Research Article Open Access
Qualitative and/or Quantitative Drinking Water Recommendations for Pediatric Obesity Treatment
| Jodi D Stookey1*, Rigoberto Del Toro1, Janice Hamer1, Alma Medina1, Annie Higa2, Vivian Ng2, Lydia Tinajero-Deck3 and Lourdes Juarez3 | |
| 1Children’s Hospital Oakland Research Institute, Oakland, CA, USA | |
| 2Pediatric Clinical Research Center, Oakland, CA, USA | |
| 3Healthy Hearts Program for Weight Management, Children’s Hospital & Research Center, Oakland, CA, USA | |
| Corresponding Author : | Jodi Dunmeyer Stookey Children’s Hospital Oakland Research Institute 5700 Martin Luther King Jr. Way, Oakland, California 94609, USA Tel: (415) 312-0237 Fax: (415) 753-9805 E-mail: jstookey@chori.org |
| Received August 04, 2014; Accepted October 04, 2014; Published October 11, 2014 | |
| Citation: Stookey JD, Del Toro R, Hamer J, Medina A, Higa A, et al. (2014) Qualitative and/or Quantitative Drinking Water Recommendations for Pediatric Obesity Treatment. J Obes Weight Loss Ther 4:232. doi:10.4172/2165-7904.1000232 | |
| Copyright: © 2014 Stookey JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Objective: The qualitative recommendation to ‘drink water instead of caloric beverages’ may facilitate pediatric obesity treatment by lowering total energy intake. The quantitative recommendation to ‘drink enough water to dilute urine’ might further facilitate weight loss by increasing fat oxidation via cell hydration-mediated changes in insulin. Methods: This 8 week randomized intervention tested whether both Qualitative-plus-Quantitative (QQ) drinking water recommendations result in more weight loss than the Qualitative recommendation alone (Q) in 25 children (9-12 y) with body mass index at or above the 85th percentile, given a reduced glycemic diet and usual physical activity. Random urine osmolality, saliva insulin, and body weight were assessed weekly. Mixed models explored if insulin mediated an effect of urine osmolality on weight loss. Results: In intention-to-treat analyses, QQ and Q participants did not differ significantly with respect to level of urine osmolality, saliva insulin, or weight loss. Only 4 out of 16 QQ participants complied with instruction to drink enough water to dilute urine, however. In completers analyses, the compliant QQ participants, who diluted urine osmolality from 910 ± 161 mmol/kg at baseline to below 500 mmol/kg over time (8 week mean ± SE: 450 ± 67 mmol/ kg), had significantly lower saliva insulin over time (8 week mean ± SE: 13 ± 8 pmol/l vs. 22 ± 4 pmol/l) and greater weight loss (mean ± SE: -3.3 ± 0.7kg vs. -2.0 ± 0.5 kg) than compliant Q participants (7 out of 9 participants) who maintained elevated urine osmolality over time (8- week mean ± SE: 888 ± 41 mmol/kg). Urine osmolality below 500 mmol/kg was significantly associated with weight loss. Change in saliva insulin partially explained the association. Conclusions: QQ recommendations may increase weight loss for those able to dilute urine. Work is warranted to pursue cell hydration effects of drinking water for pediatric obesity treatment.
Methods: This 8 week randomized intervention tested whether both Qualitative-plus-Quantitative (QQ) drinking water recommendations result in more weight loss than the Qualitative recommendation alone (Q) in 25 children (9-12 y) with body mass index at or above the 85th percentile, given a reduced glycemic diet and usual physical activity. Random urine osmolality, saliva insulin, and body weight were assessed weekly. Mixed models explored if insulin mediated an effect of urine osmolality on weight loss.
Results: In intention-to-treat analyses, QQ and Q participants did not differ significantly with respect to level of urine osmolality, saliva insulin, or weight loss. Only 4 out of 16 QQ participants complied with instruction to drink enough water to dilute urine, however. In completers analyses, the compliant QQ participants, who diluted urine osmolality from 910 ± 161 mmol/kg at baseline to below 500 mmol/kg over time (8 week mean ± SE: 450 ± 67 mmol/kg), had significantly lower saliva insulin over time (8 week mean ± SE: 13 ± 8 pmol/l vs. 22 ± 4 pmol/l) and greater weight loss (mean ± SE: -3.3 ± 0.7kg vs. -2.0 ± 0.5 kg) than compliant Q participants (7 out of 9 participants) who maintained elevated urine osmolality over time (8- week mean ± SE: 888 ± 41 mmol/kg). Urine osmolality below 500 mmol/kg was significantly associated with weight loss. Change in saliva insulin partially explained the association.
