the formation of intraepithelial blisters of the skin [14]. Other endemic foci of PF have been identified in the rural regions of Colombia [15] and Tunisia [16]. The onset and course of PF depend on both genetic predisposing and exogenous inducing factors. The involvement of environmental factors is supported by several arguments (i) normal individuals living in the endemic areas of Tunisia [17] and Brazil [18] may possess anti- Dsg1 Abs [19] (ii) a significant association between farming and the presence of these auto-Abs has been reported (iii) epidemiological study demonstrated that Tunisian endemic PF was significantly associated with certain traditional activities [20], and (iv) finally, the potential effect of parasites such as leishmania and hydatidosis in the aetiopathogenesis of endemic PF has been suggested [21,22]. |
| Taken together, these reports provide substantial evidence that the initiation and⁄or exacerbation of skin lesions could be triggered by microbial organisms. Since the epidermis constitutes the first barrier to invasive pathogens and TLR2, 3 and 4 were shown to be a variety of immune functional receptors on KCs [23], it is thus conceivable that certain microorganisms could participate in the disease process through interaction with KC’s TLRs, and the subsequent activation of antigen-presenting cells and KCs, and the adaptive immune system. |
| The aim of this study was to investigate and compare the expression of TLR2, 3 and 4 by KCs in PF and normal skin biopsies, and then to correlate TLR expression with anti-Dsg1 auto-Ab titers a direct marker of the autoimmune response. |
| Materials and Methods |
| Subjects |
| This study was performed on paraffin-embedded skin biopsies obtained from 43 PF patients recruited from endemic southern Tunisian areas and attending the Dermatology Department of Hedi Chaker University Hospital (Sfax, Tunisia). The diagnosis of PF was established according to the standard clinical, histological and immunological criteria of the disease [24]. All specimens were taken from PF patients with active disease and before treatment (T0). |
| Twenty skin specimens were obtained from normal individuals that underwent plastic surgery. None of them suffered from autoimmune or inflammatory diseases. All patients and controls were recruited from southern Tunisian areas, belonging to the same socioeconomic population stratum and exposed to similar environmental conditions. Informed consent was obtained from both patients and controls. This study was approved by the Ethical Committee of the Habib Bourguiba University-Hospital of Sfax, Tunisia (protocol number of ethical committee: 4/12). |
| Immunohistochemistry |
| For immunohistological analysis, tissue specimens were fixed in 10% formalin buffered at pH 7•0 for 24 h and paraffin-embedded. TLR2, TLR3 and TLR4 expression was detected using anti-TLR specific rabbit polyclonal IgG (Santa Cruz Biothechnology Inc®, Santa Cruz, CA, USA), diluted 1:100 in phosphate-buffered saline (PBS) containing 5% skim milk. For immunohistochemical staining, tissue sections were treated with microwave. Nonspecific binding of Abs was blocked by incubating sections with buffered casein solution for 1 h at room temperature (Power Block Universal Blocking Reagent, Bio Genex®, San Ramon, CA, USA). Sections were then incubated with the TLR specific anti-sera for 1 h at room temperature. As second step reagent, a Biotin/Streptavidin-peroxidase detection system (HRP Detection System, Lab vision®, Fremont, CA, USA) was used according to the instructions of the manufacturer with 3-amino-9 ethylkarbazole (AEC) (K346911, Dako Cytomation®, EnVision) as substrate. Finally, sections were lightly counterstained with hematoxylin and analyzed under an Olympus BX 40 microscope equipped with an Olympus DP 70 camera (Olympus®; Tokyo, Japan). |
| Expression of TLR2, TLR3 and TLR4 in the specimens was blindly evaluated by a pathologist, independently confirmed by a second examiner and graded microscopically as follows: 0 when it was the same as background (-); 0.5, when close to background (+/-); 1, focally enhanced (+); 1.5, well-marked positivity (++); 2, strong positivity (+++); 2.5, very strong positivity (++++). Data were expressed as mean ± SD of the total of about gained values. |
| Statistical analysis |
| The statistical program, “Statistical Purchase for Social Sciences” (SPSS) v13 (SPSS Inc®, Chicago; USA), was used for description and inferential statistics. Statistical analyses were performed using Mann- Whitney tests. Differences were considered significant when p<0.05. All calculations were carried out with a 95% confidence interval (95% CI). |
| Pearson’s correlation coefficients were assessed in normally distributed data to describe the relationship between TLR expression scores and anti-Dsg 1 Abs levels. |
| Results |