Conclusions: QQ recommendations may increase weight loss for those able to dilute urine. Work is warranted to pursue cell hydration effects of drinking water for pediatric obesity treatment.
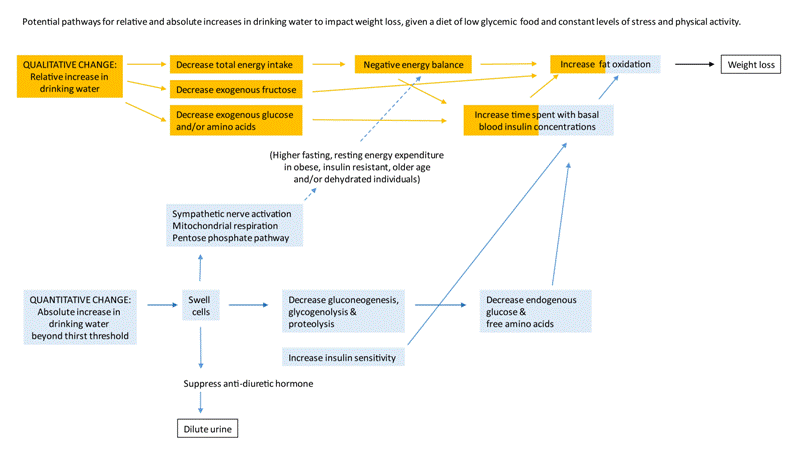
Beyond changing the type of beverage consumed to lower total energy intake, it might be possible to further impact pediatric obesity by also increasing the absolute amount of drinking water consumed to increase fat oxidation. Figure 1 describes two hypothesized pathways, whereby both qualitative and quantitative increases in drinking water might facilitate weight loss. Independent of change in total energy intake, an absolute increase in water intake may facilitate fat oxidation by swelling cells.
In healthy adults, cell swelling favors fat oxidation by limiting blood carbohydrate and insulin concentrations [4,5]. Cell swelling can lower endogenous blood glucose and free amino acid concentrations, by down-regulating gluconeogenesis, glycogenolysis, and proteolysis [6-9]. At the same time, cell swelling may accelerate glucose clearance by improving insulin sensitivity [8,9]. Elevated carbohydrate and insulin concentrations prioritize carbohydrate oxidation over fat oxidation, i.e. increase the respiratory quotient, by inhibiting the rate-limiting enzymes (hormone sensitive lipase, acylcarnitine transferase, and pyruvate carboxylase) for triglyceride breakdown, free fatty acid transport into the mitochondria, and free fatty acid oxidation by the tricarboxylic acid/Krebs cycle [10-13].
Cell swelling may also increase the overall amount of fat oxidized by increasing energy expenditure, without changing the respiratory quotient. Cell swelling can increase sympathetic nerve activity [14], stimulate the mitochondrial respiratory chain [15], shift the mitochondrial and cytosolic NADH systems to a less reduced state [16], and/or enhance flux through the pentose phosphate shunt [17].
In daily life, overnight water restriction, solute intake at meals, and insensible (via skin and lungs) and obligatory (via urine) water loss routinely create osmotic stress that draws water out of cells [18-21]. In free-living children aged 9-11 y, hyperosmotic cell shrinkage is prevalent [22] and associated with not drinking water [22]. Given that obesity is characterized by an increased Extracellular relative to Intracellular Fluid (ECF/ICF) ratio [23], there is reason to expect that obese children and adolescents may not optimally rehydrate or swell cells daily.
In overweight or obese adults, intake of 500 ml drinking water after an overnight fast increases resting fat oxidation, via hypoosmotic stimulation [24]. Similarly, in overweight or obese children, a 10 ml/kg body weight bolus of drinking water increases resting fat oxidation after overnight water restriction [25]. In both overweight or obese adults and children, the short term increases in fat oxidation after drinking water appear to be mediated by increased resting energy expenditure with a stable respiratory quotient [24,25].
In overweight dieting, pre-menopausal [26], and older adults [27], drinking water is associated with greater weight loss over 8 to 12 weeks, independent of change in total energy intake and physical activity. Cell hydration pathways conceivably mediate the extra weight loss because the effect is associated with intake of drinking water in excess of 1 L/d [26] and/or significant urine dilution [27] (specific gravity <1.01, i.e. urine osmolality <300 mmol/kg [28]). Urine dilution is a sensitive indicator of hypo-osmotic cell swelling [21,29] (Figure 1). The effect may, furthermore, reflect cell hydration-mediated changes in carbohydrate and insulin metabolism because the magnitude of extra weight loss appears to depend on the carbohydrate content of the background diet. Drinking 1+L/d water is associated with 4.7 kg additional weight loss for women on the Atkins diet, but only 2.0-3.0 kg additional weight loss for women on higher carbohydrate diets [26].
In children aged 8-15 y, drinking water interventions evaluate effects of qualitative drinking water recommendations on weight gain or obesity prevention in study populations that include normal weight or non-dieting children [30-43]. It remains to be determined if absolute increases in drinking water and cell hydration are associated with weight loss in overweight or obese children seeking obesity treatment.
The aim of this randomized intervention study was to determine if instruction to ‘drink water instead of caloric beverages’ plus instruction to ‘drink enough water to dilute urine’ (QQ: Qualitative-plus-Quantitative recommendation) results in greater weight loss over 8 weeks in overweight or obese children than the Qualitative instruction alone (Q), controlling for background diet, under conditions of usual physical activity.
The 8 week time frame was chosen, given detectable effects of drinking 1+L/d over 8 weeks in adults aged 25 to 50 y [26]. The study focused on drinking enough to dilute urine osmolality, as opposed to a specific absolute volume of water, because water requirements to swell cells are not defined for overweight or obese children. Dilute urine osmolality flags the exposure of interest-cell swelling [21], without need to know the absolute water requirement. A secondary aim of this study was to explore if insulin concentration might mediate or modify weight change effects of drinking enough water to dilute urine.
Multiple-pass recall methods were used to prompt each participant to report the timing, type, preparation, and quantity of all foods and drinks consumed on the previous day, and all physical activity. Each participant was asked about participation in organized sports, including school PE, team sports, and/or after school physical activity. The groups mean daily nutrient intakes were estimated from the 24-hour diet recalls using Nutrition Data Systems for Research software [Version 2010, NCC). The average of one-day 24-hour diet recalls provides a valid estimate for group intake [44].
All participants received their choice of plain, non-carbonated or carbonated bottled water, in large or small bottles, home-delivered for 8 weeks. Enough water was delivered to share with family members. Study participants were encouraged to take the bottled water with them to school.
The daily logs had an open space for participants to record the amount of time spent doing Moderate or Vigorous Physical Activity (MVPA) [53], moving enough to sweat (e.g. fast walking) or get out of breath (e.g. running).
Each week, the participants had a 10 min drop-in clinic visit between 10:30 am and 4 pm to turn in completed diet and activity logs, pick up new log forms and study foods for the following week, rate thirst, and collect a random urine and saliva sample.
Each week, the study coordinator reviewed the log forms, and estimated the proportion of meals consumed without a high glycemic item. Participants who did not consume a high glycemic food at more than 75% of meals (equivalent to 3 out of 4 meals per day or breakfast, lunch, and snack each day) were considered compliant with respect to the study diet. Each week, participants were encouraged to avoid high glycemic foods and caloric beverages.
The log data were used to estimate the weekly mean daily bottled water intake. Thirst was monitored as an index of hyperosmotic cell shrinkage and driver of water intake [21,54]. Participants rated their thirst on a visual analog scale from “not at all thirsty” to “extremely thirsty” [55].
The urine sample was stored on ice until processed for urine osmolality by freezing point depression osmometer (Advanced Instruments, Norwood, MA). The principal investigator reviewed the previous week’s urine osmolality with each QQ participant, and encouraged those with a urine osmolality over 500 mmol/kg to drink more water to dilute below that threshold. Midday random urine osmolality below normal (<500 mmol/kg) [56] was targeted to induce hypo-osmolality, cell swelling, and anti-diuretic hormone suppression, as opposed to iso-osmolality (no change in cell volume or antidiuretic hormone).