| Subjects |
| The patients groups consisted of 43 PF patients (39 women and 3 men; 34.5 ± 8 years). The 20 healthy controls consisted of 11 women and 9 men were 38 ± 8 years of age. |
| TLR 2 |
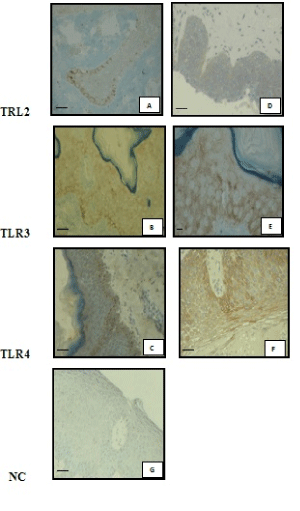
| Immunohistochemical analysis of sections from paraffin embedded normal human skin biopsies showed that only 5 out of the 20 normal skin barely expressed TLR2 (+/-). This weak immunoreactivity, close to the background, was limited to the suprabasal layers of the epidermis (Figure 1A), with a mean score value of 0.1 ± 0.2 (Table1). |
| In PF patients, the basal expression of the TLR2 was barely detected (+/-) in 2 out of the 43 biopsies, focally enhanced (+) in 3, well-marked positive (++) in 1 patient and negative in the others. However, a diffuse expression of TLR2 involving the different layers of the epidermis was detected and scored as (+) in 10 PF specimens (Figure 1D), or close to background (+/-) in 23 out of the 43. No staining was observed in 10 PF skins. Thus, in PF patients, TLR2 was predominantly expressed throughout the entire epidermis with a slight labeling in the basal layers. These results are depicted in Table 1. When TLR2 expression in PF patients was scored and compared to that of normal controls, a significant increased was observed (p<0.0001). |
| Interestingly, when the location of the immunostaining was possible, it was found to be consistently localized not on the cell surface but into the cytoplasm (Figure 1A and 1D). |
| TLR3 |
| TLR3 expression in control subject specimens and lesional skin biopsies of PF patients was shown in Figure 1B and 1E. |
| In controls, only 1 out of the 20 skin samples showed a basal and focally enhanced staining (+), whereas 10 and 9 out of those 20 controls showed a slight (+/-) (Figure 1B), or a well-marked (++) diffuse expression, respectively. The average score of this diffuse expression was 0.7 ± 0.3. |
| In the PF group, there was a stronger diffuse expression of TLR3 throughout all layers of KCs (average score: 1.094 ± 0.49), with a slight labeling of the basal layers that was scored 0.058 ± 0.27. Indeed, only 2 patient biopsies exhibited a weak (+) and 2 a well-marked (++) TLR3 basal expression, respectively, whereas the diffuse expression was well-marked (++) in 12 out of 43 (Figure 1E), focally enhanced (+) in 27 and barely (+/-) detected in 4. As observed for TLR2 expression, immunostaining was principally intra-cytoplasmic (Figure 1E). |
| Thus, a significant increase in TLR3 diffuse expression was observed in lesional biopsies of PF patients compared to controls (p<0.0001) (Table 1). |
| TLR4 |
| The TLR4 expression in PF patients and control subjects biopsies is shown in Figure 1C-1F. |
| In the control group, the immunostaining was weak and limited to the suprabasal layers (0.62 ± 0.49) (Table 1). More precisely, the expression was slight (+/-) in 8 out of 20, focally enhanced (+) in 7 and well marked in 1 out of 20 (++) (Figure 1C). |
| TLR4 expression was predominantly more pronounced in the basal layer and slightly throughout the PF’s epidermis (Figure 1F). The basal expression of TLR4 was weakly detected (+) in 21 out of the 43 PF patients, well marked (++) in 15, and strong (+++) in 2 patients. It was negative in the other 5. A diffuse expression was weak (+) in 8 patients, well marked (++) in 15, barely detected (+/-) in 5 and negative in the other 19. Again as for TLR2 and TLR3 expression, immunostaining was principally intra-cytoplasmic (Figure 1F). |
| A significant difference in TLR4 expression in the basal KCs (p<0.0001), as well as in the entire epidermis (p=0.004) in PF’s patients biopsies than in controls biopsies was observed (Table 1). |
| Correlation between expression of the different TLR in PF skin biopsies |
| Binary logistic regression was used to test the association of the diseases (PF) with TLR expression, even after adjusting for confounding factors (age, sex). The TLR3 diffuse expression and the focal TLR4 expression in basal KCs seem to be the most correlated with the disease with p values equal to 0.016 and 0.009, respectively. |
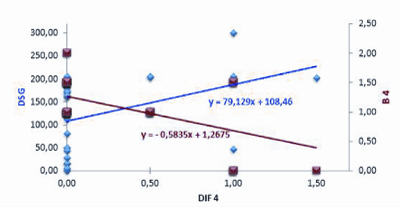
| We then looked for a correlation between the expression of the different TLRs in PF skin samples. The Pearson’s correlation study showed that the focal expression of TLR4 in basal KCs was negatively correlated with its diffuse expression throughout the epidermis (r=- 0.520; p<0.0001) (Figure 2). |
| Correlation between TLR expression and autoantibody titers |
| In patient group, anti-Dsg 1 antibodies were detected by a commercial enzyme-linked immunosorbent assay kit (MBL, Nagoya, Japan) as reported previously [17]. |