Unstimulated saliva was collected and stored at -80°C until assayed for insulin by ELISA (IBL International Corp., Toronto, Ontario, Canada). Saliva insulin, which varies with plasma insulin in healthy children and adults [57], was monitored instead of plasma insulin to avoid participant drop-out related to weekly blood sampling.
Personnel responsible for clinical measurements and specimen collection were unaware of the treatment assignments.
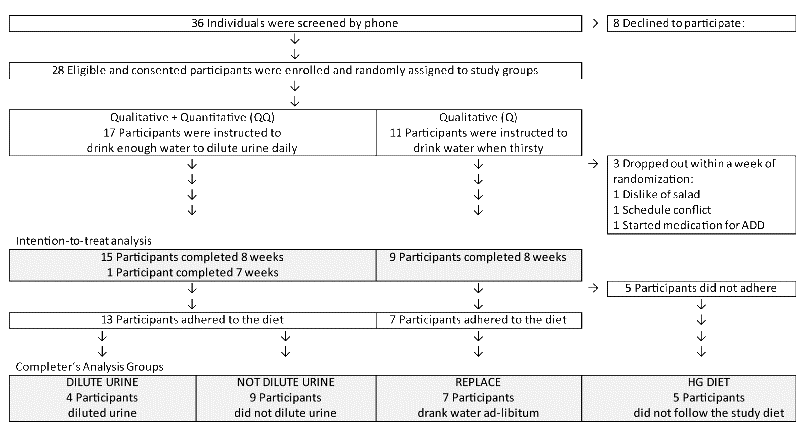
T-tests were used to evaluate baseline differences in the intervention process and outcome measures. Mixed models that included a main effect of time, group indicator variable, and the group-by-time interaction term were used to test for significant change relative to baseline. Outcome data were available for all time points for 23 out of 25 participants. Missing values were not imputed, and assumed missing at random. P-values were considered statistically significant at the two-tailed 0.05 level.
Linear regression models that controlled for demographic variables and sports participation were used to test for baseline differences in process and outcome measures between the 4 completer groups. Mixed models that included baseline status, main effects for time and the group indicator variables, the group-by-time interaction terms, and the demographic and sports covariates were used to test for significant change over time. The DILUTE URINE group was treated as reference in these models.
Table 2 describes the weight status of the intention-to-treat groups at baseline. Absolute body weight did not differ between groups. The QQ group had a significantly higher BMI percentile at baseline.
Table 1 describes changes in the weekly process measures over time for the intention-to-treat groups. After orientation, the proportion of low glycemic meals increased similarly in both the QQ and Q groups, from under 15% to over 80% of meals. The reported time spent doing moderate or vigorous physical activity did not change significantly relative to baseline for either study group. In both groups, intake of drinking water increased significantly. The change in absolute volume of drinking water did not differ by group. On average, the QQ group diluted urine to a significantly greater extent than the Q group, but only 4 out of the 17 (24%) participants assigned to the QQ group had a urine osmolality below 500 mmol/kg at half or more weekly visits. Mean urine osmolality remained over 800 mmol/kg (i.e. elevated [29]) over time for the Q group. One out of the 11 (9%) participants assigned to the Q group had urine osmolality below 500 mmol/kg at 4 visits. Saliva insulin decreased significantly relative to baseline in the QQ group.
A trend towards more weight loss in the QQ group was not statistically significant (Table 2). Change in BMI percentile was significantly greater in the Q group, with and without adjustment for the difference in initial BMI percentile.
Table 3 describes the intervention process measures over 8 weeks by completer group. The DILUTE URINE and REPLACE groups completed the study as instructed. The DILUTE URINE and REPLACE groups complied with the study diet. For 8 weeks, 3 out of 4 meals did not include a high glycemic food. Neither group increased the time spent doing moderate or vigorous physical activity.
The DILUTE URINE group significantly decreased urine osmolality to below 500 mmol/kg over time. All 4 participants in the DILUTE URINE group had urine osmolality below 500 mmol/kg, in weeks 4, 5, 6, 8, and 9. The REPLACE group did not decrease urine osmolality relative to baseline.
Saliva insulin decreased significantly by 81% in the DILUTE URINE group. Saliva insulin was below 15 pmol/L in weeks 6, 7, and 8 for all 4 participants in the DILUTE URINE group. Saliva insulin was above this value over time in the REPLACE group.
Absolute weight loss over 8 weeks was significantly greater in the DILUTE URINE group than the REPLACE group (Table 4). The completer groups did not differ significantly with respect to change in BMI percentile.
Table 5 describes the estimated weight loss attributable to urine dilution before and after control for saliva insulin. Diluting urine osmolality to below 500 mmol/kg was associated with an average ± SE 0.6 ± 0.3 kg more weight loss than having urine osmolality above that concentration, controlling for baseline weight, demographic variables, and sports participation. Addition of saliva insulin to the multivariable model reduced the estimated magnitude of effect by 17%, from 0.6 ± 0.3 kg (in Model 1) to 0.5 ± 0.2kg (in Model 2).
Saliva insulin significantly modified the association between urine osmolality and weight change (Table 5, Model 3). For individuals with a saliva insulin of 15 pmol/l or higher, having a urine osmolality below 500 mmol/kg was not associated with significant additional weight loss (0.05 ± 0.4 kg, p=0.89). For individuals with a saliva insulin below 15 pmol/l, having a urine osmolality below 500 mmol/kg was associated with 0.9 ± 0.5 kg additional weight loss (p<0.05).
Participants who were randomly assigned to the Qualitative-plus-Quantitative (QQ) group and complied (i.e. the DILUTE URINE completer group) lost significantly more weight over 8 weeks than participants who were randomly assigned to the Q group and complied (the REPLACE completer group). Given, however, that only 4 out of the 16 participants instructed to dilute urine complied, the QQ recommendation may only benefit select individuals. Weight loss did not differ significantly by intention-to-treat group. Of note, the QQ recommendation doubled the odds of drinking water to dilute urine compared to the Q recommendation.
The intention-to-treat results indicate that one-on-one orientation, free home-delivery of bottled water, and weekly feedback and encouragement to dilute urine below 500 mmol/kg were not enough for most QQ participants to meet the target drinking water condition. The failure to dilute urine cannot be attributed to impaired kidney ability to dilute urine because, when presented with a bolus of 500 ml drinking water during the baseline assessment, all participants diluted urine below 500 mmol/kg. Participants in this study reported lack of thirst and desire to avoid school bathrooms as barriers to drinking more water. Physiologic signals, such as suppressed thirst and involuntary dehydration, and environmental or social cues that discourage drinking water are known barriers to drinking water [60-63]. Participants in the NOT DILUTE URINE group reported significantly lower thirst over weeks 2-9 relative to baseline, unlike the DILUTE URINE group, which reported transiently reduced thirst in Week 3 followed by increases in thirst over weeks 4-9. The transient decrease in thirst in the DILUTE URINE group suggests adjustment to an amount of water that was initially more than thirst indicated.
The results suggest that barriers to drinking water and elevated urine osmolality may be the default or usual condition for the target population. At baseline, 88% of the study participants had a random midday urine osmolality above 500 mmol/kg; 68% of the participants had a urine osmolality above 800 mmol/kg. Without guidance about how much water to drink only 1 (<10%) of the Q participants spontaneously diluted urine osmolality below 500 mmol/kg. The mean urine osmolality above 800 mmol/kg for Weeks 2-9 for the Q group suggests chronic hyperosmotic stress and cell shrinkage.
The results of this study are consistent with insulin mediating effects of urine dilution on weight loss. Change in urine osmolality was significantly associated with change in both saliva insulin and body weight. Compared to the other groups, the DILUTE URINE group had significantly lower saliva insulin over time. The effect of having a urine osmolality below 500 mmol/kg was partially explained by having a saliva insulin below 15 pmol/l.