| Since TLR activation has been shown to modulate auto-Abs levels in certain autoimmune diseases [25], we asked whether a correlation could be observed between anti-Dsg1 Abs titers at the time of biopsy and TLR expression scores determined in PF sample biopsy. While no correlation was observed between Abs titers and TLR2 or TLR3 expression, anti-Dsg1 auto-Abs titers were positively correlated with the TLR4 diffuse expression (r=0.419; p=0.037) (Figure 2). |
| Discussion |
| This study showed that the expression of TLR2, TLR3 and TLR4 was significantly increased in KCs of Tunisian endemic PF patients compared to those of normal skins. |
| In normal skin specimens, TLR2 and TLR4 were found to be focally and weakly expressed by basal layer KCs, while TLR3 was weakly expressed throughout the epidermis. Our results are in agreement with those previously reported by immunohistochemistry [26,27]. On the other hand, it was shown that the mRNA expression of TLRs 3 and 4 was consistently detected at high intensity in cultured human KC, whereas TLR2 signal was lower and found inconsistently [28]. TLR expression is already known to be very low on cell surface [29], and, in addition, the preferential intra-cytoplasmic localization of TLR observed in the present study has also been described in a previous report [28], and might contribute to the poor response of KCs to microbial stimulation. Interestingly, a preferential intra-cytoplasmic TLR expression has also been observed in intestinal epithelial cells during the course of inflammatory bowel diseases [30]. |
| In contrast to the low TLR expression observed in normal human KCs, an increase expression was observed throughout the epidermis in PF patients. It provides evidence that in PF, in which KCs are hyperproliferative, there is more diffuse expression of the three TLR, which seems, therefore, to be dynamical process during inflammation. |
| As far as we know, this is the first report on TLRs expression in PF. We showed that TLR 2, 3 and 4 receptors were simultaneously and strongly up-regulated in lesional skin biopsies compared to controls biopsies, and that the TLR3 diffuse expression and the focal TLR4 expression in basal KCs were the most correlated to the disease (p=0.016 and 0.009, respectively). These observations could provide valuable insights into the role of TLRs in the development and progression of PF. Indeed the significant increase of those TLRs may reflect the complex environment of women living in the southern rural regions of Tunisia, who encounter a multitude of pathogenic microorganisms during their lifetime [20], In this regard, the potential role of viral [31,32] and bacterial [33] infections in the ethiopathogenesis of Tunisian PF has been previously suggested. However, the specific link between exposure to an environmental trigger and induction of a restricted of the autoimmune response has not yet been established. Herewith, the demonstration that anti-Dsg1 auto-Ab titers were significantly correlated to the TLR4 diffuse expression, argued for the potential role of a specific TLR4 ligand in the ethiopathogenesis of Tunisian endemic PF. |
| TLR4 upregulation could also result from the effects of endogenous ligands like the heat shock protein 60 (HSP60) [34,35]. Recently, it has been shown that HSPs play an important role in inflammatory and immunological responses of the skin by enhancing the signalling pathway of PF-antibody-mediated cellular responses, causing KCs adhesion inhibition [36]. Thus, TLR4 ligands might participate in pemphigus, both at the initial and the final lesional steps of the physiopathological process. |
| In conclusion, a modulation of TLR expression was demonstrated in skin lesions of PF patients. Those changes suggest that the activation of the innate immune system could represent an important event in this disorder. Most probably, microorganisms could trigger TLR3/ TLR4, which expression is increased in KCs of PF patients leading to NF-kB activation [37]. The activation of TLR pathways leads to proinflammatory cytokine secretion, which could maintain the chronic activation of the CD4+ T-cell in PF [38,39]. Thus, after stimulation by bacterial products, TLRs mediate a variety of signals not only for inducing pro-inflammatory cytokines, such as TNF-α, IL-6, INF-γ, and IL-12, but also for up-regulated co-stimulatory molecules, such as CD80 and CD86 to activate immune responses [40]. However, it have been reported that the expression of several TLRs is also specifically up-regulated by cytokines, such as TNF-α and INF-γ [41]. More investigation is needed to substantiate this theory. |
| Acknowledgements |
| We thank Pr. François Tron for his proofreading of the article. |
| This work was supported by a grant from the « Ministère de l’enseignement supérieur et de la recherche scientifique ». |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14320
- [From(publication date):
November-2013 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 9714
- PDF downloads : 4606