Saliva insulin concentrations significantly modified the effect of drinking water to dilute urine on weight loss. Saliva insulin below 15 pmol/l magnified the association between urine osmolality and weight change. Urine osmolality below 500 mmol/kg was not associated with weight loss for individuals who maintained saliva insulin above 15 pmol/l. The observed effect modification is consistent with insulin suppression of fat oxidation, as well as previous reports of greater weight loss associated with drinking water when the background diet is lower vs. higher in carbohydrate [26]. The finding implies that recommendations to drink water to dilute urine may be ineffective for weight loss without concomitant attention to background insulin concentrations.
The DILUTE URINE group included 2 participants with high baseline saliva insulin (152 pmol/l and 185 pmol/l), who might be considered insulin hyper-responders and/or more metabolically at-risk [64]. For these participants, the study diet, which limited high glycemic foods, may have been an important condition for effect.
The results warrant studies to elucidate the intermediates and pathway(s) that explain drinking water effects on fat oxidation and weight loss. Potential intermediates may include gluconeogenesis, glycogen breakdown, insulin sensitivity, mitochondrial function, ATP and NADPH generation, and sympathetic activation [6-9,14-17] (Figure 1). Reduced mitochondrial ATP production is associated with both hyperosmotic conditions and insulin resistance [15,65].
To limit participant burden, changes in total energy intake and energy expenditure were not measured. Although total energy intake at baseline did not vary by study group, and each participant was given the same study foods, it is not possible to rule out group differences in change in total energy intake as an explanation for differences in weight loss. It is unknown if the differences in weight loss reflect change in resting energy expenditure. Considering that the DILUTE URINE group reported less time doing moderate or vigorous physical activity than the other completer groups, it is unlikely that physical activity explains the greater weight loss in this group. Weight loss in this study may reflect the background 30-60 min/d of MVPA.
The small sample size limits interpretation of the results. Further work is needed to rule out bias as explanation for completer group differences, confirm the etiologic mechanism(s), and determine generalizability of the results.
Although drinking water was significantly associated with absolute weight loss, parallel relationships were not observed for change in BMI percentile. The discrepancy in results may reflect insensitivity of the BMI percentile to 10 lb weight change in children with a BMI percentile above 99%. As absolute weight loss may reflect loss of lean mass, studies involving specific measures of body fat mass and distribution are needed to confirm effects of drinking water on body fat oxidation.
Pediatric obesity treatment is notoriously resource intensive [66]. To maximize benefit for effort, further work is warranted to determine if and how multiple etiologic pathways might be leveraged to optimize drinking water effects for pediatric obesity treatment. Adding quantitative guidance about how much water to drink to qualitative advice to drink water instead of other beverages may be one way to leverage multiple pathways. Barriers to drinking water, such as lack of thirst and/or clean bathrooms, remain to be addressed.
References
- Barlow SE; Expert Committee (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 Suppl 4: S164-192.
- Academy of Nutrition and Dietetics. Tips for weight loss. Rethink your drinks.
- DellaValle DM, Roe LS, Rolls BJ (2005) Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 44: 187-193.
- Keller U, Szinnai G, Bilz S, Berneis K (2003) Effects of changes in hydration on protein, glucose and lipid metabolism in man: impact on health. Eur J Clin Nutr 57 Suppl 2: S69-74.
- Berneis K, Ninnis R, Häussinger D, Keller U (1999) Effects of hyper- and hypoosmolality on whole body protein and glucose kinetics in humans. Am J Physiol 276: E188-195.
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, et al. (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247-306.
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217: 1214-1222.
- Schliess F, Häussinger D (2003) Call volume and insulin signaling. Int Rev Cytol 225: 187-228.
- Schäfer C, Gehrmann T, Richter L, Keitel V, Köhrer K, et al. (2007) Modulation of gene expression profiles by hyperosmolarity and insulin. Cell Physiol Biochem 20: 369-386.
- Paterson CR (1987) Essentials of Human Biochemistry. Edinburg: Churchill Livingstone.
- Nutritional Biochemistry and Metabolism (1991) New York; Elsevier.
- Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF (1997) Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol 273: E768-775.
- Saris WH (2003) Sugars, energy metabolism, and body weight control. Am J Clin Nutr 78: 850S-857S.
- Badoer E, Ng CW, De Matteo R (2003) Glutamatergic input in the PVN is important in renal nerve response to elevations in osmolality. Am J Physiol Renal Physiol 285: F640-650.
- Mathai JC, Sauna ZE, John O, Sitaramam V (1993) Rate-limiting step in electron transport. Osmotically sensitive diffusion of quinones through voids in the bilayer. J Biol Chem 268: 15442-15454.
- Häussinger D, Stoll B, Morimoto Y, Lang F, Gerok W (1992) Anisoosmostic liver perfusion: redox shifts and modulation of alpha-ketoisocaproate and glycine metabolism. Biol Chem Hoppe Seyler 373: 723-734.
- Häussinger D (1996) The role of cellular hydration in the regulation of cell function. Biochem J 313 : 697-710.
- Stookey JD, Burg M, Sellmeyer DE, Greenleaf JE, Arieff A, et al. (2007) A proposed method for assessing plasma hypertonicity in vivo. Eur J Clin Nutr 61: 143-146.
- Suckling RJ, He FJ, Markandu ND, MacGregor GA (2012) Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 81: 407-411.
- Dickinson KM, Clifton PM2, Burrell LM3, Barrett PH4, Keogh JB5 (2014) Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis 232: 211-216.
- Star RA (1990) Hyperosmolar states. Am J Med Sci 300: 402-412.
- Stookey JD, Brass B, Holliday A, Arieff A (2012) What is the cell hydration status of healthy children in the USA? Preliminary data on urine osmolality and water intake. Public Health Nutr 15: 2148-2156.
- Stookey JD, Barclay D, Arieff A, Popkin BM (2007) The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr 61: 190-199.
- Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, et al. (2007) Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab 92: 3334-3337.
- Dubnov-Raz G, Constantini NW, Yariv H, Nice S, Shapira N (2011) Influence of water drinking on resting energy expenditure in overweight children. Int J Obes (Lond) 35: 1295-1300.
- Stookey JD, Constant F, Popkin BM, Gardner CD (2008) Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 16: 2481-2488.
- Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, et al. (2010) Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 18: 300-307.
- Imran S, Eva G, Christopher S, Flynn E, Henner D (2010) Is specific gravity a good estimate of urine osmolality? J Clin Lab Anal 24: 426-430.
- Manz F, Wentz A (2003) 24-h hydration status: parameters, epidemiology and recommendations. Eur J Clin Nutr 57 Suppl 2: S10-18.
- Shamah Levy T, Morales Ruán C, Amaya Castellanos C, Salazar Coronel A, Jiménez Aguilar A, et al. (2012) Effectiveness of a diet and physical activity promotion strategy on the prevention of obesity in Mexican school children. BMC Public Health 12: 152.
- Muckelbauer R, Libuda L, Clausen K, Toschke AM, Reinehr T, et al. (2009) Promotion and provision of drinking water in schools for overweight prevention: randomized, controlled cluster trial. Pediatrics 123: e661-667.
- James J, Thomas P, Cavan D, Kerr D (2004) Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ 328: 1237.
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, et al. (2006) Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 117: 673-680.
- Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, et al. (2012) A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 367: 1407-1416.
- Klesges RC, Obarzanek E, Kumanyika S, Murray DM, Klesges LM, et al. (2010) The Memphis Girls' health Enrichment Multi-site Studies (GEMS): an evaluation of the efficacy of a 2-year obesity prevention program in African American girls. Arch Pediatr Adolesc Med 164: 1007-1014.
- Cunha DB, de Souza Bda S, Pereira RA, Sichieri R (2013) Effectiveness of a randomized school-based intervention involving families and teachers to prevent excessive weight gain among adolescents in Brazil. PLoS One 8: e57498.
- Safdie M, Jennings-Aburto N, Lévesque L, Janssen I, Campirano-Núñez F, et al. (2013) Impact of a school-based intervention program on obesity risk factors in Mexican children. Salud Publica Mex 55 Suppl 3: 374-387.
- Collins CE, Dewar DL, Schumacher TL, Finn T, Morgan PJ, et al. (2014) 12 Month changes in dietary intake of adolescent girls attending schools in low income communities following the NEAT Girls cluster randomized controlled trial. Appetite 73: 147-153.
- Sichieri R, Paula Trotte A, de Souza RA, Veiga GV (2009) School randomised trial on prevention of excessive weight gain by discouraging students from drinking sodas. Public Health Nutr 12: 197-202.
- Arnberg K, Mølgaard C, Michaelsen KF, Jensen SM, Trolle E, et al. (2012) Skim milk, whey, and casein increase body weight and whey and casein increase the plasma C-peptide concentration in overweight adolescents. J Nutr 142: 2083-2090.
- Taylor RW, McAuley KA, Barbezat W, Strong A, Williams SM, et al. (2007) APPLE Project: 2-y findings of a community-based obesity prevention program in primary school age children. Am J Clin Nutr 86: 735-742.
- Rosario R, Oliveira B, Araujo A, Lopes O, Padrao P, et al. (2012) The impact of an intervention taught by trained teachers on childhood overweight. Int J Environ Res Public Health 9: 1355-1367.
- Veitch J, Singh A, van Stralen MM, van Mechelen W, Brug J, et al. (2011) Reduction in sugar-sweetened beverages is not associated with more water or diet drinks. Public Health Nutr 14: 1388-1393.
- Willett W (1998) Nutritional Epidemiology. 2nd Edition, Oxford University Press, Oxford.
- Foster-Powell K, Holt SH, Brand-Miller JC (2002) International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76: 5-56.
- Spieth LE, Harnish JD, Lenders CM, Raezer LB, Pereira MA, et al. (2000) A low-glycemic index diet in the treatment of pediatric obesity. Arch Pediatr Adolesc Med 154: 947-951.
- Thomas DE, Elliott EJ, Baur L (2007) Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev : CD005105.
- Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS (2003) A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med 157: 773-779.
- IOM (Institute of Medicine) (2010) School Meals: Building Blocks for Healthy Children. The National Academies Press, Washington DC.
- Armstrong LE (2007) Assessing hydration status: the elusive gold standard. J Am Coll Nutr 26: 575S-584S.
- Bonci L (2009) Sport nutrition for coaches. Human Kinetics, Champaign Illinois.
- Nissenberg SK, Pearl BN (2002) Eating right from 8 to 18: Nutrition solutions for parents. John Wiley & Sons, New York.
- Weston AT, Petosa R, Pate RR (1997) Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc 29: 138-143.
- Institute of Medicine of the National Academies, Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board (2005) Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press, Washington DC.
- Rolls BJ, Bell EA, Thorwart ML (1999) Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr 70: 448-455.
- Schrier RW (2007) Concentration and dilution of the urine. Disease of the Kidney & Urinary Tract, 8th edition, Wolters Kluwer, Lippincott Williams & Wilkins, Philadelphia.
- Stookey JD, Hamer J, Espinoza G, Higa A, Ng V, et al. (2012) Orange juice limits postprandial fat oxidation after breakfast in normal-weight adolescents and adults. Adv Nutr 3: 629S-635S.
- Ogden K, Kuczmarski R, Flegal K, Mei Z, Guo S, et al. (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109: 45-60.
- CDC Children’s BMI tool for schools.
- Nose H, Mack GW, Shi XR, Nadel ER (1988) Role of osmolality and plasma volume during rehydration in humans. J Appl Physiol (1985) 65: 325-331.
- Greenleaf JE, Sargent F 2nd (1965) Voluntary dehydration in man. J Appl Physiol 20: 719-724.
- Greenleaf JE (1992) Problem: thirst, drinking behavior, and involuntary dehydration. Med Sci Sports Exerc 24: 645-656.
- Millard-Stafford M, Wendland DM, O'Dea NK, Norman TL (2012) Thirst and hydration status in everyday life. Nutr Rev 70 Suppl 2: S147-151.
- Goran MI, Bergman RN, Cruz ML, Watanabe R (2002) Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes Care 25: 2184-2190.
- Ritz P, Berrut G (2005) Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab 31 Spec No 2: 5S67-65S73.
- Whitlock E, O’Conner E, Williams S, Beil TL, Lutz KW (2010) Effectiveness of Primary Care Interventions for Weight Management in Children and Adolescents: An Updated, Targeted Systematic Review for the USPSTF, Report No: 10-05144-EF-1, Agency for Healthcare Research and Quality, Rockville MD.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14312
- [From(publication date):
December-2014 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 9727
- PDF downloads : 4585
